Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HUI Min(惠敏), CUI Zhaoxia(崔朝霞), LIU Yuan(刘媛), SONG Chengwen(宋呈文)
- Transcriptome profiles of embryos before and after cleavage in Eriocheir sinensis: identification of developmental genes at the earliest stages
- Chinese Journal of Oceanology and Limnology, 35(4): 770-781
- http://dx.doi.org/10.1007/s00343-017-5364-6
Article History
- Received Dec. 18, 2015
- accepted in principle Mar. 3, 2016
- accepted for publication May. 25, 2016
2 National & Local Joint Engineering Laboratory of Ecological Mariculture, Qingdao 266071, China;
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China
Eriocheir sinensis, the Chinese mitten crab, is a delicacy in Asia, but it has been listed among the "100 World's Worst Alien Species" invading Europe and North America. Moreover, E. sinensis is an interesting model species for developmental biology because of its mosaic development with typical crustacean decapod stages, such as zoeal larval and metamorphosis stages (Kim and Hwang, 1995). In crab, embryogenesis is a complex developmental process including pivotal events such as cleavage, blastulation, and gastrulation, which are controlled by reorganization of transcriptional regulation at the genome-wide level. Research on the embryogenesis of this crab species may detect the causes of developmental defects. Such information would be useful for both aquaculture and population control.
Transcriptomic analyses are very important to clarify the molecular basis of embryogenesis. Many transcriptomic studies have focused on the early developmental stages of various animals (Vesterlund et al., 2011; Zeng et al., 2011; Lanes et al., 2013; Yang et al., 2013). Such studies have revealed the dynamics of certain transcripts shared between different developmental stages. One important discovery is that the earliest stages of development occur independently of major activity of the embryonic genome in many animal species (Flach et al., 1982; Matsuoka et al., 2013). For example, in zebrafish, a major transition in gene regulation and transcriptional activity occurs between the 512-cell and 50%-epiboly stages (Kane and Kimmel, 1993; Aanes et al., 2011; Vesterlund et al., 2011).
In the last decade, several studies have analyzed the transcriptomes of different adult tissues and whole larvae of E. sinensis (Zhang et al., 2011; Ou et al., 2012; He et al., 2013; Hui et al., 2014; Li et al., 2014; Sun et al., 2014), while other studies have focused on genes involved in the development of mitten crab (Wójcik and Normant, 2014; Li et al., 2015a; Zhao et al., 2016). Notably, researchers have identified candidate genes that potentially affect molting, metamorphosis, or muscle production/degradation, including molting-inhibiting hormone (MIH), 5-hydroxytryptamine receptor, myosin, cuticle protein, and farneosoic acid O-methyltransferase (Li et al., 2015b; Song et al., 2015), which are typical genes in crustaceans (Laufer and Biggers, 2001; Kuballa et al., 2011). Meanwhile, other studies have identified that the main morphogenic pathways related to development and growth of E. sinensis include the MAPK signaling pathway, the Wnt signaling pathway, and the hedgehog signaling pathway (Oldham et al., 2000; Ventura et al. 2013). However, there have been no reports on the embryonic transcriptomes of this crab. This lack of information has restricted our understanding of the dynamic development of E. sinensis, especially events that occur in early embryos, such as germline cell development, neurogenesis, and segmentation.
The aim of this study was to analyze the transcriptomes of the two earliest developmental stages of E. sinensis, the oosperm and the 2–4 cell stage embryo, and to identify candidate genes controlling events during early development by a comparative transcriptomic analysis. Accordingly, we can infer the events that occur during early cleavage of the crab embryo. The results of this study, combined with those of studies on other stages of development, will provide a foundation for further research on how maternal information initiates the zygotic program and the dynamics of gene expression at different developmental stages.
2 MATERIAL AND METHOD 2.1 Embryo collectionThe parent crabs (E. sinensis) were collected during their reproductive period (in December) from a farm in Panjin, Liaoning Province, China. Females and males were placed in one container filled with seawater (salinity 20) for mating at 15℃ (Sui et al., 2011). Then, the ovigerous females were sampled and cultured. The oosperms (Os) were collected immediately after discharge. The embryos were monitored under a dissecting microscope and their stage was classified according to developmental time (Du et al., 1992). The embryos were collected when they reached the 2–4 cell stage (Cs). All Os and Cs were collected separately in 1.5 mL tubes, and were then frozen and stored in liquid nitrogen until RNA extraction.
