Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Yanqing(王延清), LI Chaolun(李超伦), LIU Mengtan(刘梦坛), JIN Xin(金鑫)
- Lipids and fatty acids in Calanus sinicus during oversummering in the southern Yellow Sea
- Chinese Journal of Oceanology and Limnology, 35(4): 874-882
- http://dx.doi.org/10.1007/s00343-017-5351-y
Article History
- Received Jan. 17, 2016
- accepted in principle Mar. 21, 2016
- accepted for publication May. 30, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Ecosystem Research Network, Qingdao 266071, China
Lipids play important roles in several crucial periods of zooplankton life history including diapause, reproduction, and ontogeny (Lee et al., 2006). At high latitudes, copepods often accumulate a large amount of lipids during the relatively short summer phytoplankton bloom (Falk-Petersen et al., 2009; Kattner and Hagen, 2009). In Calanus finmarchicus and C. helgolandicus, which over-winter in Atlantic deep waters (3 000–4 000 m), storage lipids provide energy to outlast the non-feeding period and re-ascent to the surface (Rey-Rassat et al., 2002). In addition to fulfilling basic metabolic requirements, storage lipids can also be used to sustain reproduction following the termination of diapause (Niehoff et al., 2002; Gislason, 2005; Mayor et al., 2006, 2009).
Given the importance of lipids, many studies have investigated the dynamics and functions of lipids during critical periods of copepod life history (Rey-Rassat et al., 2002; Pond and Tarling, 2011; Clark et al., 2012). Based on model estimates and experimental research, a threshold theory for C. finmarchicus and for C. helgolandicus has been suggested by Jónasdóttir (1999), Rey-Rassat et al. (2002), and Irigoien (2004). According to the theory, copepods must accumulate a minimum amount of lipids before diapause is initiated, otherwise they will remain at the surface. Recent investigations into the selective metabolism of polyunsaturated fatty acids (PUFAs) provided a new perspective on the function of lipids in C. acutus and C. finmarchicus. (Pond and Tarling, 2011; Clark et al., 2012). These studies have shown that calanoid copepods may closely regulate the biochemical composition of their oil sac during both accumulation and catabolic processes. The regulated metabolism of lipids is required not only for energetic purposes, but may also be used as a signal for diapause.
In the Yellow Sea, the calanoid copepod C. sinicus is a major contributor to zooplankton biomass and plays a key role in energy and material transfer in the pelagic food web, from phytoplankton to higher trophic levels (Meng, 2003). C. sinicus survival is more difficult during the hot summer period (Zhang et al., 2007), as is the case for polar Calanus spp. during the cold winter period. In contrast to the overwintering strategies of some Calanus spp. at high latitudes, C. sinicus undergoes diapause in the Yellow Sea Cold Bottom Water (YSCBW) area during the hot summers (Sun and Zhang, 2005). Over-summering C. sinicus also accumulate large amounts of lipids and up-regulate the expression of genes related to lipid catabolism, which indicates that lipids are likely an important energy source (Sun et al., 2011; Wang et al., 2014; Zhou et al., 2016). However, little is known about the dynamics and functions of different lipid classes and fatty acids (FAs) during this period.
In this study, C. sinicus samples were collected for lipid and FA analyses from the central region of the southern Yellow Sea in June, August, and November, corresponding to the pre-, during-and post-diapause periods, respectively. Based on the data collected, the use of lipids and FAs, and their potential functions are discussed.
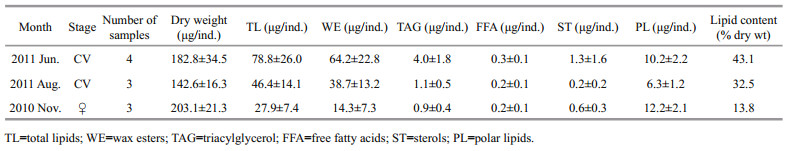
2 MATERIAL AND METHOD 2.1 Sample collectionCalanus sinicus samples were collected using a 500-μm-mesh ring net (mouth opening 0.5 m2) in the southern Yellow Sea during three cruises during 19– 30 November 2010, 13–19 June 2011, and 12–27 August 2011 (Fig. 1). The net was hauled vertically, twice at each station, from 4 m above the bottom of the seabed to the surface. Samples used to determine C. sinicus abundance and stage composition were preserved in 5% formalin seawater solutions and enumerated from CIV to CVI (the mesh size of the plankton net was too big to collect CI to CIII) under a dissecting microscope. CV or female copepods in good condition were selected from another haul for lipid analysis. These samples were filtered onto precombusted Whatman GF/C glass fiber filters with a quick flushing (10 seconds) of Mill-Q water, placed into cryotubes, and then stored in liquid nitrogen until lipid analysis was conducted. Temperature data were acquired with a Seabird Electronics 25 CTD (Laurel Technologies, USA).
