Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHAO Wen(赵文), HUO Yuanzi(霍元子), ZHANG Tianmin(张天民), WANG Shan(王珊), SHI Tingting(石婷婷)
- Effects of lithium on the survival, growth, and reproduction of Daphniopsis tibetana Sars (Crustacea: Cladocera)
- Chinese Journal of Oceanology and Limnology, 35(4): 754-762
- http://dx.doi.org/10.1007/s00343-017-6047-z
Article History
- Received Mar. 2, 2016
- accepted in principle Apr. 5, 2016
- accepted for publication May. 24, 2016
Lithium was found in trace amount in organisms (Chassard-Bouchaud et al., 1984), and the influence on the organisms and human are widely concerned because of the widespread use of lithium-ion batteries and the development of related industry. Studies in mammals have shown that its importance to health used as an essential trace element, can be prolong its life span (Schrauzer, 2002; Zarse et al., 2011), excess concentrations of lithium are toxic to biological organisms (Wang et al., 2014). In investigation of Northern Tibet, We found that lithium content is higher in salt lake of Daphniopsis tibetana occurred (e.g. Lake Bange) (Zheng et al., 1989; Zheng, 1995; Zhao et al., 2010a, b). How dose lithium influence on population growth of D. tibetana? This is worth indepth research. D. tibetana Sars, a genus of peculiar Cladocera, can be regarded as halobionta species according to Goulden (1968), Gong (1983), Beadle (1981), Williams (1983), Hammer (1986), He et al. (1989), Alonso (1990), Zhao (1991), Zhao and He (1993), Zhao et al. (1996) and Ren et al. (1996), which was a endemic species living in plateau lakes at altitude of more than 4 000 m and occurs widely in inland saline lakes in northern Tibet, Qinghai and Xinjiang, China. Zhao et al. (2005a), in a study of biological and ecological features of saline lakes in northern Tibet of China, reported that D. tibetana was recorded at five saline lakes, and the highest density was 417.2 ind./L; Zhao et al. (2002), even 7.8 ind./L of D. tibetana were there when water temperature was-1℃ in winter in Namuka Co. Other than distributions, the biology and population ecology of D. tibetana were little studied (Chiang and Du, 1979; Zhao et al., 2002, 2004, 2005b). D. tibetana can be living in the sea water (Zhao et al., 2002, 2005b; Huo et al., 2011).
In many of the saline lakes in Tibet and Xinjiang, D. tibetana is the dominant zooplankton species, and always becomes the climax species, thereby playing an important role in inland saline waters. The density and biomass of D. tibetana is typically higher in moderately saline waters (Zhao et al., 2002, 2008, 2010a, b). The rapid development and expansion of mariculture has resulted in the scarcity of live food for many cultured species. To address this, researchers have evaluated the utility of using zooplankton such as Parvocalanus crassirostris (Mckinnon et al., 2003), Temora longicornis (Helland et al., 2003) Tisbe sp. (Nanton and Castell, 1998), Gladioferens imparipes (Payne and Rippingale, 2000, 2001a, b), Calanidae copepod (Payne et al., 2001); Oithona oculata (Molejón and Alvarez-Lajonchère, 2003), Centropages namatus, Acartia spp. (Schipp et al., 1999; Marcus and Murray, 2001), and Moina mongolica (Zhao et al., 2008) as live feed. D. tibetana is a halobiont species that occupies cold water habitat (Zhao et al., 2002). The rate of embryonic development and reproductive capacity of D. tibetana is lower than that of Daphnia and Moinidae, but higher than Copepoda. Thus, D. tibetana offers potential for use as a live feed for marine fish larvae, shrimp, and crabs, particularly for cold water species.
In addition to temperature and salinity, the concentration of lithium is known to influence the population dynamics of D. tibetana because of high evaporation (>2 000 mm) and low precipitation ( < 300 mm) can be rising salinity and the concentration of lithium. In our previous result, the 24 h and 48 h half lethal concentration (LC50) was 386.34 and 325.42 mg/L, respectively (Huo et al., 2011). This study aimed primarily to determine the toxic effect of lithium to D. tibetana and the effect of lithium on growth and reproduction of D. tibetana. Additionally, this paper can supply scientific data for mass culture of D. tibetana, and also helps to explain why population seasonal dynamics of this species in Namuka Co.