2.2 RNA-seq library construction and sequencingTotal RNA was extracted from Os and Cs using a Trizol Kit (Invitrogen, Carlsbad, CA, USA). The RNA concentration and integrity were measured using a Qubit®RNA Assay Kit with a Qubit®2.0 Fluorimeter (Life Technologies, CA, USA) and a RNA Nano 6000 Assay Kit with an Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA). The same amount of total RNA from five replicate samples was pooled as input material for RNA-Seq. The mRNA was extracted from the total RNA and digested into 155-bp fragments with a TruSeq RNA Sample Prep Kit (Illumina, San Diego, CA, USA). Double-stranded cDNAs were prepared and adaptors for sequencing were ligated following the Illumina manufacturer's protocol. The ligated products were then amplified to generate cDNA libraries after purification with AMPureXP beads (Beckman Coulter, High Wycombe, United Kingdom). One cDNA library was constructed for each development stage. The libraries were sequenced by an Illumina HiSeqTM 2000 machine and paired-end reads were generated.
2.3 Sequence assemblyAll raw sequences were deposited in the NCBI Short Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/Traces/sra/). After removing adaptor sequences, clean reads were obtained by filtering the low-quality sequences ( < Q20) and sequences shorter than 50 bp using Solexa QA (Cox et al., 2010). The remaining clean reads were then assembled using Trinity software (Grabherr et al., 2011) as described for de novo transcriptome assembly without a reference genome. The longest copy of redundant transcripts was regarded as a unigene. The Os and Cs datasets were analyzed separately first, and then assembled and reanalyzed.
2.4 Sequence annotation and gene expression evaluationGene annotations were analyzed as described by Hui et al. 2014. All the assembled unigenes were first searched against the NCBI Nr (non-redundant protein sequences) database. After that, GO (Gene Ontology; http://www.geneontology.org/) annotations were assigned to classify the potential functions of the unigenes based on known orthologous gene products using Blast2GO (Conesa et al., 2005).
To estimate gene expression levels, the clean reads in each sample were first mapped to the assembled transcriptome to obtain the read count for each gene, and then the RPKM (reads per kb of exon model per million mapped reads) value was calculated (Mortazavi et al., 2008). The reads mapping and RPKM-based expression measurements were performed using SeqMap and rSeq, respectively (Jiang and Wong, 2008, 2009). The ten most abundant unigenes for the two stages were detected.
2.5 Identification of differentially expressed genesDifferentially expressed genes (DEGs) between the transcriptomes of Os and Cs were identified by the DEseq program (Anders and Huber, 2010). Genes with a significant adjusted P-value (P < 0.05) and foldchange values of >2 were considered to be differentially expressed between the two stages. Then, GO, KEGG Orthology (KO), and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analyses were performed to categorize the DEGs and investigate potential pathways they might be involved in (Kanehisa et al., 2004). In the GO and KEGG pathway enrichment analyses, a P-value of 0.05 (-Log10 (0.05)=1.3) was set as the threshold for a significant difference between Os and Cs.
By searching public databases and published literature, we manually checked all of the key DEGs related to 'signaling pathways', 'germplasm', 'nervous system', 'sensory perception', 'segment polarity', and other unigenes involved in gene activation or regulation at early embryonic stages. Unigenes specifically expressed in only Os or Cs were also analyzed.
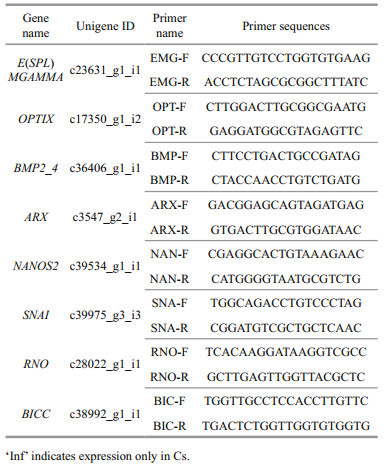
2.6 Quantitative real-time PCR (qRT-PCR) verification of RNA-seq resultsTo verify the expression level of key DEGs and the accuracy of the RNA-Seq data, new Os and Cs samples were selected for qRT-PCR. Total RNA was extracted and first-strand cDNA was synthesized using a PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). Then, the cDNA was diluted 100 times with nucleic acid-free water for use in SYBR Green Real-time RT-PCR assays, which were performed with an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Eight pairs of gene-specific primers (Table 1) were used to amplify partial cDNA sequences. The β-actin gene was amplified in parallel as an internal control. Three biological and three technical replicates were analyzed. The 2-ΔΔCt method was used to analyze the expression level of each gene. The results were subjected to one-way analysis of variance with SPSS 16.0 (where P < 0.05 indicated statistical significance).