|
| Figure 1 Map of the southern Yellow Sea and sampling locations Different lines represent the boundaries of the Yellow Sea Cold Bottom Water at different times of the year; solid line: June, dotted-and-dashed line: August, and dashed line: November. The area where the bottom water temperature was 10℃ was used to estimate the bottom boundary of the Yellow Sea Cold Bottom Water (Weng and Wang, 1982) |
Lipids were extracted from CV or female C. sinicus based on the extraction procedures of Folch et al. (1956) and Parrish (1999). Bulk samples of 40–50 C. sinicus individuals from each sampling station were lyophilized at -45℃ for 48 h and then placed on a Cahn electrobalance (±100 μg, Mettler Toledo, Switzerland) for dry weight measurements. Total lipids were extracted using 3 mL chloroform:methanol (2:1, v:v) at -20℃ for 16 h. The lower chloroform phase containing the total lipid extract was transferred to a glass vial using a 500-mL Hamilton glass syringe. After addition of 0.75 mL KCL (0.88%, w:v), the vials were whirl-mixed and centrifuged (400×g, 2 min) to promote phase separation. The lower organic layer was then removed, placed in pre-combusted glass tubes, and evaporated under a small volume of high purity nitrogen before being transferred to clean, pre-weighed 1.5-mL tapered glass vials. These vials were blown dry under high purity nitrogen and reweighed to determine total lipid content.
Each lipid class was separated by chromatography and analyzed in duplicate using an Iatroscan® MK-6 (Mitsubishi Chemical Medience, Japan) with a flame ionization detector (Hagen, 2000). Total lipid samples were applied on Chromarods-SIII (Mitsubishi Chemical Medience, Japan) using a microcapillary pipette (1 μL). These rods were developed to a height of 7.5 cm in an elution of hexane:benzene (1:1, v:v) and then re-developed to 9.5 cm in benzene: chloroform:acetic acid (50:20:0.7, v:v:v). The doubledeveloped Chromarods were scanned on the machine at a speed of 30 s/rod and a hydrogen flow rate of 160 mL/min. Following Ohman (1997), wax ester standards for calibration were purified from CV C. sinicus (~500) collected from the field in August. Commercial analytical standards were also used: triglyceride mix for triacylglycerol and phosphocholine for polar lipids.
2.3 Fatty acid analysisLipid classes were separated via column chromatography following Ohman (1997). Wax esters were obtained by eluting 1% diethyl ether in hexane, and polar lipids were extracted using 100% methanol. The separated lipids were blown dry under nitrogen and trans-esterified in 3 mL methylation reagent (methanol:sulfuric acid 99:1 v:v) at 50℃ for 16 h (with 19:0 as the internal standard). After addition of 2 mL Milli-Q water and 3 mL hexane:diethyl ether (1:1, v:v), the vials were centrifuged (1 000×g) for layer separation. The upper layer was transferred to another vial and re-washed using 1 mL 2% (w:v) NaHCO3. Following this, the organic layer was separated and evaporated under nitrogen. For both wax ester and polar lipid samples, fatty acid methyl esters were separated and purified in thin layer chromatography. Wax ester fatty alcohols were also extracted by thin layer chromatography and converted to fatty alcohol acetates. Fatty acid methyl esters and fatty alcohol acetates were analyzed on a gas chromatography instrument (Agilent 7890A, Agilent Technologies, USA), equipped with a DB-FFAP capillary column (30 m×0.25 mm inner diameter, 0.25 μm film thickness).
