Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Rongfeng(李荣锋), YU Huahua(于华华), YUE Yang(岳洋), LIU Song(刘松), XING Rong'e(邢荣娥), CHEN Xiaolin(陈晓琳), LI Pengcheng(李鹏程)
- Sulfated polysaccharides with antioxidant and anticoagulant activity from the sea cucumber Holothuria fuscogliva
- Chinese Journal of Oceanology and Limnology, 35(4): 763-769
- http://dx.doi.org/10.1007/s00343-017-5339-7
Article History
- Received Dec. 17, 2015
- accepted in principle Apr. 15, 2016
- accepted for publication May. 10, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China
Polysaccharides from marine organisms have many good activities and can be used as foods, health products, and prodrugs. For example, chitosan that is mainly prepared from the shell of shrimp and crab, is a natural polysaccharide mainly composed of β-1, 4 linked d-glucosamine, and partially of β-1, 4 linked N-acetyl-d-glucosamine (Li et al., 2012a). Chitosan is wildly applied in the fields of food, biomaterials, and drug delivery because of its various bioactivities, such as antitumor, antioxidant and antimicrobial activities (Li et al., 2013b; Younes et al., 2014). In addition, polysaccharides from seaweed were proved to have antioxidant, anticoagulant, neuroprotective, antitumor activities (Wang et al., 2011; Li et al., 2013a; Jin et al., 2013, 2014), and some were even used as important prodrugs in the clinical medicine (Wang et al., 2011).
Sea cucumber is a traditional nutritional food and medicinal resource widely consumed in China, Japan, and Korea. Polysaccharide is one of the most important bioactive components in sea cucumber. A fucoidan from Acaudina molpadioides could reverse the changes in tissue oxidation and antioxidase activities, ease regulate the signal pathways of mitogen-activated protein kinases and matrix metalloproteinases, and the damage of ethanolinduced gastric (Wang et al., 2012). A sulfated polysaccharide from Stichopus japonicus could change the morphological and proliferation of astrocytes in vitro if it combined with a basic fibroblast growth factor 2 (Sheng et al., 2011). A fucosylated chondroitin sulphate from Cucumaria frondosa exhibited anti-hyperglycaemic effects and inhibited pancreatic islets apoptosis via inhibition mitochondrial pathway (Hu et al., 2014a, b). In addition, some other polysaccharides had anticoagulant activities and could be potentially applicable as antithrombotic agents with reduced bleeding risk (Mourão et al., 1996; Matsuhiro et al., 2012; Ye et al., 2012; Luo et al., 2013; Wu et al., 2013).
Sea cucumber Holothuria fuscogliva (Phylum Echinodermata, Class Holothuroidea, order Aspidochirotida, Family of Holothuriidae) is a big sea cucumber that can reach about 50 cm long and 13 cm wide when matured (Liao, 1997). This sea cucumber is rich of bioactive polysaccharides. However, no studies have reported on the preparation and bioactivities of polysaccharides in H. fuscogliva at present. Therefore, we prepared polysaccharides from the sea cucumber in an enzyme hydrolysis method and investigated the antioxidant and anticoagulant activities. It will be significant to the development and utilization of the sea cucumber in food, health product, or even medicine industries in the future.
2 MATERIAL AND METHOD 2.1 Materials and reagentsSea cucumber H. fuscogliva was identified by Dr. N Xiao, Department of Marine Organism Taxonomy & Phylogeny, Institute of Oceanology, Chinese Academy of Sciences. The monosaccharide standards of D-galactose, L-fucose, D-mannose, L-rhamnose monohydrate, D-glucuronic acid, D-glucose, and D-xylose were purchased from Sigma (USA). The anticoagulant activity assay kits were purchased from Nanjing Jiancheng Bioengineering Institute. Other reagents used in this study were of the highest quality available from commercial vendors.