2 MATERIAL AND METHOD 2.1 Experimental animalsAdult parthenogenetic Daphniopsis tibetana were obtained from a saline lake in Namuka Co. between July and August, 2002 in northern Tibet, China. The average concentration of lithium cations was 10.41 mg/L (Zhao et al., 2010a). Female D. tibetana were taken to the laboratory and held in diluted seawater (mixing seawater and fresh water, salinity 15±0.5 g/L at 15±0.5℃) under a 14 h L:10 h D photoperiod and fed a monoculture of Dunaliella salina (3.0×105 cells/mL). The light intensity ranged from 1 800 to 2 000 lx (35.1–39.0 μE/(m2∙ s)).
2.2 Daphniidaes medium rearingThe medium for rearing the daphniidaes was prepared by diluting autoclaved, aerated, and Nucleopore-filtered seawater (FSW: 32 practical salinity unit) with distilled water to a final salinity of 15±0.5. The rearing media had the following properties: pH 7.8±0.2; disolved oxygen (DO) 7.68±0.46 mg/L; Ca2+ 213.4±3.6 mg/L; Mg2+ 697.2±3.0 mg/L; total alkalinity (ALK) 77.56± 2.84 mg/L. The initial concentration of Li+ was 79.09–80.55 μg/L. D. tibetana cultures were kept in an environmental chamber as described above. The media in each bottle was prepared with 300.8, 318.6, 357.5, 378.6, 401.1, 424.8, or 450.0 mg/L Li+.
2.3 Acute toxicity of Li+ to D. tibetanaWe prepared a stock solution of lithium (20 g/L) using seawater and AR-grade LiCl·H2O. New-born neonate D. tibetana (age < 12 h) were assigned to one of seven treatment groups (n=10 ind./group) and transferred to a glass-stoppered bottle (60 mL) containing 50 mL medium (5 bottles/treatment group). We monitored for mortality over a 48-h period. The criterion for death was failure to respond to mechanical stimulation. Survival data were used to calculate the LC50 using the Probit Method (Zhou and Zhang, 1989). In general, the safe concentration means the content of pollution matter to the biological does not produce harmful effect. It is used for acute exposure. We assessed the safe concentration value using the following formula (Zhou and Zhang, 1989):
Safe concentration=(24hLC50×0.3)/(24hLC50/48h LC50)3.
2.4 Effect of Li+ on growth and reproduction of D. tibetanaTo determine the effect of salinity on growth, life span, and neonate production, juvenile D. tibetana were assigned to one of six treatment groups that were reared long term in water containing 5, 10, 20, 40, or 60 mg/L lithium. The control group comprised D. tibetana reared at the safe concentration. Three hundred and ninety newborn neonates (age < 12 h, length 840.7±19.1 μm) were transferred to a glass stoppered bottle (volume 50 mL, n=1 ind./bottle, n=15 bottles/treatment group). Additionally, ten juveniles were placed in a single bottle and 5 such bottles were prepared for each group. The test medium in each bottle was changed daily. Salinity and pH were measured three times each day and were maintained within 0.5 g/L and 0.2 pH units of the target, that is, the maximum variation rate in salinity and pH were 3.33% and 2.56%, respectively. We added fresh algae suspension daily and supplemented the bottle with freshwater to maintain the salinity. We measured the change in length (μm) of each live specimen daily using an ocular scale under a Olympus Microscope (40×). To assess reproduction, the neonates produced in each bottle were counted and discarded daily. We then calculated the total number of neonates produced, time to first brood, numbers of broods, number of young produced per brood, mean interval between clutches, and life span (days). The experiments lasted until the death of all animals.
