Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Olga L. GASKOVA(Olga L. GASKOVA), D. Vera D.STRAKHOVENKO(KOVera D. STRAKHOVEN), Nadezhda I. ERMOLAEVA(Nadezhda I. ERMOLAEVA), Eugene Yu. ZARUBINA(Eugene Yu. ZARUBINA), Ekaterina A. OVDINA(Ekaterina A. OVDINA)
- A simple method to model the reduced environment of lake bottom sapropel formation
- Chinese Journal of Oceanology and Limnology, 35(4): 956-966
- http://dx.doi.org/10.1007/s00343-017-5345-9
Article History
- Received Dec. 18, 2015
- accepted in principle Feb. 14, 2016
- accepted for publication Apr. 28, 2016
2 Novosibirsk State University, Novosibirsk 630090, Russia;
3 Institute for Water and Environmental Problems, Siberian Branch of the Russian Academy of Sciences, Novosibirsk 630090, Russia
Change in the geochemistry of lake systems is determined more and more by climate change, i.e. water balance in their catchment areas, and by internal chemical and biological processes occurring in the lakes themselves. (Zavarzin, 2006; Leonova and Bobrov, 2012; Moiseenko et al., 2013b). Minerals play a key role in low-temperature geochemical processes involving living organisms (Calas et al., 2015). The interplay between biology, geochemistry, materials science, and environmental science constitutes one of the frontier interfaces of modern science that impact on human welfare. The lakes Kambala and Barchin (Baraba steppe, southern West Siberia, Russia) are characterized by the deposition of organic-rich sediment known as sapropel. The reasons for the sapropel formation "remain a long-standing debate in the science community, and require disentangling the roles of climatic/oceanographic processes that triggered higher primary productivity or enhanced organic matter preservation" (Plancq et al., 2015, p.30). In any case, the organic-rich sediments reflect not only the nutrient accumulation dynamics, but also the highly diverse (bio)geochemical processes and mechanisms in the operation of each lake system.
Processes of hydration and oxidation/reduction, the colloidal transport and coagulation, formation of organic complexes and deposition of sulfide and sulfate minerals take place simultaneously (Moiseenko et al., 2006), together with diverse microbiological processes (Borzenko and Zamana, 2008; Lein et al., 2013). Chemical reactions included in the diagenetic model have been published recently (Reed et al., 2011). Their components interact through a suite of reactions including (1-2), where OM is organic matter (OM) of the form (CH2O)a(NH3)b(H3PO4)c:
 (1)
(1)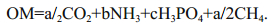 (2)
(2)The process "under anaerobic conditions, where sulfate-reducing bacteria (SRB) oxidize organic compounds using sulfate as a terminal electron acceptor and generate hydrogen sulfide (H2S) and alkalinity" is described in Diez-Ercilla et al. (2014, p.520) as a reaction (3):
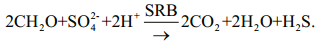 (3)
(3)The intensity of sulfide precipitation depends on SRB activity, which in turn depends on the availability of the organic carbon produced. K. L. Gallagher showed "through culture experiments, theoretical calculations, and geochemical modeling studies that the pH, alkalinity, and organomineralization potential will vary depending on the type of electron donor" (Gallagher et al., 2012, p.518). For example, they describe the effect of pH decrease on the metabolism of bacteria as a reaction (4):
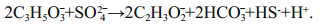 (4)
(4)Our article deals with the forward and reverse thermodynamic models of interaction between lake water and bottom sediments. Our aim has been to calculate a possible mineral composition of sediments on the base of the known solution chemistry and, vice versa, to restore the hydro-geochemical parameters of the lake water with exact chemical and mineral composition of the solid phase. Since a large amount of organic material has been found in the lake water and solid sediments, the next purpose of this study is to quantify and understand the effects of organic carbon on the Eh-pH conditions, on the precipitation of calcite CaCO3 and magnesite MgCO3, and on the type of bottom sediments as a whole. At the moment, there is an urgent need to quantify the effect of organic matter on the processes of water-bottom sediment interaction in fresh and saline lakes systems.
A computer thermodynamic model was developed for chemical interaction in water-bottom sediment systems of the Barchin (55.712 838 n.l. and 78.149 357 e.l.) and the Kambala (55.678 784 n.l. and 78.208 065 e.l.) Lakes. These lakes have the same geochemical and geomorphological landscapes, bordering the catchment area, common sources of water supply, sapropel type of precipitation with an ash content of about 50%. Nevertheless, these lakes differ in their types of overgrowing vegetation, biota species composition, and concentration of Ca and Si in the bottom sediments. They are good candidates for the development of methods for the chemical processing of sapropel.
2 STUDY AREA AND METHODMore than 20 000 lakes with sapropel are located in southern West Siberia. Field sampling and measurement of physical-chemical and hydrological parameters of the lake waters as well as core sampling of bottom sediments were carried out during 2011-2014 in 28 lakes of the Om'-Tatarsk-Vasyugan interridge-ravine lake system of the Baraba steppe. The studied lakes are small in area, and their maximum depth is no more than 3 m. Thick and wet suspension from dead algae, remnants of living organisms, soil particles and humus transform gradually into sapropel in the bottom zone of both Barchin and Kambala Lakes. Geochemical and mineralogical characteristics of lake waters and sapropel sediments of these two small lakes have already been described (Strakhovenko, 2011; Strakhovenko et al., 2014, 2015). Namely these data were used for the model thermodynamic calculations in this paper (Tables 1-3, 5, 6).