2.2 Preparation of polysaccharidesThe sulfated polysaccharides of H. fuscogliva (HfP) were prepared according to the method described by Liu et al. (2012) and Zheng et al. (2007) with some modifications. Briefly, dry H. fuscogliva without viscus was cut into small pieces and then immersed in 30 times (v/w) of pH 6.0, 0.1 mol/L NaAc buffer, containing 5% papain, 5 mmol/L EDTA and 5 mmol/L cysteine, and incubated at 60℃ for 24 h. The mixture was centrifuged at 3 000×g for 15 min, and then added 2 times of ethanol into the supernatant, standing at 4℃ for 12 h. Then, the precipitate was collected and washed by ethanol. After freeze-drying, the crude polysaccharides were dissolved in distilled water and adjusted to pH 10.0 with 0.1 mol/L KOH. The polysaccharides solution was added with 1/7 v: v 30% H2O2, and incubated at 60℃ for 24 h. The solution was then centrifuged at 3 000×g for 15 min and the supernatant was adjusted to pH 2.0 with 1 mol/L HCl. After centrifugation at 3 000×g for 15 min, the supernatant was added with KAc to 1.5 mol/L, standing at 4℃ for 12 h. After centrifugation at 3 000×g for 15 min, the precipitate was washed with ethanol and then freeze-dried to obtain polysaccharide of HfP.
2.3 Molecular weight assayAn Agilent 1260 HPLC system in high performance gel permeation chromatography of TSKgel G3000 PWXL column (7.8 mm×30.0 cm) was employed to measure the molecular weight of HfP by eluting with 0.2 mol/L Na2SO4 at a flow rate of 1.0 mL/min. The eluate was monitored by a refractive index detector, and the column calibration was performed with standard dextrans Mw 670 000, 270 000, 80 000, 50 000, 25 000, 12 000, 5 000 and 1 000 Da (Sigma, USA).
2.4 Monosaccharide composition and sulfated content analysisThe molar ratio of monosaccharide composition of HfP was determined following Zhang et al. (2009). Briefly, 10 mg/mL sample was hydrolyzed with 2 mol/L trifluoroacetic acid in a 10-mL ampoule. It was then sealed in nitrogen atmosphere and hydrolyzed at 110℃ for 4 h and adjusted to pH 7.0 with 0.1 mol/L NaOH. Later, it was converted into its 1-phenyl-3-methyl-5-pyrazolone derivatives and analyzed by a HPLC chromatography, using Ribose as the internal standard. Sulfate content was analyzed with barium chloride-gelatin method (Kawai et al., 1969).
2.5 FTIR and NMR spectroscopyFourier transform infrared (FTIR) spectra of HfP was measured in the 4 000–400/cm regions using a Thermo Scientific Nicolet iS10 FT-IR spectrometer in KBr discs. Twenty mg HfP was co-evaporated with D2O twice. Then it was dissolved in D2O containing 0.1 μL deuterated acetone. A Bruker AVANCE Ⅲ 600 MHz was employed to analyze its NMR spectra at 25℃ and the internal standard of deuterated acetone was used as the chemical shifts.
2.6 Antioxidant activities assay 2.6.1 Hydroxyl radical scavenging activityThe hydroxyl radical scavenging activity of HfP was determined according to Guo et al. (2005). Briefly, the reaction mixture, total volume 4.5 mL, containing different concentrations of HfP, was incubated with 220 μmol/L EDTA–Fe2+, 0.23 μmol/L safranine O, 60 μmol/L H2O2 in 150 mmol/L, pH 7.4 PBS for 30 min at 37℃ and then measured the absorbance at 520 nm. As the hydroxyl radicals can bleach the safranine O, the increased absorbance indicated the capability of the hydroxyl radical scavenging activity of the sample, which can be calculated by the follow equation:
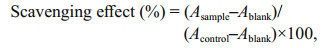
Ablank is the absorbance of the blank, using double distilled water (ddH2O) instead of the samples and Acontrol is the absorbance of the control, using dd H2O instead of H2O2.
2.6.2 Superoxide radical scavenging activityThe superoxide radical scavenging activity of HfP was determined according to the method of Nishikimi et al. (1972). Briefly, the reaction mixture, containing different concentrations of HfP, 30 μmol/L PMS, 338 μmol/L NADH, and 72 μmol/L NBT in 16 mmol/L, pH 8.0 Tris-HCl buffer, was incubated 5 min at room temperature and then measured at 560 nm. The capability of superoxide radical scavenging activity was calculated by the following equation:
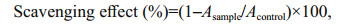
Acontrol is the absorbance of the control, using Tris-HCl instead of NADH.