2.3 Data analysisWe calculated the relative growth rate using length data (GR), intrinsic rate of population increase (rm) (Gotelli, 1995) and reproductive value (VX) (Krebs, 1994). The intrinsic rate of increase (rm), net reproduction rate (R0), generation time (T) and finite rate of increase (λ) were calculated according to Krebs (1994):
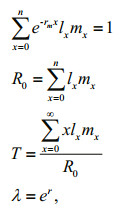
where lx is the age-specific survival probability, mx is the fecundity (the number of juveniles produced per female during the interval x), and x is larval age (days). All data were analyzed using the statistical software SPSS v.17.0 for Windows (SPSS, Chicago, IL). Two-way ANOVA and one way ANOVA were followed by Duncan's multiple range tests or Fisher's least significant difference for comparisons among treatment means. Data are expressed as the mean±standard error. Differences were considered significant or highly significant if P < 0.05 or P < 0.01, respectively.
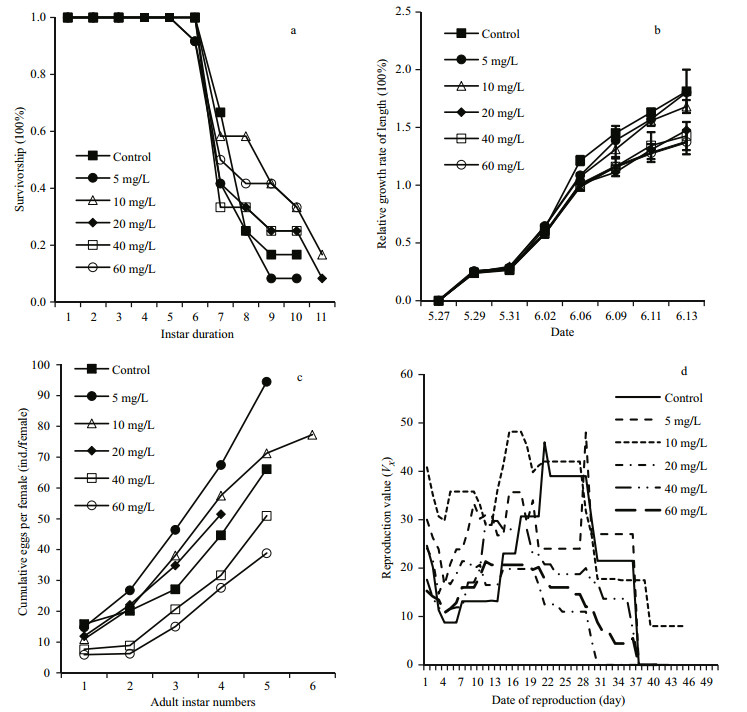
3 RESULT 3.1 Survival and growthThe safe concentration value was 69.3 mg/L. Survival was high (100%) in all treatment groups prior to the first adult instar, but declined rapidly after the sixth instar (Fig. 1a). Overall statistical analysis showed that the survival rate in 10 mg/L Li+ was significantly higher than the other groups (P < 0.05). On day 17, the GR was highest in the control group (181.4%), followed by the 5 mg/L (180.4%) and 10 mg/L (168.2%) groups. GR in control group and 5, 10 mg/L Li+ treatment group were significantly higher than the other treatment groups (P < 0.05) (Fig. 1b). D. tibetana that were reared at ≥20 mg/L lithium grew at a significantly slower speed than those reared at concentrations of ≤10 mg/L Li+ (P < 0.05, n=15) (Fig. 1c–d).