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, v. 13.0 IBM, Armonk, NY, USA).
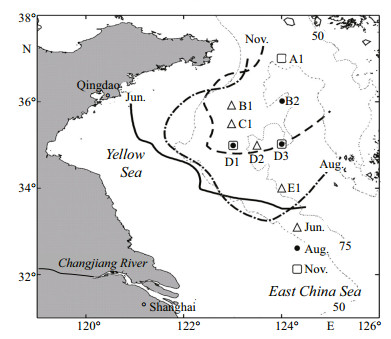
3 RESULT 3.1 Hydrographic conditionsThe temperature profiles of all three seasons showed a similar pattern, with a cold water mass near the bottom indicating the YSCBW (Fig. 2). However, the surface water temperature exhibited clear seasonal variations, being high ( > 20℃) in August, and low in June (17–19℃) and November ( < 15℃). In August, the surface temperature increased above the optimum temperature value for C. sinicus (10 to 20℃, Huang and Zheng, 1986). In November, the thermocline layer reached a depth of approximately 50 m and the temperature of the upper layer decreased to below 15℃, while the bottom water remained relatively cool (~10℃; Fig. 2c).
|
| Figure 2 Vertical temperature profiles at each sampling station in (a) June, (b) August, and (c) November |
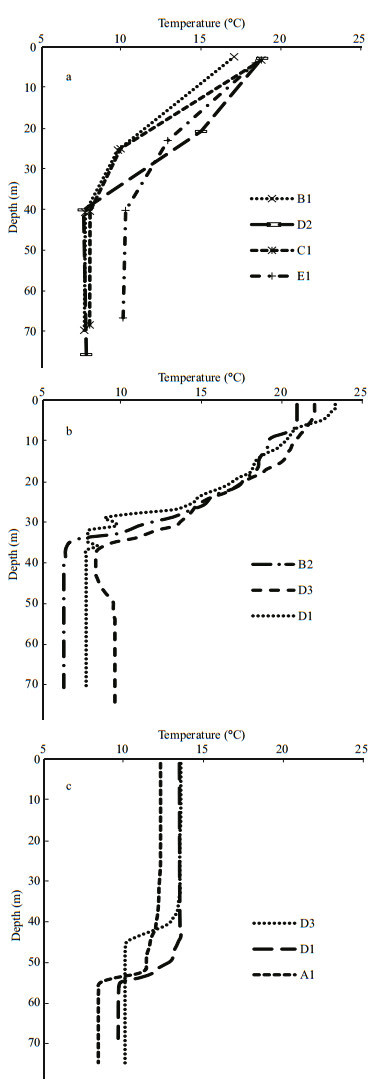
The abundance and composition of late stage C. sinicus samples are shown in Fig. 3. Mean abundance through the water column was 310 ind./m3 in June, 254 ind./m3 in August, and 121 ind./m3 in November. Samples mainly comprised CIVs and CVs in June (48% and 41%, respectively), were gradually dominated by CVs in August (74%–83%), and contained a high abundance of females in November (30%–63%).
|
| Figure 3 Abundance (×103 ind./m3) (a) and copepod stage composition (b) at each sampling station of Calanus sinicus in the southern Yellow Sea in June, August, and November |
Total lipid levels and the dominant storage lipid classes, wax ester and triacylglycerol, all exhibited a general monthly decreasing trend, with the highest amounts present in June and lowest in November (Table 1). Wax ester was the major lipid class detected in the copepods and was also the most variable, declining from 64.2 μg/ind. in June to 38.7 μg/ind. in August, and to only 14.3 μg/ind. in November (Table 1). In contrast with wax ester, another main lipid class, polar lipids, varied only slightly as a proportion of dry weight (5.6% in June, 4.4% in August and 6.0% in November).