2.7 Anticoagulant activities assay 2.7.1 Activated partial thromboplastin time (APTT)The APTT of HfP was measured according to the manufacturer's instructions. Briefly, 40 μL plateletpoor plasma was mixed with 10 μL solutions of HfP at different concentrations in 0.9% NaCl, and added 50 μL preheated APTT into the mixture, followed by an incubation at 37℃ for 5 min. At the same time, 0.025 mol/L CaCl2 was also preheated at 37℃. Then, these two preheated solutions were mixed, and the time for clot formation was recorded by an automatic coagulation analyzer (Healife HF6000-4, China). The controls were used 0.9% NaCl instead.
2.7.2 Prothrombin time (PT)The PT of HfP was tested according to the manufacturer's instructions. Briefly, 100 μL PT reagent was incubated for 5 min. Then, 10 μL HfP with different concentrations in 0.9% NaCl was mixed with 40 μL platelet-poor plasma. The mixed solution was preheated for 3 min. Then, these two preheated solutions were mixed, and recorded the time for clot formation by an automatic coagulation analyzer (Healife HF6000-4, China). The controls were used 0.9% NaCl instead.
2.7.3 Thrombin time (TT)The TT of HfP was tested following the manufacturer's instructions with some modifications. Briefly, 90 μL platelet-poor plasma was mixed with 10 μL HfP with different concentrations in 0.9% NaCl. After preheating for 3 min, the solution was then mixed with 100 μL TT reagent and recorded the time for clot formation was recorded by an automatic coagulation analyzer (Healife HF6000-4, China). The controls were used 0.9% NaCl instead.
2.8 Statistical analysisAll data in this study were presented as means± standard deviation and the statistical differences between the experimental groups and controls with P < 0.05 was considered to be statistically significant.
3 RESULT AND DISCUSSION 3.1 General analysisThe average molecular weight of HfP was about 1 867.1 Da using an Agilent 1260 HPLC system with a TSKgel G3000 PWXL column. The molar ratio of monosaccharide composition of HfP showed that Man: Rha: Glc A: Glc: Gal: Xyl: Fuc=0.083 6: 0.437: 0.134: 0: 1.182: 0.748: 1 (Fig. 1), and the sulfate content of HfP is about 20.7%.
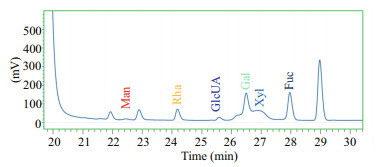
|
| Figure 1 HPLC analysis of monosaccharide composition in HfP Man: mannose; Rha: rhamnose; GlcUA: glucuronic acid; Gal: galactose; Xyl: xylose; Fuc: fucose. |
The FTIR spectra of HfP and its methylated derivative were showed in Fig. 2. In the 4 000–1 800/ cm region, there was a peak at 3 431.96/cm, which was contributed by NH2, NHAc, or OH group. We also observed two small peaks at 2 926.50/cm and 2 854.87/cm, which should be assigned to CHO, CH3, CH2, and CH group. In the 1 800–400/cm region, we can find a peak at 1 656.70/cm, which was obviously contributed by COOH group. Moreover, the peaks at 590/cm and 1 240.88/cm were assigned to the SO4 group. As FTIR spectra could provide only primary information about the functional groups, more information was confirmed by 1H NMR (Fig. 3). Similar spectra containing characteristic signals, the main peaks were assigned. Specifically, signals at 1.30×10-6 was readily assigned to the methyl protons of Fuc (CH3) (Wu et al., 2013). Signals at 1.98×10-6 was assigned to the Acetyl galactosamine protons of GalNAc (CH3), which was coincidence with the previous results (Dong et al., 2014).