|
| Figure 1 Survivorship (a), relative growth rate (mean±SD length) (b), rate of egg reproduction (c), and reproductive value (d) of D. tibetana cultured in different concentrations of lithium |
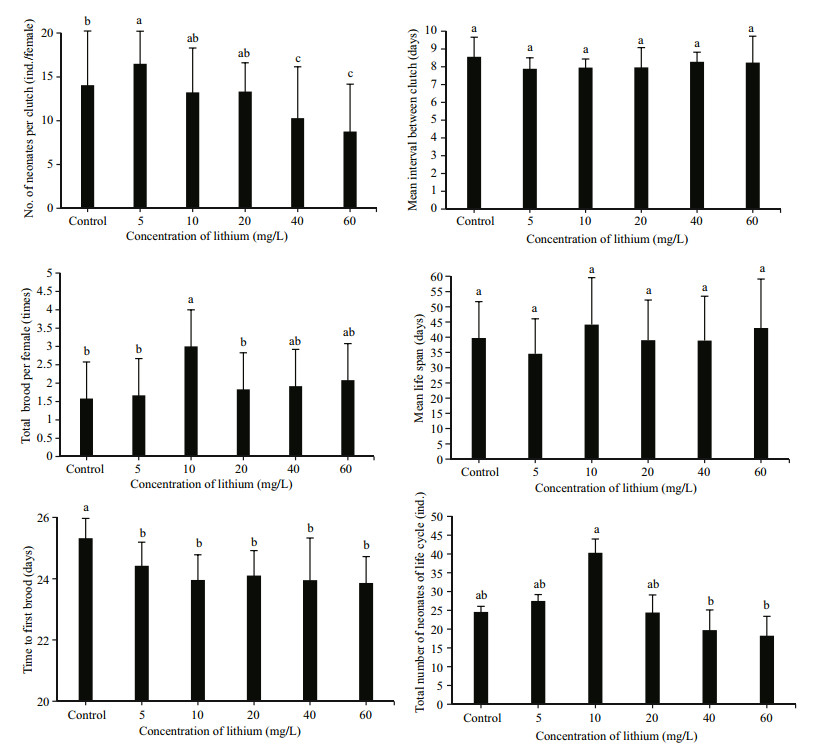
The time to the first brood was 25.3 d for the control group, which was significantly longer than the time for the remaining groups (23.9–24.1 d). Females reared in 60 mg/L lithium produced significantly fewer neonates per clutch (8.8 ind.) over their lifetime than those reared at lower concentrations. In contrast, D. tibetana that were reared at 5 mg/L produced 16.5 ind. per clutch, which was significantly higher than all other groups (P < 0.01). The total number of neonates per female and number of reproductive events per female were significantly higher for individuals reared in 10 mg/L lithium (40.3±6.7 ind. and 3.0±1.95, respectively) than those reared at the remaining concentrations (P < 0.01). There was no difference in the mean interval between broods (7.9–8.6 d) and mean life span (34.6–44.2 d) between groups (Fig. 2).
|
| Figure 2 Effect of lithium concentration during culture on reproductive indices (mean±SD) in D. tibetana Different letters indicate significant differences (P < 0.05). |
The cumulative neonatal production of D. tibetana was plotted against the number of adult instars to determine the rate of egg production, represented by the slope (a) of the regression line (Murugan and Sivaramakrishnan, 1976), where the intercept (b) represents the number of neonates in the first brood. The rate of egg production (a) was 1.203 1 and 1.242 6 for D. tibetana reared at 40 and 60 mg/L, respectively, but the number of neonates in the first brood (b) was 0.771 4 and 0.638 4, which was significantly lower than in the control. Conversely, the a values for females reared in 5, 10, and 20 mg/L lithium (1.152 8, 1.156 0, and 1.042 5, respectively) were significantly higher than in the control (0.860 0), whereas the b values were similar to that of the control (Fig. 1c). The VX was higher 19, 27, and 13 d after maturation in the 5, 10, and 20 mg/L groups than in the control (Fig. 1d)
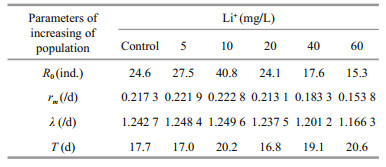
3.3 Life tableThe intrinsic rate of population increase (rm), net reproductive rate (R0), and finite rate of increase (λ) were highest in the 10 mg/L group followed by the 5 mg/L group (Table 1). The values of these population indices were significantly lower for the 40 and 60 mg/L groups compared with the control (Table 1).
|
Parameters include the intrinsic increasing rate of population (rm), net reproductive rate (R0), the finite rate of increase (λ) and generation time (T).