|

|

|

|
A much more interesting feature of these lakes is biota diversity, but the precise reason for this phenomenon is not currently known. Diatoms are among the most common types of phytoplankton in the Kambala Lake. The diatom cells incorporate skeletons composed of hydrated SiO2 dioxide, so this lake contains siliceous type sapropel. Small-celled colonial calcareous cyanobacteria capable of depositing calcium carbonate inside or on the surface of the mucous sheath that surrounds each cell or multicellular colony (Bilan and Usov, 2001) sharply dominate in the Barchin Lake with calcium type sapropel. The Kambala Lake, compared to the Barchin Lake, is marked by higher productivity of zooplankton (829 and 159 g/m3 per year, respectively).
During the study period, the main species of zooplankton in the Kambala Lake was large filtering Cladocera, whereas Rotifera and small Cladocera dominated in the Barchin Lake. Zooplankton active life is the cause of the high content of Humic Substances (HS) in the Kambala Lake sapropel (38%) contrary to the Barchin Lake (18%). We showed previously that "an active role in the formation of sapropel belongs to microorganisms. The studied lakes are characterized by the abundant bacteria of the genus Sphaerotilus, which reduce Fe actively, and sulfate-reducing bacteria (SRB), which play the leading role in the formation of hydrogen sulfide and in the deposition of sulfide minerals" (Strakhovenko et al., 2015, p.1 164). Pyrite was found in both lakes in the first mm of the sapropel silts. Air-water plants also have a big impact on Ca and Si accumulation in the sediments. The soils around lakes are salinized and solodized; the surrounding territory is swampy. The bedrocks are characterized as loess-like clay loams and lake clay alluvium (Strakhovenko et al., 2014).
Analysis of group composition of both sapropel organic substances showed significant differences in total easily hydrolyzable materials (EHM) and HS (Strakhovenko et al., 2015). EHM (polysaccharides) are present in large quantities in the Barcin sapropel (22.2%); they are synthesized by higher plants and cyanobacteria. Carbonic anhydrase enzyme localized in the outer layers of cyanobacteria is involved in photosynthetic carbon assimilation and the process of CaCO3 deposition. The Kambala Lake sapropel is characterized by a low amount of EHM (2.0%) and a high content of HS (38%). Zooplankton (large filtering Cladocera) in the waters of this lake readily absorb EHM and forms HS. The mechanism of biochemogenic carbonate deposition does not occur.
The Barchin Lake has the border's type of overgrowth (width is 20-30 m). It is dominated by Phragmites australis. The area overgrown with macrophytes in the Kambala Lake is 30% (width is 120-150 m). It is dominated by Phragmites australis and Ceratophyllum demersum. The lake is located in Baraba steppe, so leaf litter is not supplied to the water. Allochthonous organic matter is retained by the semi-submerged macrophyte barrier and does not reach the center of the lake. This means that the bottom sediments must be formed by almost 100% autochthonous organic matter. This is indirectly confirmed by the ratio of Сorg/Norg. For the lakes of Western Siberia South, the ratio below 14 corresponds to organic matter of autochthonous origin (Moiseenko et al., 2013a). In both studied lakes this ratio is in the range from 12 to 13. Such factors as concentration of nutrients (N, P) in both lakes are very similar because they are on the same flood basin; sunlight and temperature are the same, whereas the dominant species of biotic components of an ecosystem in each lake are significantly different. Namely the species composition of aquatic organisms has resulted in a different sapropel composition (Yermolaeva et al., 2016).
The average chemical composition of the Barchin and Kambala bottom sediments has been determined by X-ray fluorescence (XRF) and atomic absorption spectroscopy (AAS) analyses and is shown in Table 1. The losses on ignition (LOI), which vary from 59.9% in the Barchin sapropel to 48.7% in the Kambala sapropel, can be used to estimate the organic-matter content of these bottom sediments. A common assumption is that LOI is a loss of water, organic substances, CO2 of carbonates and adsorbed gases. Calcite decomposition occurs in the temperature interval of 708-778℃. A quantitative determination of the sapropel trace-elements content is shown in Table 2. The trace-element contents of sapropel from different lakes are "different but remain within the standard deviation from the average values for Siberian bottom sediments, i. e., at the level of the regional background" (Strakhovenko, 2011, p.16). Results of X-ray diffraction analyses of the representative layers of lake bottom sediments and content of the selected elements are shown in Table 3.