Fertilization naturally transforms two highly differentiated gamete cells, the sperm and egg, into a single totipotent cell that gives rise to a complete individual. This represents the most radical programmed change in cellular potency. After fertilization, many biological processes occur at the early embryonic developmental stages, resulting in diverse changes in cellular potency. The transcriptomes of E. sinensis before and after cleavage (Os and Cs) were analyzed using the Illumina sequencing platform. Transcriptome data analyses and comparisons between the two transcriptomes revealed for the first time the various genes and pathways that correspond to the earliest development of embryos in crabs. This information is a useful genomic resource for E. sinensis.
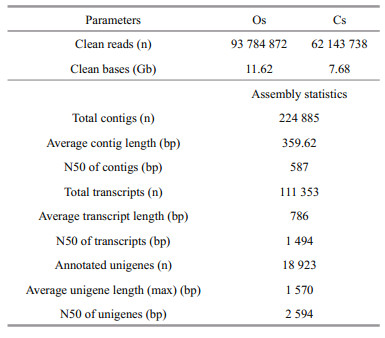
3.1 Overview of the two transcriptomesIn total, 57 135 691 and 36 954 342 raw reads were obtained for Os and Cs of E. sinensis. After filtering, 46 892 436 (11.62 Gb) and 31 071 869 (7.68 Gb) clean reads remained for Os and Cs, respectively (Table 2). After combining the two transcriptomic datasets, 224 885 contigs were generated with an average length 359.62 bp and an N50 of 587 bp. Assembly of the contigs resulted in 111 353 transcripts with an average length of 786 bp and an N50 of 1 494 bp. BlastX searches against the NR database resulted in 18 923 unigenes with annotations (E-value < 1E-05). The unigene length ranged from 201 bp to 17 773 bp with an N50 of 2 594 bp, and the average length was 1 570 bp (Table 2). The length distribution of the unigenes is shown in Supplementary Fig. 1. All raw reads sequence data from E. sinensis have been deposited at the NCBI under the BioProject number PRJNA293485.

|
| Figure 1 GO distribution of all unigenes in transcriptomes of Eriocheir sinensis |
|
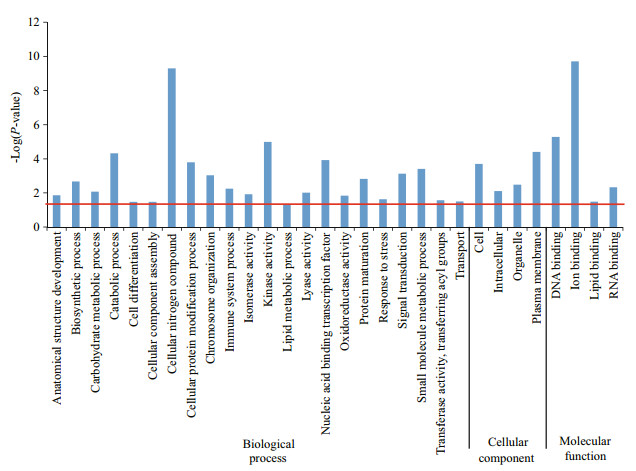
In the GO analysis, 15 201 unigenes out of the total 18 923 unigenes (80.33%) were assigned GO terms in the three major functional groups 'biological process', 'cellular component', and 'molecular function' (Fig. 1). For the two transcriptomes, the three most abundant GO terms in 'biological process' were biosynthetic process, cellular nitrogen compound metabolic process, and transport, similar to those in the larval transcriptomes of E. sinensis (Hui et al., 2014). The following numbers of unigenes (out of a total of 403) matched with GO terms related to developmental processes: GO:0009653 anatomical structure morphogenesis, 8; GO:0007275 multicellular organismal development, 210; GO:0009790 embryo development, 62; GO:0030154 cell differentiation, 54; and GO:0000003 reproduction, 69 (Supplementary Table 1).
The ten most abundant unigenes were almost identical between the Os and Cs transcriptomes (Table 3). The GO annotations of these genes and searches of the Uniprot database revealed that CTRB, UB, and GPLCA are related to protein or carbon catabolic process, and ODRP, PABPC1L-1, OP, and OHSS are involved in processes related to reproductive development, such as oocyte maturation, ovary development, and spermatogenesis. MFAS, SP5, and ARK play roles as transcriptional activators or components of signaling pathways in embryo development, including axonogenesis. SOD is involved in the destruction of toxic free radicals that are produced within cells. These results indicate that the dominant processes at the earliest developmental stages of crab are sex regulation, substance metabolism, and other embryonic developmental events. Many genes expressed in the two transcriptomes (Supplementary Table 1) might be maternal genes, especially those expressed in Os. These data are important to complete the transcriptomic resources of E. sinensis at different developmental stages.