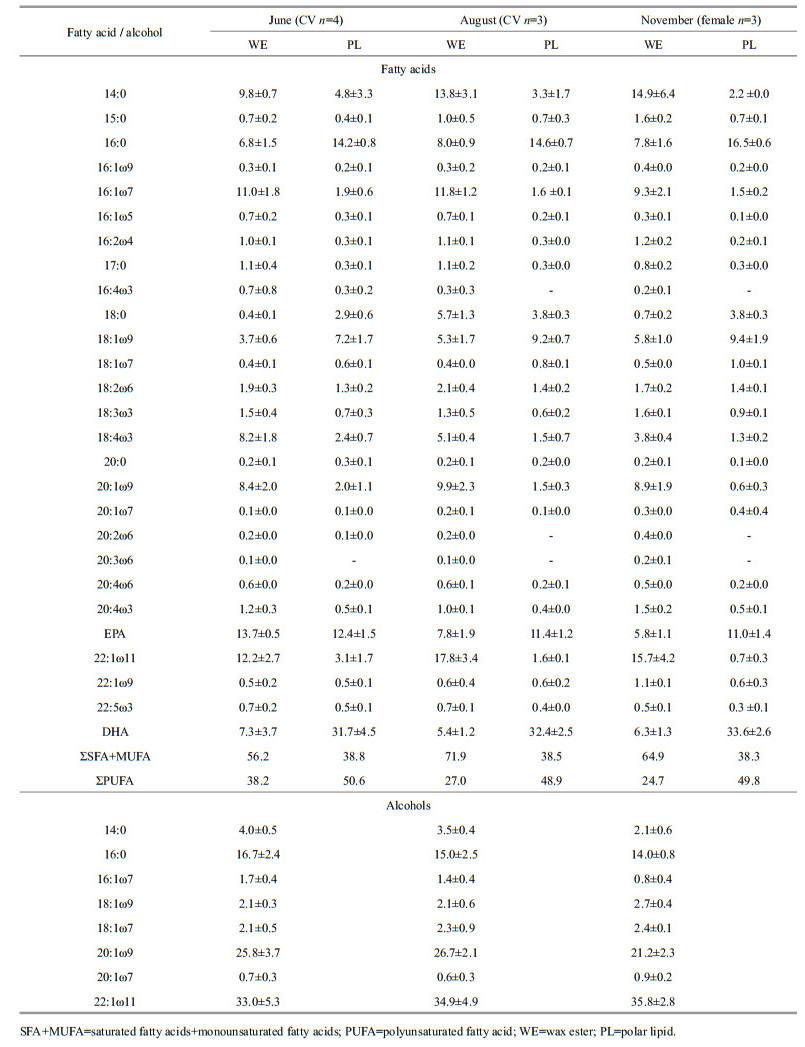
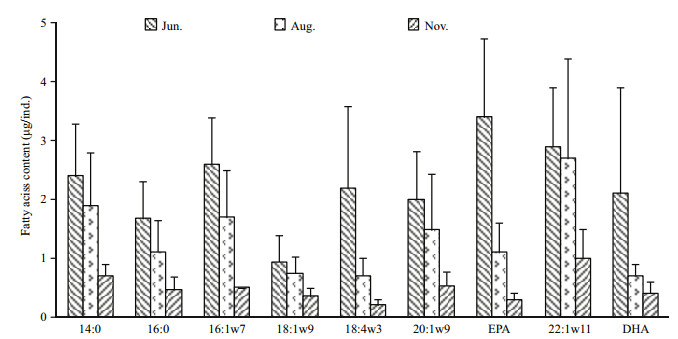
The dominant wax ester FAs in all samples comprised 14:0, 16:0, 16:1ω7, 18:1ω9, 18:4ω3, 20:1ω9, eicosapentaenoic acid (EPA), 22:1ω11, and docosahexaenoic acid (DHA), typically constituting > 2% of total FAs (Table 2). The percentage composition of the two groups of FAs showed apparent variations in trends (Table 2). Between June and August, the percentage PUFA decreased from 38.2% to 27.0%, largely because of the reduction of 18:4ω3, EPA, and DHA. In contrast, the percentage composition of saturated fatty acids (SFA) + monounsaturated fatty acids (MUFA) showed a minimum value of 56.2% in June and increased in August (mean 71.9%). Between June and August, PUFA decreased more rapidly than SFA+MUFA. This was also apparent from the changes in the absolute amounts of FAs (Fig. 4). The reductions in 18:4ω3, EPA, and DHA were greater than in the other FAs (mean 1.5 μg/ind., 2.3 μg/ind., and 1.4 μg/ind., respectively). Between August and November, both percentage composition and absolute amounts of SFA+MUFA and PUFA decreased. The amounts of 14:0, 16:1ω7, and 22:1ω11 decreased more than the other FAs. In contrast to wax ester FAs, major alcohols comprised 16:0, 20:1ω9, and 22:1ω11, and the percentage composition remained stable during the sampling periods.