Thermodynamic equilibrium modeling at 25℃ and 1 bar total pressure was performed with the "HCh" code and the UNITHERM database using a Gibbs free energy minimization algorithm (Shvarov, 2008). In this case there is no need to describe the mass balance of dissolved and particulate chemical species by a suite of reactions (Reed et al., 2011). We modeled the heterophase 20-component system H-OC-Сl-S-P-Na-Ca-Mg-K-Al-Fe-Mn-Sr-Ba-Li-Hg-BeCu-Zn. The dissolved species and solid phases thermodynamic data (specific to the type of mineral lakes studied) were incorporated into the UNITHERM database using data borrowed from the literature elsewhere. Activity coefficients were calculated with the third approximation of the Debye-Hückel equation, which is valid for an ionic strength I < 0.5 mol/L. Specific Eh-, pH-data, the equilibrium composition of the solution (including elements speciation) and solid phases as well as under/ supersaturation indices (SI) with respect to all phases are the results of each simulation. There are spatial dependences in the characterization of the sediments, but the thermodynamic modeling is applied to the sediment as a whole because they do not change substantially to a depth of 13 cm. This finding also describes the near constancy of conditions in the water-rock system. There are "two known factors contributing to carbonate mineral precipitation—the saturation index and the presence of nucleation sites. Both the above factors can be influenced by microorganisms, which can either alter SI through their metabolisms, or produce and consume organic substances such as extracellular polymeric substances (EPS) that can affect nucleation" (Gallagher et al., 2012, p.518).
Direct thermodynamic calculation of bacterial influence (or total organic influence) on the geochemical processes is currently impossible. The corresponding redox reaction could be modeled using CH2O as a generally accepted model of organic matter and an electron donor (reaction 3). So simple model excluding the quantity and quality of DOM (molecular weight and elemental composition) could only equilibrate the redox conditions without clarifying the exact mechanism of the observed phenomena. So our results are only semiquantitative. For example, in some waters nearly 90%-100% of calcium ions may be associated with humic acids. Molecular weight and heteroatom content of the humic substances correlated well with calcite growth inhibition, whereas carboxyl content and their aliphatic nature did not correlate at all (Hoch et al., 2000).
Method of calculation of the complexation of calcium and magnesium with FA (fulvic acid) and HA (humic acid) is described below. Humic substances (HS), including FA, HA and humin, are a general class of biogenic, refractory dissolved organic matter that is ubiquitous in terrestrial and aquatic environments (Hoch et al., 2000). In our thermodynamic model FA and HA could dissociate and phenolic and carboxylic acid functional groups are the ligands to form complexes with Ca2+ and Mg2+. The complexation in turn would decrease the saturation index (SI) and inhibit calcite growth. Carboxyl functional groups are among the most abundant and chemically reactive components in humic substances. Table 4 shows the dissociation constants of FA and HA and stability constants of organic complexes of calcium and magnesium. These constants are a synthesis of many authors' data where N is the number of binding sites per molecule of FA or HA. For example, if a solution contains 50 mg/L of FA or HA, so 0.36 or 0.25 mmol/L of organic acid binding sites are capable of complexation with cations. "High-molecular weight organic acids are known to play a double role: they can decrease the solubility of metals, binding them into surface complexes with HA of sediments, or increase it by complexation with dissolved FA" (Gas'kova and Sklyarova, 2013, p.639).
3 RESULT AND DISCUSSIONThe brackish lake Kambala water salinity (0.53 g/L) has a pH of 9.3 (Tables 5, 6). The water of this lake is sodium-bicarbonate-rich. The molar ratio of Na/(Ca+Mg) ranges from 1.4 to 1.7. The Ca/Mg ratio ranges from 0.75 to 0.85, a (Ca+Mg)/HCO3- —from 0.55 to 0.62 for different years. As mentioned above, the parent material for catchment-area soil is the covering loess-like clay loam and lake clay alluvium. Sedimentary rocks (shale, sandstone, limestone) are the reason that the fresh waters are low in dissolved silica (less than 20 mg/L) and (Ca+Mg)/ HCO3 ratios higher than 0.5 (Yan et al., 2002).
Thermodynamic calculations showed that there was a small water supersaturation with respect to quartz, calcite (~0.93 mg/L) and magnesite (Table 7, 2 and 3 columns). Traces of chlorite (1.11×10-6 gmol/L), goethite, pyrolusite, strontianite and apatite-F may occur in the suspension. X-ray diffraction analyses revealed that the X-ray amorphous phase is a significant part of the mineral composition of the Kambala bottom sediments (Table 3). The rest of the composition is represented mainly by quartz with minor admixture of pyrite, illite, feldspars and clay minerals. Traces of low Mg-calcite were found in the upper layers of bottom sediments. The complexation of Ca2+ and Mg2+ with organic acids in solution and solid phases (TOC is 16.5 mg/L) may be responsible for the small amounts of carbonates in the sediments where the surface water is supersaturated with respect to calcite and magnesite (pH 9.3).