3.4 Postembryonic developmentEach individual passed through 5 preadult instars and 4–6 adult instars. In general, the cumulative duration of each instar was longer in the control than in the remaining groups (P < 0.05, Fig. 3a). The duration of the first preadult instar and the 2th adult instar only was significantly longer in the control than in the remaining groups (P < 0.01, Fig. 3b)

|
| Figure 3 Effect of lithium concentration during culture on the mean (±SD) cumulative duration of each instar (a) and duration of each instar (b) in D. tibetana |
A recent study of the biological and ecological features of saline lakes in northern Tibet by Zhao et al. (2005a) documented the environmental conditions experienced by D. tibetana in five localities. Except for Ca2+, the conditions experienced by D. tibetana in our study were within the ranges observed in the wild. The concentration of total hardness (TH, 34.34 mmol/L) was within the range needed for D. tibetana to survive (5.5–53.0 mmol/L). The salinity (15 g/L) and temperature (15℃) were close to the optimum values for D. tibetana survival and reproduction (Zhao et al., 2008), and were similar to the values observed in Namuka Co during August (Zhao et al., 2010b). Thus, our results in the laboratory can be used to infer the effects of lithium on the seasonal population dynamics of D. tibetana observed in saline lakes.
Under specific environmental conditions, a small change in the environment can be reflected by changes in the intrinsic rate of population increase (rm), an important index that describes the reproductive capacity of species (Lin, 1963; Krebs, 1994; Manuel, 1999). The rate of egg production (a) is positively correlated with the number of adult instars and mean neonates per brood (Murugan and Sivaramakrishnan, 1976; Huang, 1983, 1985). The reproductive value (VX) is important in determining the contribution to the future population that an individual female will make (Krebs, 1994; Sun, 2001). In terms of rm, VX, a, R0, and λ, experimental results can be used to evaluate the factors affecting the survival, growth, and reproduction of D. tibetana. The values of these indices are a reflection of the physiological state of D. tibetana because exogenous behavior operates endogenously by affecting genes, enzymes and metabolism (Zheng, 1990). Thus, the optimum concentrations of lithium for D. tibetana can be determined by measuring these parameters. In our study, the mean number of neonates per brood, the number of clutches per female, the rate of egg production, and the total number of neonates per female of were highest in D. tibetana reared in 5 or 10 mg/L Li+. Similarly, the values for rm, R0, and λ were better in these two groups than for the remaining groups. Additionally, the VX value peaked rapidly within 19–28 d after maturation in the 5 and 10 mg/L Li+ groups, suggesting that a larger population can be obtained in a shorter time when D. tibetana are reared in media containing between 5 and 10 mg/L of lithium. In summary, our results suggest that the optimum lithium concentration for rearing D. tibetana is between 5 and 10 mg/L.
A small quantity of trace metals such as Cu2+, Zn2+, Co2+, or Ni2+ can accelerate the growth, reproduction, and development of plankton and other crustacean species (Gao et al., 1995; Yuan et al., 1995; Yuan et al., 1998; Liu et al., 2001; Wang et al., 2001; Wang et al., 2003) because of their positive effects on enzyme activity (e.g., alkaline phosphatase, ATPase, digestive enzymes). However, few studies have evaluated the effects of lithium on aquatic animals. Schou (Mertz, 1994) concluded that Li+ may play a role in amine and electrolyte metabolism, adenylate cyclase activity, and carbohydrate metabolism. Anke et al. (1983) noted that supplementation of goat feed with 24 μg/g lithium resulted in increased growth and rates of impregnation relative to goats that only received 3.3 μg/g lithium in the diet. This is consistent with our observations in which low doses of lithium (5– 10 mg/L) can accelerate growth, reproduction, and development in D. tibetana.
Huo et al (2011) stated that the 48 h LC50 of lithium was 325.4 mg/L for D. tibetana and may reflect adaptations to coping with metal stressors. Gasso (1998) noted out that animals experiencing environmental stress exhibit changes in morphophysiological indices, including total protein and lipids, lipid fractions, DNA and RNA levels, and mixed function oxygenases. Populations within a species may also have different levels of adaptation reflected by the population structure, reproductive strategy, and metabolic rate (Wang et al., 2005). Our results suggest that D. tibetana adapted to exposure to a lithium stressor by shortening the duration of development, accelerating growth, and rapidly reaching peak VX. Over the long term, these changes may result in the evolution of D. tibetana that are adapted to different environments. On the other hand, D. tibetana has adapted to the lithium at their original site and this is reflected in the data observed.