|
|
| Figure 4 Wax ester fatty acid content (μg/ind.) in Calanus sinicus during each sampling month DHA=docosahexaenoic acid; EPA=eicosapentaenoic acid. Error bars represent standard errors. |
The percentage fatty acid composition of polar lipids for the three sampling months is shown in Table 2. Compared with wax esters, the fatty acid composition of polar lipids was relatively stable between months. In contrast to wax ester FAs, dominant FAs in polar lipids comprised 16:0, EPA, and DHA (representing ~60% of the total FAs). Polar lipids contained more PUFAs, (DHA in particular; mean=32.6%), than SFA + MUFA.
4 DISCUSSION 4.1 Lipid consumption during over-summeringAccumulation of lipids is a common characteristic for many diapausing Calanoid copepods that are exposed to short but intense phytoplankton blooms (Lee et al., 2006). The accumulated lipids of polar/ subpolar Calanoid copepods can reach 50%–65% of their dry weight (Kattner and Hagen, 2009) and play an important role in their over-wintering strategy. In this study, C. sinicus total lipids comprised ~43.1% of dry weight in June. This level of lipid content is lower than polar species, but higher than other mid-latitude Calanus spp. (8%–26% of dry weight) as reported by Lee et al. (2006). This may be related to the specific over-summering strategy of C. sinicus, which undergoes diapause during summer (Sun, 2005). However, it only applies to C. sinicus in the YSCBW and further studies should be conducted for the populations that do not perform diapause during summer, such as in the Bohai Sea, China and in Japanese coastal and shelf waters (Huang et al., 1993; Zhang et al., 2006). Similar to the deep dark polar/ subpolar waters in winter, the resting water mass of the YSCBW is also food limited (Li et al., 2004). C. sinicus in the YSCBW may therefore have to accumulate lipids to fulfill energy requirements, similar to polar Calanus spp.
Our results show that wax esters are the predominant storage lipid in C. sinicus, as for many polar Calanus spp. such as C. finmarchicus and C. hyperboreus (Lee et al., 2006). From June to August, the wax ester content decreased by approximately one third (from 64.2 μg/ind. to 38.7 μg/ind.). This rate of lipid reduction was higher than the polar Calanus spp. C. finmarchicus only used 5% of its storage lipids to sustain diapause during the over-wintering period according to modeling by Jónasdóttir (1999). Furthermore, other studies have shown that dormant Calanus spp. do not use much of their lipid reserves for diapause, instead they channel this energy source into reproductive processes in late winter/early spring (Rey-Rassat et al., 2002; Clark et al., 2012). There are two potential explanations for the large reduction in lipids from June to August. One is that wax esters were used rapidly during that period. However, this does not appear to be the case, because in addition to wax esters, the content of polar lipids also decreased. Polar lipids are usually related to membrane function (Lee et al., 2006), and are not typically used as energy reserves in resting calanoid copepods. The decrease in polar lipid content suggests that there may be other reasons for the reduction of wax esters. A more plausible potential explanation is that a proportion of the CV copepods from August were derived from the CIVs (or earlier stages) in June. CIVs (or earlier stages) in June may gradually develop into CVs before the surface temperature and food supply become unsuitable, and therefore may accumulate relatively smaller amounts of lipids before diapause.
Similar to the earlier resting period from June to August, the wax ester also showed a large reduction between August and November. This reduction may be caused by the greater energy needs of C. sinicus during the latter period. In November, the increased proportion of female C. sinicus suggested that the CVs began to terminate diapause and molt into adults. The energetic costs of diapause, molting, and gonad formation may lead to increased lipid use. For C. finmarchicus, it has been suggested that approximately 95% of the storage lipids are used for gonad formation and ascent migration at the end of the diapause period (Jónasdóttir, 1999). Although the energy allocation for maintaining diapause development and reproduction in C. sinicus cannot yet be clarified, it can be concluded that C. sinicus was in an energy shortage phase during the later diapause period.