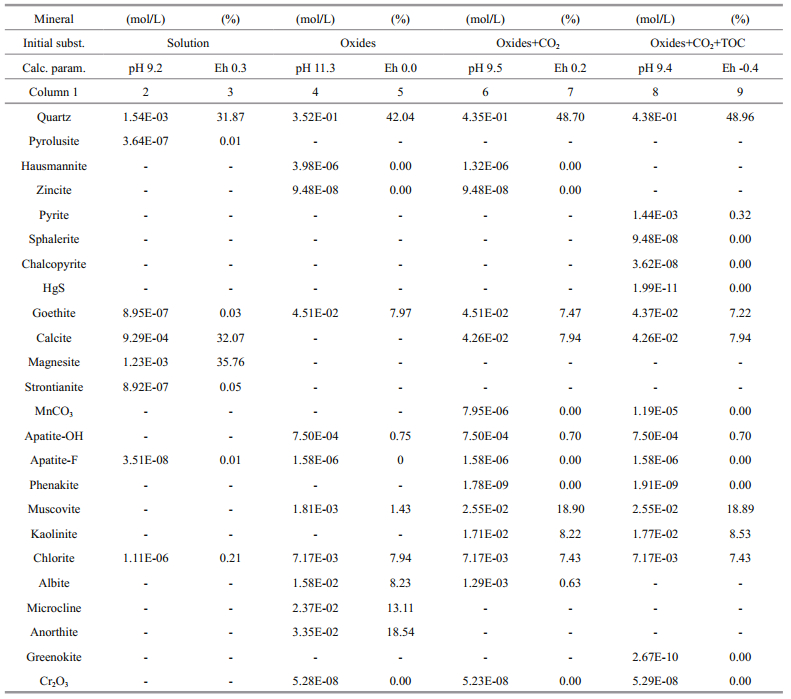
The mineral composition of the bottom sediments of the Kambala Lake has been recalculated according to X-ray fluorescence and AAS analyses (Tables 1, 2). Recall that the loss on ignition to 900℃ of the Kambala sediment samples was 48.7% (LOI). Elemental analysis indicates that the Corg content is about 15% and Cinorg is about 4% in the sapropel sediments. The content of Corg increases in the lower part of the bottom sediments and the content of Cinorg decreases from 7.2% to 3.9% with depth of the sediments cross-section (Table 3). If neither CO2 nor CH2O are taken into account in the calculation, the mineral association consists mainly of quartz, all feldspars, chlorite, goethite with minor muscovite (illite), apatite, hausmannite Mn2O4 and zincite ZnO (Table 7, 4 and 5 columns). The equilibrium of this association with water occurs at the alkaline рН 11.3 and low-reduced Eh=0.0 V. However, the introduction of two grams of CO2 into the system composition leads to a decrease in pH to 9.5 (identical one measured) and calcite (7.94%) appears inevitably in the association mainly with quartz (48.7%), muscovite (18.9%), kaolinite (8.22%), goethite and chlorite (Table 7, 6 and 7 columns). Only albite (the most stable of feldspars) remains, that increases the proportion of micaceous minerals on account of other feldspars. One could see that magnesite MgCO3(s) is absent, but low-Mg calcite like Ca0.98Mg0.02CO3 has near equilibrium saturation index, logSI=-0.122 (not shown in Table 7).
The influence of organic matter was modeled by introducing of 1.5 gram of virtual CH2O component (Table 7, columns 8 and 9). The calculation results showed that this approach was methodologically correct and TOC oxidation immediately causes precipitation of pyrite and many other sulfides at рН 9.4 and Eh -0.4 V:
 (5)
(5)Of course, nano-contents of HgS (1.99×10-11 mol/L) could not be determined microscopically but calculations point to its presence in the Kambala bottom sediments. The same applies to sphalerite, chalcopyrite and greenockite CdS. It is noticeable, that traces of gypsum CaSO4∙2H2O were identified in the low part of natural Kambala sediments (Table 3, KS-9-10 and KS-13) instead of low-Mg calcite. It means that redox conditions are marked by SO42-/HSequilibrium at fixed pH, Eh, CH2O and HCO3- activities (5). Nevertheless, low total Ca and SO3 content (Table 1) determines the absence of gypsum in calculated model association (logSI≤-3 for gypsum).
It is necessary to find any peculiarities in the composition of the Barchin lake water in equilibrium with the carbonate-containing sapropel sediments.
The brackish Barchin Lake water has a salinity of 0.57 g/L and a pH of 8.9 (Tables 5, 6). The water of this lake is clearly bicarbonate-sodium-rich, with a molar ratio Ca/Mg that ranges from 0.43 to 0.62. The Na/(Ca+Mg) molar ratio ranges from 1.9 to 2.3, (Ca+Mg)/HCO3- molar ratio ranges from 0.32 to 0.45 for different years (2010-2013). Note that the (Ca+Mg)/HCO3- ratio is lower than 0.5 in comparison to the Kambala Lake water. The content of dissolved organic carbon is 26.6 mg/L. The mineral part of the Barchin Lake sapropel consists of low-magnesian calcite up to 80 wt.%. Elemental analysis indicates (Table 3) that Corg content is about 11.5% and Cinorg is about 29% in the sapropel sediments. Their content is about the same throughout the bottom cross-section. Cinorg content in the Barchin Lake sediments is 5 times higher than in the Kambala Lake and SiO2 content is 3 times lower depending on the type of sapropel. It is noteworthy that SO3 content is also ~13 times higher (Table 1). Significant amounts of sulfates enter the water bodies in the process of organisms dying, oxidation of terrestrial and aquatic substances of plant and animal and with an underground drain. The findings of aragonite are very interesting and indicative. Aragonite originates from portions of shells of various kinds of shellfish and it is absent in the siliceous sapropel of the Kambala Lake.