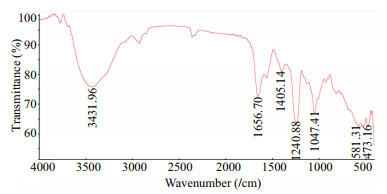
|
| Figure 2 FTIR spectra of the HfP FTIR was measured in the 4 000–400/cm regions using a Thermo Scientific Nicolet iS10 FT-IR spectrometer in KBr disc. |

|
| Figure 3 1H NMR spectrum of HfP The 1H NMR spectra were recorded using a Bruker AVANCE Ⅲ 600 MHz at 25℃. |
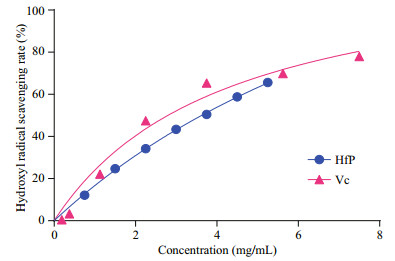
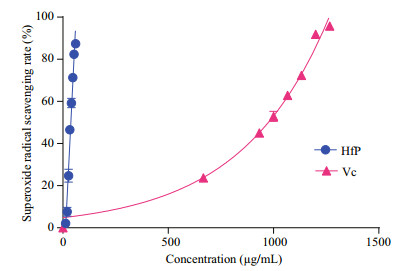
Hydroxyl radical scavenging activity and superoxide anion radical scavenging activity are the most important indicators for the antioxidant activities of the bioactive substances. Hydroxyl radical is responsible for oxidative injury to biomolecules generated by reaction of Fe (Ⅱ) complex with H2O2 in the presence of acid. As shown in Fig. 4, both HfP and Vc have obvious hydroxyl radical scavenging activity and the EC50 of HfP and Vc was 3.74 and 2.72 mg/mL, respectively. The hydroxyl radical scavenging activity of HfP is close to that of Vc, which means that HfP and Vc has a similar scavenging ability for hydroxyl radical. In the PMS/NADH-NBT system, superoxide anion radicals derived from dissolved oxygen by the PMS-NADH coupling reaction reduce NBT. At 560 nm, the decrease of absorbance with antioxidants indicates the consumption of superoxide anion radicals in the reaction mixture. Figure 5 shows that the % scavenging effect on superoxide anion radicals of HfP and the EC50 values, defined as the concentration for 50% of maximal scavenging radical, were usually used to express the capacity of radical scavenging. In present study, the EC50 of HfP and Vc was about 0.037 8 and 0.93 mg/mL, respectively.
|
| Figure 4 Hydroxyl radicals scavenging activity of HfP HfP: polysaccharides of H. fuscogliva; Vc: vitamin C. All results were representative of three parallel experiments and expressed as mean±SD. |
|
| Figure 5 Superoxide radicals scavenging activity of HfP HfP: polysaccharides of H. fuscogliva; Vc: vitamin C. All results were representative of three parallel experiments and expressed as mean±SD. |
Reactive oxygen species, such as the superoxide anion radical, hydroxyl radical, can be generated inevitably in aerobic organisms during respiration. It is necessary for the normal cell function at physiological concentrations. However, it can cause a number of pathological events such as aging, cellular injury, lipids damage, proteins and DNA degradation when excessively or the antioxidant defense systems are depressed (Söğüt et al., 2003). Therefore, the body needs some antioxidant to help to scavenge them in vivo. Although, the synthetic antioxidants, such as butylated hydroxytoluene, butylated hydroxyanisole, and tert-butylhydroquinone, have a strong capacity of scavenging reactive oxygen species nowadays. They were used under strict regulation because of their toxic effects on human's enzyme systems (Hatate et al., 1990; Chen et al., 1996; Yu et al., 2005; Li et al., 2012b). Therefore, HfP as a good natural antioxidant from sea cucumber H. fuscogliva can be applied to enrich health products.
3.3 Anticoagulant activities assayAnticoagulant activities often involve three indicators of APTT, PT, and TT. The coagulation factors Ⅷ, Ⅸ, Ⅺ, Ⅻ and prekallikrein in the intrinsic blood coagulation pathway were often used to evaluate the anticoagulant activity of bioactive substances in the APTT assay. In addition, PT indicated the extrinsic coagulation factors to assess the capacity of the anticoagulant activity. TT represents blood coagulation status that transforming fibrinogen into fibrin (Lin et al., 2007).