5 CONCLUSIONThrough the research in this thesis, following conclusion can be drawn: The safe concentration value was 69.3 mg/L. Time to first brood of D. tibetana was lowest at lithium content of 5 mg/L. Mean neonates per female of D. tibetana was 16.0 ind. in 5 mg/L of Li+. 5–10 mg/L of lithium can accelerate growth and reproduction of D. tibetana.
| Alonso M, 1990. Anostraca, cladocera and copepoda of Spanish saline lakes. Hydrobiologia, 197(1): 221–231. Doi: 10.1007/BF00026952 |
| Anke M, Groppel B, Kronemann H, Gruen M. 1983. Evidence of essentiality of lithium in goats. In: Anke et al. eds. Lithium: Spurenelement Symposium. F. Schiller Universitaet, Jena. p. 58-64. |
| Beadle L C. 1981. The Inland Waters of Tropical Africa. 2nd edn. Lo Ecological Principal of Animal ngman, London, New York. 212p. |
| Chassard-Bouchaud C, Galle P, Escaig F, Miyawaki M, 1984. Bioaccumulation of lithium by marine organisms in European, American, and Asian coastal zones:microanalytic study using secondary ion emission. Comptes Rendus de l'Academie des Sciences. Serie Ⅲ, Sciences de la vie, 299(18): 719–724. |
| Chiang X C, Du N S. 1979. Fauna Sinica: Crustacea: Freshwater Cladocera. Science Press, Peking. p. 122-124. (in Chinese) |
| Gao C N, Qu K M, Zhang D X, Yuan Y X, 1995. Optimum zinc ion activity in seawater for egg hatching and nauplius metamorphosis of Penaeus chinensis. Journal of Fishery Sciences of China, 2(3): 1–7. |
| Gasso V Y, 1998. P4F142-different levels of reptile homeostasis under conditions of environmental stress. Toxicology Letters, 95(S1): 238. |
| Gong X J. 1983. Rotifera of Tibetan plateau. In: The Chinese Academy of Sciences of the Qinghai-Tibet Plateau Comprehensive Scientific Expedition ed. Aquatic Invertebrates of the Tibetan Plateau. Science Press, Beijing. 492p. (in Chinese) |
| Gotelli N J. 1995. A Primer of Ecology. Sinauer Associates, Sunderland, MA, USA. 365p. |
| Goulden C E, 1968. The systematics and evolution of the Moinidae. Transaction of the American Philosophical Society, 58(6): 1–101. Doi: 10.2307/1006102 |
| Hammer U T. 1986. Saline Lake Ecosystem of the World. Springer, Dordrecht. 330p. |
| He Z H, Qin J G, Wang H Q, Wang Z Y, Xia X, 1989. Studies on the saline and hypersaline zooplanktons from Jinnan and Yinchuan regions. Acta Hydrobiologia Sinica, 13(1): 24–37. |
| Helland S, Terjesen B F, Berg L, 2003. Free amino acid and protein content in the planktonic copepod Temora longicornis compared to Artemia franciscana. Aquaculture, 215(1-4): 213–228. Doi: 10.1016/S0044-8486(02)00043-1 |
| Huang X F, 1983. Effect of temperature on the development, growth and egg production in Moina affinis (Cladocera, Moinidae). Acta Hydrobiologica Sinica, 8(1): 105–112. |
| Huang X F, 1985. Studies on the biology of three freshwater Cladoceran species. Oceanologia et Limnologia Sinica, 16(3): 189–194. |
| Huo Y Z, Zhao W, Wei J, 2011. Acute and joint toxicity of Cu2+, Zn2+, Co2+, Ni2+ and Li+ to Daphniopsis tibetana Sars. Journal of Fishery Sciences of China, 18(2): 466–471. |
| Krebs C J. 1994. Ecology: The Experimental Analysis of Distribution and Abundance. 4th edn. Harper Collins College Publishers, New York. p. 184-186. |
| Lin C S, 1963. Theoretical and laboratory studies of animal population dynamics Ⅰ. The significance and application of laboratory populations and of mathematical models in the studies of animal population dynamics. Acta Zoologica Sinica, 15(3): 371–381. |