|
Of the total 18 923 unigenes, 432 (2.28%) were significantly differentially expressed between Os and Cs, including 147 down-regulated and 285 upregulated genes at the Cs stage (Supplementary Table 2). The qRT-PCR analyses estimated lower expression levels for most of these genes, but showed the same trends in expression as those detected in the RNA-Seq analysis (Fig. 2), confirming the accuracy of RNASeq. Compared with other transcriptome studies of E. sinensis (He et al., 2013; Li et al., 2015b), our study detected lower proportions of DEGs in the transcriptomes of Os vs. Cs. This observation highlights the transitional nature of the embryonic transcriptomes during these periods, where there were comparatively small changes between these two earliest embryonic developmental stages.

|
| Figure 2 Comparison of gene expression results obtained by qRT-PCR and RNA-Seq 'Fold change' indicates value of gene expression of Cs: Os (above axis) or Os: Cs (below axis); 'Inf': infinite; minus (-) indicates that expression is higher in Os than in Cs of Eriocheir sinensis. |
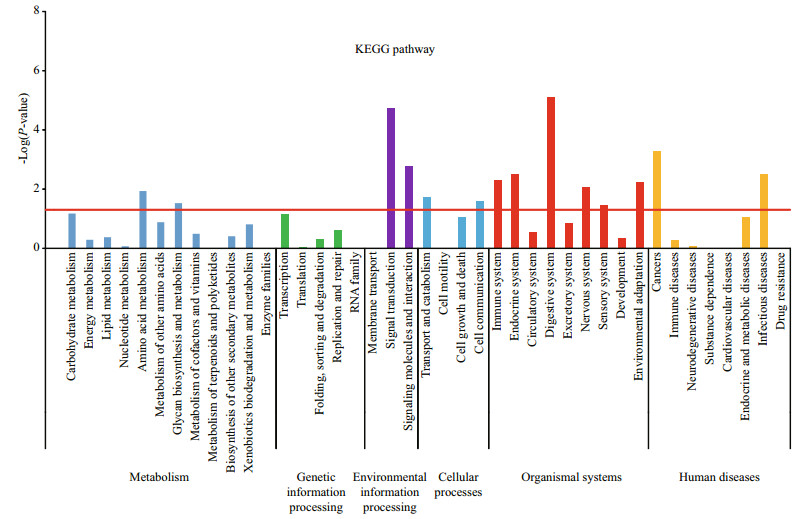
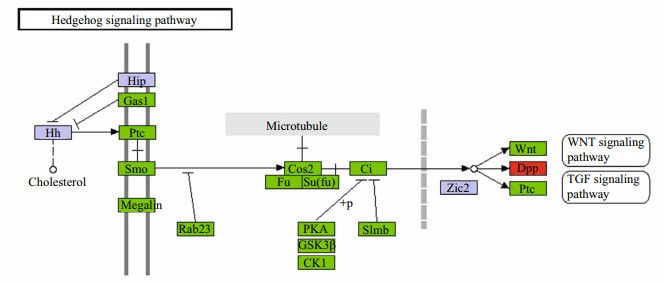
To understand the functional distribution of the DEGs, they were further classified by GO and KEGG enrichment analyses. Based on GO, 30 major functional groups were found to be significantly different between the two stages (P-values < 0.05;-Log10(0.05)=1.3) (Fig. 3), including 'cellular nitrogen' and 'ion binding'. KEGG enrichment analysis revealed that 14 KO terms (Fig. 4) differed substantially between the two stages, including 'amino acid metabolism', 'signal transduction', 'transport and catabolism', and 'digestive system'. Because the focus of this study was on crab development, we paid particular attention to the hedgehog signaling pathway and the Wnt signaling pathway (Figs. 5, 6), which were shown to be related to periodic morphological changes in the prawn Macrobrachium rosenbergii (Ventura et al., 2013). Most genes involved in the two pathways were found in both transcriptomes and were highly expressed in Cs. For example, BMP2_4 showed a 332-fold difference in expression levels between the two transcriptomes (Table 4). When we compared the results with those of another study on different development stages of E. sinensis (from the megalopal stage to the first juvenile stage) (Song et al., 2015), greater changes in expression were found between Os to Cs. This illustrated that these two pathways regulating development possibly play different roles at different developmental stages. Together, the main functional groups of DEGs may play important roles at an early stage of embryo development of E. sinensis. These results are useful for identifying genes involved in the major changes that occur from Os to Cs.