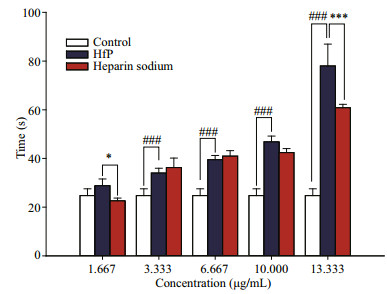
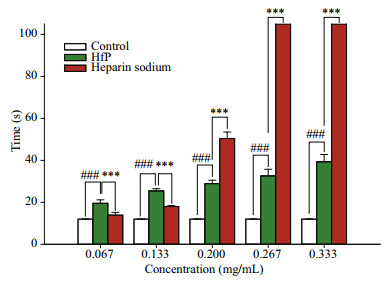
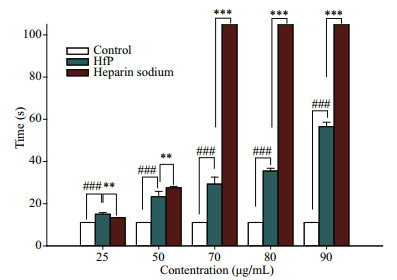
As shown in Fig. 6, APPT of HfP is longer than that of heparin sodium at most concentration. Especially, the APPT of HfP and heparin sodium is 78.022 5 s and 60.866 7 s, respectively. Figure 7 shows that the PT of HfP is higher than that of heparin sodium when the concentration is lower than 0.133 mg/mL, while the PT of heparin sodium is much higher than that of HfP when the concentration is above 0.133 mg/mL. Therefore, the anticoagulant activity evaluated by PT of HfP is also higher than that of heparin sodium, with the concentration lower than 0.133 mg/mL. Figure 8 shows that the TT of HfP and heparin sodium. The TT of HfP is higher than that of heparin sodium when the concentration is lower than 50 μg/mL. But, the TT of heparin sodium is much higher than that of HfP when the concentration is higher than 50 μg/mL. It may be related to the sulfate content and the molecular weight. The sulfated group plays an important role in anticoagulant biological activity (Borsig et al., 2007; Ye et al., 2012). But, the molecular weight may be more important when the concentration is lower than 50 μg/mL. Therefore, the anticoagulant activity evaluated by TT of HfP is also higher than that of heparin sodium, with the concentration lower than 50 μg/mL.
|
| Figure 6 Activated partial thromboplastin time (APTT) analysis of HfP Control: the blank control; HfP: polysaccharides of H. fuscogliva; Heparine sodium: the positive control with heparine sodium (n=4, */# P < 0.05, **/## P < 0.01, ***/### P < 0.001). |
|
| Figure 7 Prothrombin time (PT) analysis of HfP Control: the blank control; HfP: polysaccharides of H. fuscogliva; Heparine sodium: the positive control with heparine sodium (n=4, */# P < 0.05, **/## P < 0.01, ***/### P < 0.001). |
|
| Figure 8 Thrombin time (TT) analysis of HfP Control: the blank control; HfP: polysaccharides of H. fuscogliva; Heparine sodium: the positive control with heparine sodium (n=4, */# P < 0.05, **/## P < 0.01, ***/### P < 0.001). |
At present, cardiovascular and cerebrovascular disease is threatening human health seriously for a long time and become one of the major causes for the death. Most patients, who have thromboembolism problems, require anticoagulation therapy, and heparin is a currently available anticoagulant polysaccharide drug (Moll and Roberts, 2002). However, heparin also has many limitations clinically, including short half-life, osteoporosis, and continuous injection. Sea cucumber was used as a traditional Chinese medicine for many years. Polysaccharides are one of the most important bioactive components in sea cucumber and showed good anticoagulant activities in present study. Especially, APTT of HfP is much higher than that of heparin sodium. In addition, PT and TT of HfP are very close to that of heparin sodium at a low concentration. Therefore, HfP will have a good potential to be a drug for the prevention or therapy of thromboembolism in the future.
4 CONCLUSIONIn present study, we prepared sulfated polysaccharides HfP from sea cucumber H. fuscogliva in protease hydrolysis method. The mean molecular weight was 1 867.1 Da with sulfate content of about 20.7%. The molar ratio of monosaccharide composition of HfP was Man: Rha: Glc A: Glc: Gal: Xyl: Fuc=0.083 6: 0.437: 0.134: 0: 1.182: 0.748: 1. HfP showed notable antioxidant activities including hydroxyl radical scavenging activity and superoxide radical scavenging activity in vitro. In addition, HfP had strong anticoagulant activities. Especially, the APTT of HfP was much higher than that of heparin sodium, and the PT and TT of HfP was close to that of heparin sodium at a low concentration. Therefore, HfP will have a good application future in health product or medicine industry.