| Liu C Q, Wang A L, Wang W N, Liu Y, 2001. Influences of metal ions in seawater on activities of alkaline phosphatase (AKP) and ATPase in mysis and postlarvae of Penaeus chinensis. Journal of Fisheries of China, 25(4): 298–303. |
| Manuel Jr C M. 1999. Ecology: Concepts and Applications. McGraw-Hill Companies, Inc, New York. p. 218. |
| Marcus N H, Murray M, 2001. Copepod diapause eggs:a potential source of nauplii for aquaculture. Aquaculture, 201(1-2): 107–115. Doi: 10.1016/S0044-8486(01)00514-2 |
| McKinnon A D, Duggan S, Nichols P D, Rimmer M A, Semmens G, Robino B, 2003. The potential of tropical paracalanid copepods as live feeds in aquaculture. Aquaculture, 223(1-4): 89–106. Doi: 10.1016/S0044-8486(03)00161-3 |
| Mertz W. 1994. Trace Elements in Human and Animal Nutrition. Zhu L Z trans. Qingdao Press, Qingdao. p. 315-330. (in Chinese) |
| Molejón O G H, Alvarez-Lajonchère L, 2003. Culture experiments with Oithona oculata Farran, 1913(Copepoda Cyclopoida), and its advantages as food for marine fish larvae. Aquaculture, 219(1-4): 471–483. Doi: 10.1016/S0044-8486(02)00644-0 |
| Murugan N, Sivaramakrishnan K G, 1976. Laboratory studies on the longevity, Instar duration, growth, reproduction and embryonic development in scapholeberis kingi sars (1903) (Cladocera:Daphnidae). Hydrobiologia, 50(1): 75–80. Doi: 10.1007/BF00016844 |
| Nanton D A, Castell J D, 1998. The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture, 163(3-4): 251–261. |
| Payne M F, Rippingale R J, Cleary J J, 2001. Cultured copepods as food for West Australian dhufish (Glaucosoma hebraicum) and pink snapper (Pagrus auratus) larvae. Aquaculture, 194(1-2): 137–150. Doi: 10.1016/S0044-8486(00)00513-5 |
| Payne M F, Rippingale R J, 2000. Evaluation of diets for culture of the calanoid copepod Gladioferens imparipes. Aquaculture, 187(1-2): 85–96. Doi: 10.1016/S0044-8486(99)00391-9 |
| Payne M F, Rippingale R J, 2001a. Effects of salinity, cold storage and enrichment on the calanoid copepod Gladioferens imparipes. Aquaculture, 201(3-4): 251–262. Doi: 10.1016/S0044-8486(01)00609-3 |
| Payne M F, Rippingale R J, 2001b. Intensive cultivation of the calanoid copepod Gladioferens imparipes. Aquaculture, 201(3-4): 329–342. Doi: 10.1016/S0044-8486(01)00608-1 |
| Ren M L, Quo Y, Wang J L, Su R, Li H, Ren B. 1996. Survey of Artemia Ecology and Resources in Inland Salt Lakes in Northwest China. Heilongjiang Science and Technology Press, Harbin. 260p. (in Chinese) |
| Schipp G R, Bosmans J M P, Marshall A J, 1999. A method for hatchery culture of tropical calanoid copepods, Acartia spp. Aquaculture, 174(1-2): 81–88. Doi: 10.1016/S0044-8486(98)00508-0 |
| Schrauzer G N, 2002. Lithium:occurrence, dietary intakes, nutritional essentiality. Journal of the American College of Nutrition, 21(1): 14–21. Doi: 10.1080/07315724.2002.10719188 |
| Sun R Y. 2001. Ecological Principal of Animal. 3rd edn. Peking Normal University Press. p. 146-161. (in Chinese) |
| Wang H B, Shu W S, Lan C Y, 2005. Ecology for heavy metal pollution:recent advances and future prospects. Acta Ecologica Sinica, 25(3): 596–605. |
| Wang J M, Ren W, Wang W N, Wang A L, 2003. The effects of Co2+ and NP2+ in seawater on the hatchability and metamorphosis of Artemia saline. Journal of Aquaculture, 24(1): 34–38. |