Overall, from June to November, most of the storage lipids (predominantly wax esters) were used by C. sinicus. In the models presented by Lee et al.(2006), C. finmarchicus used most of its lipids during winter and accumulated lipids during the following year's spring/summer phytoplankton bloom. C. sinicus also used most of its lipids during over-summering. This type of lipid use is similar to C. finmarchicus, despite the differences in life-histories, with one diapausing in summer and one in winter.
Compared with wax esters, polar lipid levels were less variable. This may be because polar lipids are usually used as a structural component of biomembranes, the contents of which should be relatively stable to ensure normal function.
4.2 Fatty acid changes during over-summeringThe composition of the major fatty acid classes of C. sinicus, wax esters and polar lipids, were similar to those in the polar/subpolar Calanus spp. (such as C. finmarchicus), comprising the following major FAs: 14:0, 16:0, 16:1ω7, 18:1ω9, 18:4ω3, 20:1ω9, EPA, 22:1ω11, and DHA (Lee et al., 2006). The relationship between copepod FAs and food source FAs has been well studied and it is usually believed that most dietary FAs are incorporated, unmodified, into storage lipids (Lee et al., 1971). In this study, the contents of both diatom marker FAs (16:1ω7 and EPA) and dinoflagellate markers (18:4ω3 and DHA) in wax esters were high, indicating that both diatoms and dinoflagellates are important food sources for C. sinicus. In addition, the contents of selfbiosynthesized FAs (20:1ω9 and 22:1ω11) in wax esters, together with the corresponding fatty alcohols, were also high and emphasize the herbivorous feeding habits of C. sinicus.
Wax ester FAs, particularly 18:4ω3 and 20:5ω3, exhibited greater variation than any other FA between June and August. As FA composition of copepod wax esters was closely related to the FA composition of their diets (Lee et al., 1971), variations in FA composition should primarily be due to changes in diet. In this study, the influence of dietary change was difficult to evaluate and the population overlap (CIVs in June that developed into CVs in August) made this more complicated. However, in a laboratory study, Liu et al. (2011) found that C. sinicus cultures selectively used PUFAs under starvation conditions. This indicates that the large variation in PUFAs during C. sinicus diapause may also be caused by selective metabolism, because food in the YSCBW is limited (Li et al., 2004). Clark et al. (2012) also observed significant reductions in PUFAs, in addition to 18:4ω3 and 20:5ω3, in C. finmarchicus during the overwintering period. They suggested that resting copepods may precisely regulate the metabolism of PUFAs during diapause. Generally, PUFAs are considered essential dietary nutrients for marine zooplankton (Harrison, 1990; Kattner et al., 2007; Chen et al., 2012) and are not often readily metabolized for energetic requirements. However, all of these studies report a selective use, which may explain the large variation of PUFAs in our results. A starvation culture experiment, or a more accurate and continuous observation of diapause in C. sinicus should be performed to confirm this.
The FA composition of polar lipids was relatively stable during the three months of this study, although it was variable for wax ester FAs. This may be because polar lipids typically play functional and structural roles (Albers et al., 1996; Lee et al., 2006). Therefore, maintaining a stable composition of polar lipids may be important for basic metabolic activities.
5 CONCLUSIONIn comparing the management of lipid reserves in C. sinicus with C. finmarchicus, both species accumulate a large amount of lipids, predominantly wax esters, before diapause and most of these lipids are used by the end of diapause. Both C. finmarchicus and C. sinicus selectively use wax ester PUFAs in diapause, even though C. sinicus in the YSCBW diapauses in summer whereas C. finmarchicus diapauses in winter.