Thermodynamic calculations have shown that there are no major differences in the composition of the minerals to which the Barchin Lake water is supersaturated in comparison with the Kambala Lake water. These are mainly calcite and magnesite, minor quartz, trace of goethite and chlorite like as seen in the second column of Table 7. Alternative calculations when the series of CaxMg1-xCO3 solid solutions were considered instead of pure CaCO3 or MgCO3 have shown that the lake water could be supersaturated with respect to low-magnesian calcite.
The mineral composition of the bottom sediments of the Barchin Lake has been recalculated according to X-ray fluorescence and AAS analyses (Tables 1, 2). If neither CO2 nor CH2O are taken into consideration in the calculation, portlandite Ca(OH)2(s) could be formed and an equilibrium of this association with water occurs at the alkaline рН of 12.0. It is obviously a wrong conclusion. Due to the high LOI content (59.9%), 14 grams of CO2 were added in the system and mainly low-magnesian calcite (62%), quartz (17%), chlorite (8%), gypsum (5%), muscovite (1.5%) were formed at рН 9.9 and Eh 0.25 V in association with trace goethite, apatite, hausmannite Mn2O4, zincite and even barite (6.8×10-8 gmol). Magnesite MgCO3(s) is absent because of high chlorite Mg4.5Al3Si2.5O10(OH)8 content. The influence of organic matter was modeled by introducing 1 gram of virtual CH2O component. The calculation results showed that TOC oxidation causes pyrite and many other heavy metal sulfides to precipitate at рН 8.9 and Eh -0.3V. Calcite is the main mineral (66.3%), gypsum is absent, but its logSI=-0.02 (near equilibrium saturation). Consequently, the occurrence of a new mineral, gypsum CaSO4∙2H2O, though it was not identified by X-ray investigation in carbonate sapropel of the Barchin Lake, is a characteristic of the Barchin association (Table 3). So the important process controlling the evolution of closed lakes is poorly treated in the current model. This is the removal of dissolved SO42- according to different rate of sulfate bacterial reduction to produce H2S and alkalinity (Yan et al., 2002).
Calcite-gypsum equilibrium is important as an indicative. A strong vertical redox gradient within a water column separates sulfide bearing bottom water (silt water) from overlying oxygenated water. Pyrite may form immediately below the redox chemocline. Nevertheless, depending on the Eh, sulfate sulfur can dominate in solution, causing the formation of gypsum together with pyrite. So, the silt water is not necessarily euxinic (anoxic and sulfidic water). Our calculations show that at the measured pH of 9.3-8.9, sulfate sulfur may prevail at Eh (-0.3)-(-0.4) V (Fig. 1). Then reaction (5) should be supplemented by reaction (6).
 (6)
(6)

|
| Figure 1 Sulfate and sulfide sulfur concentrations in equilibrium with indicated mineral associations depending on pH, Eh at 25℃ Py: pyrite; Ct: calcite; Gt: goethite; Gyp: gypsum. |
The concentration of selected trace elements and anions in model solution equilibrated with pyritecontaining bottom sediments in comparison with the surface water of the Barchin Lake are shown in Table 8. It is obvious that in solving the inverse problem (recovery of the solution composition on the basis of known chemical composition of the sediments) much less cations are in the model solution than in the surface water. The exception is sodium, which probably was brought with clastic material. Its behavior is controlled by the process of dissolution of aluminosilicate minerals (that have been included as oxides in Table 1) until saturation with respect to the Na-salt minerals is achieved. As described above, lake waters are supersaturated with respect to calcite and magnesite. This means that the equilibrium concentrations of Ca and Mg in model solutions are significantly lower than the one measured in the lake waters. For example, Ca is 6.14 and 28 mg/L and Mg is 1.1×10-4 and 39 mg/L respectively in the model and natural waters of the Barchin Lake. At the same time, Na+ and K+ are conservative elements (the concentration of these elements normalized to salinity is constant) and this is a reason of a change of the cation ratio in the model solution, i.e. Na>K>Ca>Mg (mg/L). Model associations recalculated on the base of Tables 1 and 2 contain Ab-An, Kfsp, kaolinite and muscovite (illite) as natural associations (Table 3). It means that the substance that has been brought in to the bottom sediments with clastic materials comes back into the solution. This fact should be taken into account when calculating the mass balance.