| Wang W N, Wang A L, Sun R Y, 2001. Effects of Cu2+, Zn2+, Fe3+, Co2+ in freshwater on digestive enzyme and alkaline phosphatase activity of Macrobrachium nipponense. Acta Zoologia Sinica, 47(S): 72–77. |
| Wang Y, Zhang Y, Zhang H J, Liu D X, Liu Z X, Cheng X J, Lu Y, Jia J C, Liu J T, Pan F, 2014. The toxic effect of lithium ion on neurons (PC12 cells) and Aβ42 molecules. Biological Trace Element Research., 159(1-3): 410–415. Doi: 10.1007/s12011-014-9949-z |
| Williams W D. 1983. Life in Inland Waters. Blackwell Scientific Publications, Melbourne. 252p. |
| Yuan Y X, Qu K M, Liu L B, Gao C N, Zhang D D, 1995. Optimum copper ion activity in seawater for egg hatching and nauplius metamorphosis of Penaeus chinensis. Acta Oceanologica Sinica, 17(1): 83–89. |
| Yuan Y X, Qu K M, Xin F Y, 1998. Optimum concentration of microelements in seawater culture solution of marine algae culture. Journal of Fishery Sciences of China, 5(2): 45–51. |
| Zarse K, Terao T, Tian J, Iwata N, Ishii N, Ristow M, 2011. Low-dose lithium uptake promotes longevity in humans and metazoans. European Journal of Nutrition, 50(5): 387–389. Doi: 10.1007/s00394-011-0171-x |
| Zhao W, He Z H, Ren S R. 2008. Biology and Technology of Culture and Utilization in Marine Water for Cladocera in Inland Saline Waters. Science Press, Beijing. 163p. (in Chinese) |
| Zhao W, He Z H, 1993. Rotifera from inland saline waters in northern China. Journal of Dalian Fisheries University, 8(2-3): 20–31. |
| Zhao W, Jiang H, He Z H, 1996. Planktonic Crustaceans of inland saline waters in Sanbei District, Northern China. Journal of Dalian Fisheries University, 11(1): 1–13. |
| Zhao W, Wang Q H, Zheng M P, Zhao Y Y, Wang H L, 2002. A preliminary study on the biology of Daphniopsis tebitana Sars. Journal of Dalian Fisheries University, 17(3): 209–214. |
| Zhao W, Ying X W, Peng Z, Wei J. 2010a. Ecology of Inland Saline Lakes in China. Science Press, Beijing. (in Chinese) |
| Zhao W, Zhang L, Hu Y Z, 2005b. The effect of salinity, temperature and body length on oxygen consumption of Daphniopsis tibetana Sars. Acta Ecologica Sinica, 25(7): 1549–1553. |
| Zhao W, Zhang P, Huo Y Z, Wang H L, 2004. Study of karyotype on Daphniopsis tibetana Sars (Cladocera:Daphniidae). Journal of Dalian Fisheries University, 19(3): 167–170. |
| Zhao W, Zhao Y Y, Wang Q H, Zheng M P, Zhang P, He Z H, 2010b. Spatial and temporal patterns of plankton assemblage structure in a high altitude saline lake, Namuka Co in Northern Tibet, China. Clean-Soil, Air, Water, 38(10): 960–968. Doi: 10.1002/clen.v38:10 |
| Zhao W, Zheng M P, Xu X Z, Liu X F, Guo G L, He Z H, 2005a. Biological and ecological features of saline lakes in northern Tibet, China. Hydrobiologia, 541(1): 189–203. Doi: 10.1007/s10750-004-5707-0 |
| Zhao W, 1991. A review on the Cladocera in inland saline waters. Journal of Dalian Fisheries University, 6(2): 31–41. |
| Zheng M P, Xiang J, Wei X J, Zheng Y. 1989. Saline Lakes on the Qinghai-Xizang (Tibet) Plateau. Beijing Scientific and Technical Publishing House, Beijing. (in Chinese) |
| Zheng M P, 1995. On "saline lake agriculture". Acta Geoscientic Sinica(4): 404–418. |
| Zheng Z, 1990. Reproductive capacity of crustacea in relation to the environment, Ⅰ. Cladocera. Journal of Ecology, 9(5): 36–41. |
| Zhou Y X, Zhang Z S. 1989. Toxicity Experiment Method for Aquatic Living. China Agriculture Press, Beijing. 266p. (in Chinese) |
 2017, Vol. 35
2017, Vol. 35