|
| Figure 3 Significantly different GO terms of differentially expressed genes (DEGs) between Os and Cs transcriptomes in Eriocheir sinensis Horizontal line indicates significance threshold (P < 0.05). |
|
| Figure 4 Significantly different functional groups of differentially expressed genes (DEGs) between Os and Cs transcriptomes in Eriocheir sinensis based on KEGG analysis Horizontal line indicates significance threshold (P < 0.05). |
|
| Figure 5 Hedgehog signaling pathway based on KEGG pathway analysis Up-regulated and down-regulated genes are labeled in green and red, respectively, and purple represents genes showing no difference in expression between Os and Cs transcriptomes of Eriocheir sinensis. |

|
| Figure 6 Wnt signaling pathway based on KEGG pathway analysis Up-regulated and down-regulated genes are shown in green and red, respectively, and purple indicates genes showing no difference in expression between Os and Cs transcriptomes of Eriocheir sinensis. |

|
Stage-specific expressed genes (SSGs)
Genes with specific expression at one developmental stage and not the other were considered to be preferentially expressed at that stage. We identified nine SSGs in total, with two genes specifically expressed in Os, and seven in Cs (Table 5). It was noteworthy that among the SSGs of Cs, PRD, ZNF354C, OPTIX, and E(SPL)MGAMMA are all related to embryo development (defining the polarity of embryonic segments, development of head sensory organs, and involvement in early neurogenesis). These genes could serve as molecular signatures to distinguish the two developmental stages.

|
Germline genes
A central character of the continuation of life in animals with sexual reproduction is the circulation of the germline through generation after generation. Germ cells may be specified through the localization of germline determinants to specific cells in early embryogenesis (Extavour, 2005). Several genes that are known to function in germline development were differentially expressed between Os and Cs (Table 4). The NANOS gene was up-regulated in Cs. This gene has been shown to play a critical role in the sex differentiation of germline cells by improving the male fate and suppressing the female fate in many species (Kobayashi et al., 1996; Calvo et al., 2005; Extavour et al., 2005; Dearden, 2006). BICC is required maternally for specifying anterior position during early Drosophila development. Also, during oogenesis, it is involved in the correct targeting of the migrating anterior follicle cells and the establishment of anterior-posterior polarity in the oocyte (Mahone et al., 1997; Chicoine et al., 2007). GPR64 may be involved in the signal transduction pathway controlling reproductive organ function and male fertility (Obermann et al., 2003). These results indicate that changes in the expression patterns of certain genes could initiate the specification of germ cells at an early stage during crab development.
Genes related to neurogenesis and sensory perception
Thus far, little attention has been paid to understanding embryonic neurogenesis and sensory perception development in crustaceans. In this study, seven genes related to neurogenesis and five related to sensory perception were differentially expressed between Os and Cs, and most of them were upregulated in Cs (Table 4). For example, among the neurogenesis-related genes, CHL1 plays an important role in nervous system development and synaptic plasticity (Lutz et al., 2014; Tang et al., 2015). ARX is a transcription factor required for normal brain development, and ROBO is involved in neural development in axonal navigation of the neural tube (Jen et al., 2004). In several animals, transcriptomes from different developmental stages have been compared to identify genes related to sensory perception (Chen et al., 2014; Li et al., 2015b; Song et al., 2015). Some genes related to visual sense or auditory stimulus show differential expression between stages, such as protein purity of essence and nicotinic acetylcholine receptor subunit alpha 10. Here, comparison of the Os and Cs transcriptomes revealed up-regulation of IR25A and EYG (related to detection of chemical stimulus and the light response) and OPN4B and CDH23 (related to auditory receptor cell differentiation). Therefore, at the early developmental stages of embryos, development of the nervous system and other sensory organs, such as the eye, may have already started.
Segmentation genes
The genes necessary for segmentation have been genetically characterized in Drosophila. Three homologs of Drosophila genes involved in segmentation were up-regulated in Cs compared with Os in E. sinensis: PRD, HB, and BAB (Table 4). Among them, PRD is expressed in a segmentally repeating pattern to define the polarity of embryonic segments, and BAB is required for the specification of the tarsal segment. It has been reported that segmentation in insect embryos is initiated by maternally derived information, which is stored in the developing oocyte (Tautz, 1988). Another DEG, the gap gene hb, belongs to the first zygotic group of genes, which are thought to respond to the spatial cues provided by maternal genes. This gene has also been identified in another crustacean, Penaeus japonicas (Sellars et al., 2015) and this is the first report of its existence in crab. These results suggest that segmentation in the crab embryo may also be initiated by maternally derived molecular information.