| Borsig L, Wang L C, Cavalcante M C M, Cardilo-Reis L, Ferreira P L, Mourão P A S, Esko J D, Pavão M S G, 2007. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber-Effect on tumor metastasis and neutrophil recruitment. Journal of Biological Chemistry, 282(20): 14984–14991. Doi: 10.1074/jbc.M610560200 |
| Chen H M, Muramoto K, Yamauchi F, Nokihara K, 1996. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. Journal of Agricultural and Food Chemistry, 44(9): 2619–2623. Doi: 10.1021/jf950833m |
| Dong X D, Pan R J, Deng X Y, Chen Y T, Zhao G M, Wang C H, 2014. Separation, purification, anticoagulant activity and preliminary structural characterization of two sulfated polysaccharides from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochemistry, 49(8): 1352–1361. Doi: 10.1016/j.procbio.2014.04.015 |
| Guo Z Y, Xing R E, Liu S, Yu H H, Wang P H, Li C P, Li P C, 2005. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorganic & Medicinal Chemistry Letters, 15(20): 4600–4603. |
| Hatate H, Nagata Y, Kochi M, 1990. Antioxidative effect of bovine serum albumin hydrolyzates and their synergistic effect with antioxidants. Journal of Japan Oil Chemists' Society, 39(1): 42–46. Doi: 10.5650/jos1956.39.42 |
| Hu S W, Wang J F, Xu H, Wang Y M, Li Z J, Xue C H, 2014a. Fucosylated chondroitin sulphate from sea cucumber inhibits high-fat-sucrose diet-induced apoptosis in mouse pancreatic islets via down-regulating mitochondrial signaling pathway. Journal of Functional Foods, 7: 517–526. Doi: 10.1016/j.jff.2014.01.004 |
| Hu S W, Xu L L, Shi D, Wang J F, Wang Y M, Lou Q M, Xue C H, 2014b. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic effects via activating phosphoinositide 3-kinase/protein kinase B signal pathway. Journal of Bioscience and Bioengineering, 117(4): 457–463. Doi: 10.1016/j.jbiosc.2013.09.005 |
| Jin W H, Zhang Q B, Wang J, Zhang W J, 2013. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydrate Polymers, 91(1): 1–6. Doi: 10.1016/j.carbpol.2012.07.067 |
| Jin W H, Zhang W J, Wang J, Yao J T, Xie E Y, Liu D C, Duan D L, Zhang Q B, 2014. A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. International Journal of Biological Macromolecules, 67: 336–342. Doi: 10.1016/j.ijbiomac.2014.03.031 |
| Kawai Y, Seno N, Anno K, 1969. A modified method for chondrosulfatase assay. Analytical Biochemistry, 32(2): 314–321. Doi: 10.1016/0003-2697(69)90091-8 |
| Li B, Liu S, Xing R E, Li K C, Li R F, Qin Y K, Wang X Q, Wei Z H, Li P C, 2013a. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydrate Polymers, 92(2): 1991–1996. Doi: 10.1016/j.carbpol.2012.11.088 |
| Li K C, Liu S, Xing R E, Qin Y K, Li P C, 2013b. Preparation, characterization and antioxidant activity of two partially N-acetylated chitotrioses. Carbohydrate Polymers, 92(2): 1730–1736. Doi: 10.1016/j.carbpol.2012.11.028 |
| Li K C, Xing R E, Liu S, Qin Y K, Meng X T, Li P C, 2012a. Microwave-assisted degradation of chitosan for a possible use in inhibiting crop pathogenic fungi. International Journal of Biological Macromolecules, 51(5): 767–773. Doi: 10.1016/j.ijbiomac.2012.07.021 |
| Li R F, Yu H H, Xing R E, Liu S, Qing Y K, Li K C, Li B, Meng X T, Cui J H, Li P C, 2012b. Isolation, identification and characterization of a novel antioxidant protein from the nematocyst of the jellyfish Stomolophus meleagris. International Journal of Biological Macromolecules, 51(3): 274–278. Doi: 10.1016/j.ijbiomac.2012.05.015 |
| Liao Y. 1997. Fauna Sinica: Phylum Echinodermata Class Holothuroidea. Science Press, Beijing, China, p. 119-121. (in Chinese) |
| Lin C Z, Guan H S, Li H H, Yu G L, Gu C X, Li G Q, 2007. The influence of molecular mass of sulfated propylene glycol ester of low-molecular-weight alginate on anticoagulant activities. European Polymer Journal, 43(7): 3009–3015. Doi: 10.1016/j.eurpolymj.2007.04.015 |