|
The next question concerns the causes of supersaturation of many surface waters with respect to magnesite, calcite and aragonite, despite the fact that the last mineral abiogenic deposition is relatively rare. Propensity of Ca2+ and Mg2+ to form stable supersaturated solutions in the surface waters is their characteristic feature (Gas'kova et al., 2011; 'Gaskova and Sklyarova, 2013). Fresh and ocean waters are commonly supersaturated with respect to carbonate phases with no observed mineral precipitation (Berner et al., 1978; Gas'kova et al., 2015). It is known that in nature, dissolved organic matter (DOM) acts as a growth inhibitor to calcium carbonate crystal growth. "Although many other ions are present in the complex water systems", Chen et al. (2005, p.e1342) showed "that the calcite growth rate could often be well described by considering inhibition mainly from Mg2+ (in addition to DOM)". Magnesium ions could be adsorbed to the calcite surface thus blocking crystal growth sites. Nevertheless, Mg2+ is an effective inhibitor at relatively low content. A reasonable method to reproduce this effect by a thermodynamic modeling is not found elsewhere. Let us try to evaluate the effect of calcium and magnesium complexation with humic acids on the precipitation of CaCO3(s) and MgCO3(s) taking into account a number of assumptions about the thermodynamic properties of these highmolecular substances and their stoichiometry (Table 4).
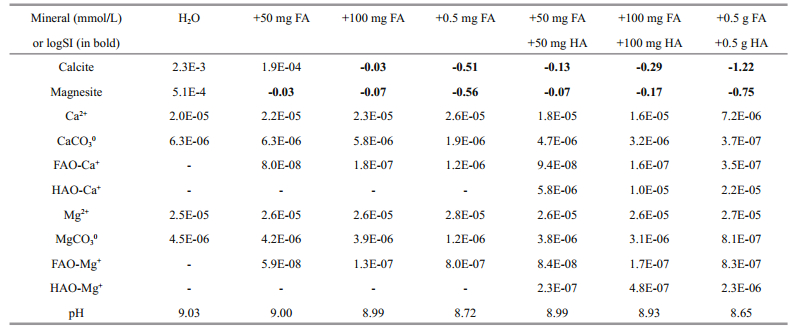
Let us assume that the solution is slightly supersaturated with respect to calcite and magnesite and that 0.002 3 gmmol calcite and 0.000 5 gmmol of magnesite are precipitated from every liter of water (Table 9). If the solution contains 100 mg of FA (column 4), it becomes undersaturated with respect to these minerals (logSI -0.03 and -0.07 respectively). Nevertheless only 0.6% Ca and 0.4% Mg are in the species like FA-Ca+ and FA-Mg+. If the solution contains 100 mg of FA and HA (column 7), a share of calcium associates with HA and is able to deposit into the bottom sediments at 34.5%. For magnesium, the effect is small even in this case and the share of FAMg+ is equal to 1.6% only. This is due to the fact that calcium forms much more stable complexes with organic acids, compared to magnesium. Experimental "addition of calcium to the DOC extracts resulted in flocculation of up to 50% of the DOC originally present in the samples" (Römkens and Dolfing, 1998, p.363).
|
In summary we could say, that despite the fact that the investigated sediments are significantly different in their content of CaO (2.6%-19.8%), SiO2 (34.1%-11.2%), Fe2O3 (3.6%-1.0%) and SO3 (0.23%-3.0%) for the Kambala and Barchin Lakes respectively, the model phase composition variability consists mainly of diverse proportions between calcite, quartz and pyrite. Nevertheless, the sapropel sediments of the Barchin Lake have higher Ba, Sr, Mn, U contents, whereas higher V, Cr, Co, Ni, Cu, Zn, Cd, Li contents are typical of the Kambala Lake according to the XRF and AAS analysis. It is not clear exactly if this is a result of the trace elements accumulation during recent biochemical sedimentation from the lake water or that the elements have been carried from the catchment areas by terrigenous materials. According to our investigation, the rates of accumulation of organic components in the inland lakes of western Siberia are comparable to the supply of terrigenous materials, resulting in the formation of sapropel deposits. Dating of the bottom sediments was made by the activity of radioisotopes 137Cs and 210Pb, which permitted estimation of the sedimentation rates in lakes in different regions of Siberia: 0.35 cm/year in the south and 0.25-0.3 cm/year in the north (Strakhovenko et al., 2010, p.1 167). It is interesting that the homogeneous source rocks of the Baraba steppe have higher contents of V, Cr, Co, Ni, Cu, Zn, Cd and Li than the upper continental crust. Maybe the Kambala Lake sapropel is more affected by clastic rocks of the lake catchment areas, and the Barchin Lake sapropel more by the accumulation of biogenic carbonates, including aragonite. Very interesting "geochemical meaning of elemental ratios" have been discussed recently (Lash and Blood, 2014; Tachikawa et al., 2015). They suppose that sediments that accumulated beneath areas of high primary productivity are often enriched in Ba-bearing solid phases. Barite dissolves after burial under strongly reducing conditions, where sulfates are unstable. Barium passes into the interstitial pore waters, limiting the application of Ba and barite as an indicator of paleo productivity (Lash and Blood, 2014).