6 ACKNOWLEDGEMENTWe would like to thank J NING and the crew of the R/Vs Dongfanghong 2 and Kexue 3 for their support during sampling. We thank Prof. YU F (Institute of Oceanology, Chinese Academy of Sciences) and Prof. WANG H G (Ocean University of China) for providing temperature data.
| Albers C S, Kattner G, Hagen W, 1996. The compositions of wax esters, triacylglycerols and phospholipids in Arctic and Antarctic copepods:evidence of energetic adaptations. Mar. Chem., 55(3-4): 347–358. Doi: 10.1016/S0304-4203(96)00059-X |
| Chen M R, Liu H B, Chen B Z, 2012. Effects of dietary essential fatty acids on reproduction rates of a subtropical calanoid copepod, Acartia erythraea. Mar. Ecol. Prog. Ser., 455: 95–110. Doi: 10.3354/meps09685 |
| Clark K A J, Brierley A S, Pond D W, 2012. Composition of wax esters is linked to diapause behavior of Calanus finmarchicus in a sea loch environment. Limnol. Oceanogr., 57(1): 65–75. Doi: 10.4319/lo.2012.57.1.0065 |
| Falk-Petersen S, Mayzaud P, Kattner G, Sargent J R, 2009. Lipids and life strategy of Arctic Calanus. Mar. Biol. Res., 5(1): 18–39. Doi: 10.1080/17451000802512267 |
| Folch J, Lees M, Sloane Stanley G H, 1956. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem., 226(1): 497–509. |
| Gislason A, 2005. Seasonal and spatial variability in egg production and biomass of Calanus finmarchicus around Iceland. Mar. Ecol. Prog. Ser., 286: 177–192. Doi: 10.3354/meps286177 |
| Hagen W. 2000. Lipids. In: Harris R P, Wiebe P H, Lenz J, Skjoldal H R, Huntley M eds. ICES Zooplankton Methodology Manual. Academic Press, San Diego, California. p. 113-119. |
| Harrison K E, 1990. The role of nutrition in maturation, reproduction and embryonic development of decapod crustaceans:a review. J. Shellfish Res., 9(1): 1–28. |
| Huang C, Uye S, Onbé T, 1993. Geographic distribution, seasonal life cycle, biomass and production of a planktonic copepod Calanus sinicus in the Inland Sea of Japan and its neighboring Pacific Ocean. J. Plankton Res., 15(11): 1 229–1 246. Doi: 10.1093/plankt/15.11.1229 |
| Huang J Q, Zheng Z, 1986. The effects of temperature and salinity on the survival of some copepods from Xiamen Harbour. Oceanol. Limnol. Sin., 17(2): 161–167. |
| Irigoien X, 2004. Some ideas about the role of lipids in the life cycle of Calanus finmarchicus. J. Plankton Res., 26(3): 259–263. Doi: 10.1093/plankt/fbh030 |
| Jónasdóttir S H, 1999. Lipid content of Calanus finmarchicus during overwintering in the Faroe-Shetland Channel. Fish. Oceanogr., 8(S1): 61–72. |
| Kattner G, Hagen W, Lee R F, Campbell R, Deibel D, FalkPetersen S, Graeve M, Hansen B W, Hirche H J, Jónasdóttir S H, Madsen M L, Mayzaud P, Müller-Navarra D, Nichols P D, Paffenhöfer G A, Pond D, Saito H, Stübing D, Virtue P, 2007. Perspectives on marine zooplankton lipids. Can. J. Fish. Aquat. Sci., 64(11): 1 628–1 639. Doi: 10.1139/f07-122 |
| Kattner G, Hagen W. 2009. Lipids in marine copepods: latitudinal characteristics and perspective to global warming. In: Kainz M, Brett M T, Arts M T eds. Lipids in Aquatic Ecosystems. Springer, New York. p. 257-280. |
| Lee R F, Hagen W, Kattner G, 2006. Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser., 307: 273–306. Doi: 10.3354/meps307273 |
| Lee R F, Nevenzel J C, Paffenhöfer G A, 1971. Importance of wax esters and other lipids in the marine food chain:phytoplankton and copepods. Mar. Biol., 9(2): 99–108. Doi: 10.1007/BF00348249 |