| Liu X, Sun Z L, Zhang M S, Meng X M, Xia X K, Yuan W P, Xue F, Liu C H, 2012. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydrate Polymers, 90(4): 1664–1670. Doi: 10.1016/j.carbpol.2012.07.047 |
| Luo L, Wu M Y, Xu L, Lian W, Xiang J Y, Lu F, Gao N, Xiao C, Wang S M, Zhao J H, 2013. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Marine Drugs, 11(2): 399–417. Doi: 10.3390/md11020399 |
| Matsuhiro B, Osorio-Román I O, Torres R, 2012. Vibrational spectroscopy characterization and anticoagulant activity of a sulfated polysaccharide from sea cucumber Athyonidium chilensis. Carbohydrate Polymers, 88(3): 959–965. Doi: 10.1016/j.carbpol.2012.01.052 |
| Moll S, Roberts H R, 2002. Overview of anticoagulant drugs for the future. Seminars in Hematology, 39(3): 145–157. Doi: 10.1053/shem.2002.34087 |
| Mourão P A S, Pereira M S, Pavão M S G, Mulloy B, Tollefsen D M, Mowinckel M C, Abildgaard U, 1996. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm-Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. Journal of Biological Chemistry, 271(39): 23973–23984. Doi: 10.1074/jbc.271.39.23973 |
| Nishikimi M, Appaji Rao N, Yagi K, 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications, 46(2): 849–854. Doi: 10.1016/S0006-291X(72)80218-3 |
| Sheng X H, Zhang N N, Song S L, Li M, Liang H, Zhang Y J, Wang Y S, Ji A G, 2011. Morphological transformation and proliferation of rat astrocytes as induced by sulfated polysaccharides from the sea cucumber Stichopus japonicus. Neuroscience Letters, 503(1): 37–42. Doi: 10.1016/j.neulet.2011.08.003 |
| Söğüt S, Zoroğlu S S, Özyurt H, Yılmaz H R, Özuğurlu F, Sivaslı E, Yetkin Ö, Yanık M, Tutkun H, Savaş H A, Tarakçioğlu M, Akyol Ö, 2003. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clinica Chimica Acta, 331(1-2): 111–117. Doi: 10.1016/S0009-8981(03)00119-0 |
| Wang J, Zhang Q B, Jin W H, Niu X Z, Zhang H, 2011a. Effects and mechanism of low molecular weight fucoidan in mitigating the peroxidative and renal damage induced by adenine. Carbohydrate Polymers, 84(1): 417–423. Doi: 10.1016/j.carbpol.2010.11.055 |
| Wang J, Zhang Q B, Zhang Z S, Hou Y, Zhang H, 2011b. Invitro anticoagulant activity of fucoidan derivatives from brown seaweed Laminaria japonica. Chinese Journal of Oceanology and Limnology, 29(3): 679–685. Doi: 10.1007/s00343-011-0181-9 |
| Wang Y C, Su W, Zhang C Y, Xue C H, Chang Y G, Wu X L, Tang Q J, Wang J F, 2012. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chemistry, 133(4): 1414–1419. Doi: 10.1016/j.foodchem.2012.02.028 |
| Wu N, Ye X Q, Guo X, Liao N B, Yin X Z, Hu Y Q, Sun Y J, Liu D H, Chen S G, 2013. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydrate Polymers, 93(2): 604–614. Doi: 10.1016/j.carbpol.2012.12.044 |
| Ye L B, Xu L, Li J R, 2012. Preparation and anticoagulant activity of a fucosylated polysaccharide sulfate from a sea cucumber Acaudina molpadioidea. Carbohydrate Polymers, 87(3): 2052–2057. Doi: 10.1016/j.carbpol.2011.10.014 |
| Younes I, Hajji S, Frachet V, Rinaudo M, Jellouli K, Nasri M, 2014. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. International Journal of Biological Macromolecules, 69: 489–498. |
| Yu H H, Liu X G, Xing R E, Liu S, Li C P, Li P C, 2005. Radical scavenging activity of protein from tentacles of jellyfish Rhopilema esculentum. Bioorganic & Medicinal Chemistry Letters, 15(10): 2659–2664. |
| Zhang J J, Zhang Q B, Wang J, Shi X J, Zhang Z S, 2009. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chinese Journal of Oceanology and Limnology, 27(3): 578–582. Doi: 10.1007/s00343-009-9205-0 |
| Zheng A C. Chen J, Peng C Y, 2007. Extraction and purification of acidic mucopolysaccharide from Holothuria scabra Jaeger. Modern Food Science and Technology, 23(5): 65–67. |
 2017, Vol. 35
2017, Vol. 35