4 CONCLUSIONKambala and Barchin Lakes have similar geochemical and geomorphological landscapes and a sapropel type of bottom sediments with an ash content of about 50%. Nevertheless, these lakes are different in types of overgrowing vegetation and biota speciation and have siliceous and calcium types of sapropel sediments respectively. Thermodynamic calculations have shown that there is no major difference in the mineral compositions with respect to which lakes have supersaturated water. It means that biological activity causes the formation of different mineral phases in environmentally relevant situations. Analysis of group composition of both sapropel organic substances showed significant differences in total easily hydrolyzable materials (EHM) and Humic Substances (HS). EHM are presented in large quantities in the Barcin sapropel. Carbonic anhydrase enzyme localized in the outer layers of cyanobacteria is involved in photosynthetic carbon assimilation and CaCO3 deposition in the Barcin Lake sapropel. The Kambala Lake sapropel is characterized by a low amount of EHM and a high content of HS. Zooplankton (large filtering Cladocera) in the waters of this lake readily absorbs EHM and forms HS. The mechanism of biochemogenic carbonate deposition is not occurring.
The influence of organic matter was modeled by introducing a virtual CH2O component. The calculation results showed that this approach was methodologically correct and CH2O oxidation is responsible for the precipitation of pyrite and many other heavy metal sulfides at Eh < 0 V. This clearly demonstrates an obvious disequilibrium between surface and silt waters. Calcite-gypsum equilibrium is important because pyrite may form within the water column immediately beneath the sulfide chemocline. It was shown that depending on the Eh, sulfate sulfur can dominate in solution, causing the formation of gypsum together with pyrite and calcite (at pH of 9.3-8.9, SO42- prevails at Eh ~0.3 V). The model of highmolecular weight organic acids was supplemented as FA and HA virtual components are able to complex with calcium and magnesium. They can decrease the saturation indexes of carbonate minerals binding them in complexes.
The results confirm the need to better understand the role of the biota in the formation of sapropel as well as the ability to simulate this process with the help of thermodynamic code.
5 ACKNOWLEDGMENTWe thank the editor and two anonymous reviewers for their constructive comments, which helped us to improve the manuscript.
| Berner R A, Westrich J T, Graber R, Smith J, Martens C S, 1978. Inhibition of aragonite precipitation from supersaturated seawater; a laboratory and field study. Am. J. Sci., 278(6): 816–837. Doi: 10.2475/ajs.278.6.816 |
| Bilan M I, Usov A I, 2001. Polysaccharides of calcareous algae and their effect on the calcification process. Russian Journal of Bioorganic Chemistry, 27(1): 2–16. Doi: 10.1023/A:1009584516443 |
| Borzenko S V, Zamana L V, 2008. Sulfate reduction as factor of the formation of soda waters in Lake Doroninskoe, eastern Transbaikalia. Vestn. Tomsk. Gos. Univ., 312: 188–193. |
| Calas G, McMillan P F, Bernier-Latmani R, 2015. Environmental mineralogy:new challenges, new materials. Elements, 11(4): 247–252. Doi: 10.2113/gselements.11.4.247 |
| Chen T, Neville A, Yuan M D, 2005. Assessing the effect of Mg2+ on CaCO3 scale formation-bulk precipitation and surface deposition. Journal of Crystal Growth, 275(1-2): e1341–e1347. Doi: 10.1016/j.jcrysgro.2004.11.169 |
| Diez-Ercilla M, Sá nchez-España J, Yusta I, Wendt-Potthoff K, Koschorreck M, 2014. Formation of biogenic sulphides in the water column of an acidic pit lake:biogeochemical controls and effects on trace metal dynamics. Biogeochemistry, 121(3): 519–536. Doi: 10.1007/s10533-014-0020-0 |
| Gallagher K L, Kading T J, Braissant O, Dupraz C, Visscher P T, 2012. Inside the alkalinity engine:the role of electron donors in the organomineralization potential of sulfatereducing bacteria. Geobiology, 10(6): 518–530. Doi: 10.1111/j.1472-4669.2012.00342.x |
| Gas'kova O L, Isupov V P, Vladimirov A G, Shvartsev S L, Kolpakova M N, 2015. Thermodynamic modeling of the behavior of uranium and arsenic in mineralized ShaazgaiNuur Lake (Northwest Mongolia). Doklady Earth Sciences, 465(1): 1159–1163. Doi: 10.1134/S1028334X15110094 |
| Gas'kova O L, Sklyarova O A, 2013. Influence of natural organic acids on the Mg/Ca ratio in the bottom sediments of highly mineralized lakes. Russian Geology and Geophysics, 54(6): 637–645. Doi: 10.1016/j.rgg.2013.04.013 |
| Gas'kova O L, Solotchina E P, Sklyarova O A, 2011. Reconstruction of solution chemistry evolution based on the sedimentary record of salt lakes in the Olkhon region. Russian Geology and Geophysics, 52(5): 548–554. Doi: 10.1016/j.rgg.2011.04.007 |