| Li C, Sun S, Wang R, Wang X, 2004. Feeding and respiration rates of a planktonic copepod (Calanus sinicus) oversummering in Yellow Sea Cold Bottom Waters. Mar.Biol., 145(1): 149–157. |
| Liu M T, Li C L, Sun S, 2011. Identification of trophic relationships between marine algae and the copepod Calanus sinicus in a fatty acid approach. Acta Ecol. Sin., 31(4): 933–942. |
| Mayor D J, Anderson T R, Irigoien X, Harris R, 2006. Feeding and reproduction of Calanus finmarchicus during nonbloom conditions in the Irminger Sea. J. Plankton Res., 28(12): 1 167–1 179. Doi: 10.1093/plankt/fbl047 |
| Mayor D J, Anderson T R, Pond D W, Irigoien X, 2009. Egg production and associated losses of carbon, nitrogen and fatty acids from maternal biomass in Calanus finmarchicus before the spring bloom. J. Mar. Syst., 78(4): 505–510. Doi: 10.1016/j.jmarsys.2008.12.019 |
| Meng T X, 2003. Studies on the feeding of anchovy (Engraulis japonicus) at different life stages on zooplankton in the Middle and Southern Waters of the Yellow Sea. Mar. Fish. Res., 24(3): 1–9. |
| Niehoff B, Madsen S, Hansen B, Nielsen T, 2002. Reproductive cycles of three dominant Calanus species in Disko Bay, West Greenland. Mar. Biol., 140(3): 567–576. Doi: 10.1007/s00227-001-0731-3 |
| Ohman M D, 1997. On the determination of zooplankton lipid content and the occurrence of gelatinous copepods. J. Plankton Res., 19(9): 1 235–1 250. Doi: 10.1093/plankt/19.9.1235 |
| Parrish C C. 1999. Determination of total Lipid, lipid classes, and fatty acids in aquatic samples. In: Arts M T, Wainman B C eds. Lipids in Freshwater Ecosystems. Springer, New York, US. p. 4-20. |
| Pond D W, Tarling G A, 2011. Phase transitions of wax esters adjust buoyancy in diapausing Calanoides acutus. Limnol. Oceanogr., 56(4): 1 310–1 318. Doi: 10.4319/lo.2011.56.4.1310 |
| Rey-Rassat C, Irigoien X, Harris R, Carlotti F, 2002. Energetic cost of gonad development in Calanus finmarchicus and C. helgolandicus. Mar. Ecol. Prog. Ser., 238: 301–306. Doi: 10.3354/meps238301 |
| Sun S, Wang S W, Li C L, 2011. Preliminary study on the oil storage of Calanus sinicus fifth copepodites (C5) in the Yellow Sea. Oceanol. Limnol. Sin., 42(2): 165–169. |
| Sun S, 2005. Over-summering strategy of Calanus sinicus. GLOBEC Int. Newsl., 11: 34. |
| Wang Y Q, Li C L, Liu M T, Jin X, Wang X D, Sun Y K, 2014. Lipid content and composition of Calanus sinicus in the Yellow Sea in spring and autumn. Acta Oceanol. Sin., 36(2): 99–107. |
| Weng X C, Wang C M. 1982. Determining the boundary and range of temperature and salinity of Yellow Sea Cold Water Mass. In: Hydrometeorology. Chinese Society of Oceanology and Limnology Science, Beijing. p. 61-70. (in Chinese with English abstract) |
| Zhang G T, Li C L, Sun S, Zhang H Y, Sun J, Ning X R, 2006. Feeding habits of Calanus sinicus (Crustacea:Copepoda) during spring and autumn in the Bohai Sea studied with the herbivore index. Scientia Marina, 70(3): 381–388. Doi: 10.3989/scimar.2006.70n3 |
| Zhang G T, Sun S, Yang B, 2007. Summer reproduction of the planktonic copepod Calanus sinicus in the Yellow Sea:influences of high surface temperature and cold bottom water. J. Plankton Res., 29(2): 179–186. Doi: 10.1093/plankt/fbm005 |
| Zhou K L, Sun S, Wang M X, Wang S W, Li C L, 2016. Differences in the physiological processes of Calanus sinicus inside and outside the Yellow Sea Cold Water Mass. J. Plankton Res., 38(3): 551–563. Doi: 10.1093/plankt/fbw011 |
 2017, Vol. 35
2017, Vol. 35