4 CONCLUSIONIn total, 18 923 unigenes were obtained from transcriptomes of embryos before and after cleavage in E. sinensis. Among them, 403 genes matched with GO terms related to developmental processes and 432 DEGs were detected between the two stages. The hedgehog signaling pathway and Wnt signaling pathway, which are related to morphology, showed large differences in gene expression between the two stages, and most genes involved in the two pathways were highly expressed in Cs. A number of DEGs related to 'germplasm', 'nervous system', 'sensory perception' and 'segment polarity' were identified, and most of them were up-regulated in Cs. Therefore, these embryonic developmental events may begin at the cleavage period in crab, and the genes expressed in the two transcriptomes might be maternal genes, especially those expressed at the Os stage. Comparative transcriptome analyses should be conducted for more embryo developmental stages to clarify the transition from maternal to embryonic control. The results of this study provide useful genetic resources and a foundation for further research on crab development.
| Aanes H, Winata C L, Lin C H, Chen J P, Srinivasan K G, Lee S G P, Lim A Y M, Hajan H S, Collas P, Bourque G, Gong Z Y, Korzh V, Aleström P, Mathavan S, 2011. Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res., 21(8): 1328–1338. Doi: 10.1101/gr.116012.110 |
| Anders S, Huber W, 2010. Differential expression analysis for sequence count data. Genome Biol., 11(10): R106. Doi: 10.1186/gb-2010-11-10-r106 |
| Calvo E, Walter M, Adelman Z N, Jimenez A, Onal S, Marinotti O, James A A, 2005. Nanos (nos) genes of the vector mosquitoes, Anopheles gambiae, Anopheles stephensi and Aedes aegypti. Insect Biochem. Mol. Biol., 35(7): 789–798. Doi: 10.1016/j.ibmb.2005.02.007 |
| Chen S L, Zhang Z J, Shao C W, et al, 2014. Whole-genome sequence of a flatfish provides insights into zw sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics, 46(3): 253–260. Doi: 10.1038/ng.2890 |
| Chicoine J, Benoit P, Gamberi C, Paliouras M, Simonelig M, Lasko P, 2007. Bicaudal-C recruits CCR4-NOT deadenylase to target mRNAs and regulates oogenesis, cytoskeletal organization, and its own expression. Dev. Cell, 13(5): 691–704. Doi: 10.1016/j.devcel.2007.10.002 |
| Conesa A, Götz S, García-Gómez J M, Terol J, Talón M, Robles M, 2005. Blast2GO:a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18): 3674–3676. Doi: 10.1093/bioinformatics/bti610 |
| Cox M P, Peterson D A, Biggs P J, 2010. SolexaQA:At-aglance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics, 11: 485. Doi: 10.1186/1471-2105-11-485 |
| Dearden P K, 2006. Germ cell development in the Honeybee (Apis mellifera); Vasa and Nanos expression. BMC Dev.Biol., 6(1): 6. Doi: 10.1186/1471-213X-6-6 |
| Du N S, Zhao Y L, Lai W. 1992. Studies on the embryonic development of Eriocheir sinensis. In: Memoir of Crustacean (the Third Set). Ocean University of Qingdao Press, Qingdao, p. 128-135. (in Chinese) |
| Extavour C G, Pang K, Matus D Q, Martindale M Q, 2005. Vasa and nanos expression patterns in a sea anemone and the evolution of bilaterian germ cell specification mechanisms. Evol. Dev., 7(3): 201–215. Doi: 10.1111/(ISSN)1525-142X |
| Extavour C G, 2005. The fate of isolated blastomeres with respect to germ cell formation in the amphipod crustacean Parhyale hawaiensis. Dev. Biol., 277(2): 387–402. Doi: 10.1016/j.ydbio.2004.09.030 |
| Flach G, Johnson M H, Braude P R, Taylor R A, Bolton V N, 1982. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J., 1(6): 681–686. |
| Grabherr M G, Haas B J, Yassour M, Levin J Z, Thompson D A, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q D, Chen Z H, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren B W, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A, 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol., 29(7): 644–652. Doi: 10.1038/nbt.1883 |