| Hoch A R, Reddy M M, Aiken G R, 2000. Calcite crystal growth inhibition by humic substances with emphasis on hydrophobic acids from the Florida Everglades. Geochim. Cosmochim. Acta, 64(1): 61–72. Doi: 10.1016/S0016-7037(99)00179-9 |
| Lash G G, Blood D R, 2014. Organic matter accumulation, redox, and diagenetic history of the Marcellus Formation, southwestern Pennsylvania, Appalachian basin. Marine and Petroleum Geology, 57: 244–263. Doi: 10.1016/j.marpetgeo.2014.06.001 |
| Lein A Yu., Makkaveev P N, Savvichev A S, Kravchishina M D, Belyaev N A, Dara O M, Ponyaev M S, Zakharova E E, Rozanov A G, Ivanov M V, Flin M V, 2011. Transformation of suspended particulate matter into sediment in the Kara Sea in September of 2011. Oceanology, 53(5): 570–606. |
| Leonova G A, Bobrov V A. 2012. Geochemical Role of Plankton of the Continental Water Bodies of Siberia in Concentrating and Biosedimentation of Microelements. Academic Publishing House "GEO", Novosibirsk, 314p. |
| Moiseenko T I, Gashkina N A, Dinu M I, Kremleva T A, Khoroshavin V Y, 2013a. Aquatic geochemistry of small lakes:effects of environment changes. Geochem. Int., 51(13): 1031–1148. Doi: 10.1134%2FS0016702913130028#/page-1 |
| Moiseenko T I, Gashkina N A, Kudryavtseva L P, Bylinyak Y A, Sandimirov S S, 2006. Zonal features of the formation of water chemistry in small lakes in European Russia. Water Resources, 33(2): 144–162. Doi: 10.1134/S0097807806020047 |
| Moiseenko T I, Skjelkvåle B L, Gashkina N A, Shalabodov A D, Khoroshavin V Y, 2013b. Water chemistry in small lakes along a transect from boreal to arid ecoregions in European Russia:effects of air pollution and climate change. Applied Geochemistry, 28: 69–79. Doi: 10.1016/j.apgeochem.2012.10.019 |
| Plancq J, Grossi V, Pittet B, Huguet C, Rosell-Melé A, Mattioli E, 2015. Multi-proxy constraints on sapropel formation during the late Pliocene of central Mediterranean(southwest Sicily). Earth and Planetary Science Letters, 420: 30–44. Doi: 10.1016/j.epsl.2015.03.031 |
| Reed D C, Slomp C P, de Lange G J, 2011. A quantitative reconstruction of organic matter and nutrient diagenesis in Mediterranean Sea sediments over the Holocene. Geochim. Cosmochim. Acta, 75(19): 5540–5558. Doi: 10.1016/j.gca.2011.07.002 |
| Römkens P F A M, Dolfing J, 1998. Effect of Ca on the solubility and molecular size distribution of DOC and Cu binding in soil solution samples. Environ. Sci. Technol., 32(3): 363–369. Doi: 10.1021/es970437f |
| Shvarov Y V, 2008. HCh:new potentialities for the thermodynamic simulation of geochemical systems offered by Windows. Geochem. Int., 46(8): 834–839. Doi: 10.1134/S0016702908080089 |
| Strakhovenko V D, Ovdina E A, Ermolaeva N I, Zarubina E Yu, Taran O P, Boltenkov V V. 2015. Assessing the role of organic matter in precipitation of authigenic minerals in sediments of the Barchin and Kambala Lakes (Baraba Steppe). Water-Rock Interaction: Geological Evolution. In: Proceedings of the Second Russian Scientific Conference with International Participation. Vladivostok, p. 616-619. (in Russian) |
| Strakhovenko V D, Shcherbov B L, Malikova I N, Vosel Y S, 2010. The regularities of distribution of radionuclides and reare-earth elements in bottom sediments of Siberian lakes. Russian Geology and Geophysics, 51(11): 1167–1178. Doi: 10.1016/j.rgg.2010.10.002 |
| Strakhovenko V D, Taran O P, Ermolaeva N I, 2014. Geochemical characteristics of the sapropel sediments of small lakes in the Ob'-Irtysh interfluve. Russian Geology and Geophysics, 55(10): 1160–1169. Doi: 10.1016/j.rgg.2014.09.002 |
| Strakhovenko V D. 2011. Geochemistry of Bottom Sediments in the Small Continental Lakes of Siberia. Extended Abstract Doctoral (Geol. -Mineral. ) Dissertation. IGM SORAN, Novosibirsk (in Russian), 32p. |
| Tachikawa K, Vidal L, Cornuault M, Garcia M, Pothin A, Sonzogni C, Bard E, Menot G, Revel M, 2015. Eastern Mediterranean Sea circulation inferred from the conditions of S1 sapropel deposition. Climate of the Past, 11(6): 855–867. Doi: 10.5194/cp-11-855-2015 |
| Yan J P, Hinderer M, Einsele G, 2002. Geochemical evolution of closed-basin lakes:general model and application to Lakes Qinghai and Turkana. Sedimentary Geology, 148(1-2): 105–122. Doi: 10.1016/S0037-0738(01)00212-3 |
| Yermolaeva N I, Zarubina E Y, Romanov R E, Leonova G A, Puzanov A V, 2016. Hydrobiological conditions of sapropel formation in Lakes in the South of Western Siberia. Water Resources, 43(1): 129–140. Doi: 10.1134/S0097807816010073 |
| Zavarzin G A, 2006. Does evolution make the essence of biology?. Herald of the Russian Academy of Sciences, 76(3): 292–302. Doi: 10.1134/S101933160603004X |
 2017, Vol. 35
2017, Vol. 35