| He L, Jiang H, Cao D D, Liu L H, Hu S N, Wang Q, 2013. Comparative transcriptome analysis of the accessory sex gland and testis from the Chinese Mitten Crab (Eriocheir sinensis). PLoS One, 8(1): e53915. Doi: 10.1371/journal.pone.0053915 |
| Hui M, Liu Y, Song C W, Li Y D, Shi G H, Cui Z X, 2014. Transcriptome changes in Eriocheir sinensis megalopae after desalination provide insights into osmoregulation and stress adaption in larvae. PLoS One, 9(12): e114187. Doi: 10.1371/journal.pone.0114187 |
| Jen J C, Chan W M, Bosley T M, et al, 2004. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science, 304(5676): 1509–1513. Doi: 10.1126/science.1096437 |
| Jiang H, Wong W H, 2008. SeqMap:mapping massive amount of oligonucleotides to the genome. Bioinformatics, 24(20): 2395–2396. Doi: 10.1093/bioinformatics/btn429 |
| Jiang H, Wong W H, 2009. Statistical inferences for isoform expression in RNA-Seq. Bioinformatics, 25(8): 1026–1032. Doi: 10.1093/bioinformatics/btp113 |
| Kane D A, Kimmel C B, 1993. The zebrafish midblastula transition. Development, 119(2): 447–456. |
| Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M, 2004. The KEGG resource for deciphering the genome. Nucl. Acids Res., 32(S1): D277–D280. |
| Kim C H, Hwang S G, 1995. The complete larval development of the mitten crab Eriocheir sinensis H. Milne Edwards, 1853 (Decapoda, Brachyura, Grapsidae) reared in the laboratory and a key to the known zoeae of the Varuninae. Crustaceana, 68(7): 793–812. Doi: 10.1163/156854095X00953 |
| Kobayashi S, Yamada M, Asaoka M, Kitamura T, 1996. Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature, 380(6576): 708–711. Doi: 10.1038/380708a0 |
| Kuballa A V, Holton T A, Paterson B, Elizur A, 2011. Moult cycle specific differential gene expression profiling of the crab Portunus pelagicus. BMC Genomics, 12(1): 147. Doi: 10.1186/1471-2164-12-147 |
| Lanes C F C, Bizuayehu T T, de Oliveira Fernandes J M, Kiron V, Babiak I, 2013. Transcriptome of Atlantic cod (Gadus morhua L.) early embryos from farmed and wild broodstocks. Mar. Biotechnol., 15(6): 677–694. |
| Laufer H, Biggers W J, 2001. Unifying concepts learned from methyl farnesoate for invertebrate reproduction and postembryonic development. Am. Zool., 41(3): 442–457. |
| Li E C, Wang S L, Li C, Wang X D, Chen K, Chen L Q, 2014. Transcriptome sequencing revealed the genes and pathways involved in salinity stress of Chinese mitten crab, Eriocheir sinensis. Physiol. Genomics, 46(5): 177–190. Doi: 10.1152/physiolgenomics.00191.2013 |
| Li Q, Xie J, He L, Wang Y L, Duan Z L, Yang H D, Wang Q, 2015a. Identification of ADAM10 and ADAM17 with potential roles in the spermatogenesis of the Chinese mitten crab, Eriocheir sinensis. Gene, 562(1): 117–127. Doi: 10.1016/j.gene.2015.02.060 |
| Li Y D, Hui M, Cui Z X, Liu Y, Song C W, Shi G H, 2015b. Comparative transcriptomic analysis provides insights into the molecular basis of the metamorphosis and nutrition metabolism change from zoeae to megalopae in Eriocheir sinensis. Comp. Biochem. Phys. Part D, 13: 1–9. |
| Lutz D, Wolters-Eisfeld G, Schachner M, Kleene R, 2014. Cathepsin E generates a sumoylated intracellular fragment of the cell adhesion molecule L1 to promote neuronal and Schwann cell migration as well as myelination. J. Neurochem, 128(5): 713–724. Doi: 10.1111/jnc.2014.128.issue-5 |
| Mahone M, Saffman E E, Lasko P F, 1997. Localized BicaudalC RNA encodes a protein containing a KH domain, the RNA binding motif of FMR1. EMBO J., 16(13): 4152. Doi: 10.1093/emboj/16.13.4152 |
| Matsuoka T, Ikeda T, Fujimaki K, Satou Y, 2013. Transcriptome dynamics in early embryos of the ascidian, Ciona intestinalis. Dev. Biol., 384(2): 375–385. Doi: 10.1016/j.ydbio.2013.10.003 |
| Mortazavi A, Williams B A, McCue K, Schaeffer L, Wold B, 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods, 5(7): 621–628. Doi: 10.1038/nmeth.1226 |
| Obermann H, Samalecos A, Osterhoff C, Schröder B, Heller R, Kirchhoff C, 2003. HE6, a two-subunit heptahelical receptor associated with apical membranes of efferent and epididymal duct epithelia. Mol. Reprod. Dev., 64(1): 13–26. Doi: 10.1002/mrd.v64:1 |
| Oldham S, Böhni R, Stocker H, Brogiolo W, Hafen E, 2000. Genetic control of size in Drosophila. Philos. Trans. R. Soc. Lond. B Biol. Sci., 355(1399): 945–952. Doi: 10.1098/rstb.2000.0630 |
| Ou J T, Meng Q H, Li Y, Xiu Y J, Du J, Gu W, Wu T, Li W J, Ding Z F, Wang W, 2012. Identification and comparative analysis of the Eriocheir sinensis microRNA transcriptome response to Spiroplasma eriocheiris infection using a deep sequencing approach. Fish Shellfish Immunol., 32(2): 345–352. Doi: 10.1016/j.fsi.2011.11.027 |
| Sellars M J, Trewin C, McWilliam S M, Glaves R S E, Hertzler P L, 2015. Transcriptome profiles of Penaeus (Marsupenaeus) japonicus animal and vegetal halfembryos:identification of sex determination, germ line, mesoderm, and other developmental genes. Mar. Biotechnol., 17(3): 252–265. Doi: 10.1007/s10126-015-9613-4 |
| Song C W, Cui Z X, Hui M, Liu Y, Li Y D, Li X H, 2015. Comparative transcriptomic analysis provides insights into the molecular basis of brachyurization and adaptation to benthic lifestyle in Eriocheir sinensis. Gene, 558(1): 88–98. Doi: 10.1016/j.gene.2014.12.048 |
| Sui L Y, Wille M, Cheng Y X, Wu X G, Sorgeloos P, 2011. Larviculture techniques of Chinese mitten crab Eriocheir sinensis. Aquaculture, 315(1-2): 16–19. Doi: 10.1016/j.aquaculture.2010.06.021 |
| Sun Y, Zhang Y C, Liu Y C, Xue S X, Geng X Y, Hao T, Sun J S, 2014. Changes in the organics metabolism in the hepatopancreas induced by eyestalk ablation of the Chinese mitten crab Eriocheir sinensis determined via transcriptome and DGE analysis. PLoS One, 9(4): e95827. Doi: 10.1371/journal.pone.0095827 |
| Tang D Y, Yu Y, Zhao X J, Schachner M, Zhao W J, 2015. Single chain fragment variable antibodies developed by using as target the 3rd fibronectin type Ⅲ homologous repeat fragment of human neural cell adhesion molecule L1 promote cell migration and neuritogenesis. Exp. Cell Res., 330(2): 336–345. Doi: 10.1016/j.yexcr.2014.10.021 |
| Tautz D, 1988. Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature, 332(6161): 281–284. Doi: 10.1038/332281a0 |
| Ventura T, Manor R, Aflalo E D, Chalifa-Caspi V, Weil S, Sharabi O, Sagi A, 2013. Post-Embryonic transcriptomes of the prawn Macrobrachium rosenbergii:multigenic succession through metamorphosis. PLoS One, 8(1): e55322. Doi: 10.1371/journal.pone.0055322 |
| Vesterlund L, Jiao H, Unneberg P, Hovatta O, Kere J, 2011. The zebrafish transcriptome during early development. BMC Dev. Biol., 11(1): 30. Doi: 10.1186/1471-213X-11-30 |
| Wójcik D, Normant M, 2014. Gonad maturity in female Chinese mitten crab Eriocheir sinensis from the southern Baltic Sea-the first description of ovigerous females and the embryo developmental stage. Oceanologia, 56(4): 779–787. Doi: 10.5697/oc.56-4.779 |
| Yang H X, Zhou Y, Gu J L, Xie S Y, Xu Y, Zhu G F, Wang L, Huang J Y, Ma H, Yao J H, 2013. Deep mRNA sequencing analysis to capture the transcriptome landscape of zebrafish embryos and larvae. PLoS One, 8(5): e64058. Doi: 10.1371/journal.pone.0064058 |
| Zeng V, Villanueva K E, Ewen-Campen B S, Alwes F, Browne W E, Extavour C G, 2011. De novo assembly and characterization of a maternal and developmental transcriptome for the emerging model crustacean Parhyale hawaiensis. BMC Genomics, 12(1): 581. Doi: 10.1186/1471-2164-12-581 |
| Zhang W, Wan H L, Jiang H, Zhao Y L, Zhang X W, Hu S N, Wang Q, 2011. A transcriptome analysis of mitten crab testes (Eriocheir sinensis). Genet. Mol. Biol., 34(1): 136–141. Doi: 10.1590/S1415-47572010005000099 |
| Zhao J C, Wang Y L, Li Q, Zhu ML, Sun W J, Wu T M, Wang Q, He L, 2016. Molecular cloning and characterization of p38 gene in the Chinese mitten crab, Eriocheir sinensis. Aquac. Res., 47(4): 1353–1363. Doi: 10.1111/are.2016.47.issue-4 |
 2017, Vol. 35
2017, Vol. 35