Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Yan(李俨), QIN Yukun(秦玉坤), LIU Song(刘松), LI Pengcheng(李鹏程), XING Rong'e(邢荣娥)
- Preparation, characterization, and antifungal activity of hymexazol-linked chitosan derivatives
- Chinese Journal of Oceanology and Limnology, 35(5): 1079-1085
- http://dx.doi.org/10.1007/s00343-017-6100-y
Article History
- Received Apr. 21, 2016
- accepted in principle Jun. 1, 2016
- accepted for publication Jul. 5, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
Chitosan (CS) consists of glucosamine β-1, 4-linked with various N-acetyl glucosamine residues (Muzzarelli, 1983) and is a natural cationic polysaccharide. Chitosan is a kind of natural renewable resource and exhibits beneficial properties, such as biodegradability, biocompatibility, and bioactivities. Because of its special molecular structure and bioactivities, chitosan is applied in many areas of production, such as in the pharmaceutical and food industries and agriculture (Liu et al., 2001). Chitosan has been found to have broad-spectrum antifungal activity against plant pathogenic fungi (Kumar et al., 2016). Allan and Hadwiger (1979) have found that chitosan shows antifungal activity against 32 isolates from 46 tested fungi. Bautista et al. have described the antifungal activity of chitosan against Colletotrichum gloeosporioides in vitro. Chitosan (3%) has been shown to completely inhibit C. gloeosporioides growth (Bautista-Baños et al., 2006). However, chitosan also possesses negative features that hinder its further application. Chitosan's antifungal effect is weaker than many chemical fungicides and, thus, many methods, such as chitosan chemical modifications, have been proposed to improve its antifungal activity (Rinaudo, 2006). Different kinds of functional groups have been chemically linked to chitosan polymer chains to achieve new chitosan derivatives, such as carboxymethyl chitosan (Alves and Mano, 2008), sulfate chitosan (Xing et al., 2005), chitosan quaternary ammonium salt (Li et al., 2014), and hydroxypropyl chitosan (Peng et al., 2005).
Recently, synthesis and property studies of chitosan conjugates have received increased attention. Molecules with different bioactivities have been chemically linked to chitosan molecules (Du et al., 2014). For example, chitosan complexation with metal ions exhibits enhanced antibacterial activity (Varma et al., 2004; Higazy et al., 2010) and conjugation of antioxidant molecules and chitosan improves antioxidant activity (Liu et al., 2013). However, there has been little attention paid to improving chitosan antifungal activity by linking it to antifungal agents (Qin et al., 2012, 2014).
Hymexazol (HML) is a fungicide widely used in agriculture (Harveson et al., 2007) and is also a soil disinfectant and plant growth-regulating agent. Owing to its multiple abilities as a broad-spectrum antifungal agent, exhibiting special effects against the growth of mastigomycetes, ascomycetes, and basidiomycetes, HML represents a large share of the commercial fungicide market. HML's antifungal mechanism is to inhibit spore germination and normal growth of pathogenic fungi mycelium (Nakanishi and Sisler, 1983). HML does not inhibit actinomyces and other bacteria in the soil, except for pathogenic fungi, thus making it an environmental agent.
To improve chitosan's antifungal activity, the conjugation (Jia et al., 2014) of chitosan and HML was studied, in which HML was linked onto chitosan polymer chains for the first time. The resulting HMLchitosan derivatives were characterized by Fourier transform infrared (FT-IR) and elemental analysis (EA) to confirm the conjugation. Linkage ratios were detected by HPLC and the derivatives' antifungal activities assessed.
2 MATERIAL AND METHOD 2.1 Materials and reagentsChitosan (86.3% deacetylated), with an average molecular weight of 1 060 kDa, was purchased from Qingdao Yunzhou Biochemical Co., Ltd. (Qingdao, China). HML (>96% purity) was purchased from Kechuang Chemical Corp. (Yantai, China). Chloroacetyl chloride was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Chloropropionyl chloride and 4-(chloromethyl) benzoyl chloride were purchased from Aladdin Industrial (Shanghai) Co., Ltd. (Shanghai, China). Isopropanol, dichloromethane, trichloromethane, potassium carbonate, glacial acetic acid, methanol, sodium hydroxide, ethyl acetate, and Tween 80 were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and all analytical grade. Methanol for HPLC analysis was purchased from Merck KGaA, Darmstadt, Germany and chromatographic grade.
2.2 Analytical methodsFourier transform infrared (FT-IR) analysis was performed using a Thermo Scientific Nicolet iS10 FTIR spectrometer (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA) with attenuated total reflection intelligent components in the spectral range of 4 000– 400/cm. EA was performed on a Vario EL-Ⅲ elemental analyzer (Elementar Analysesysteme GmbH, Hanau, Germany) and the carbon, nitrogen, and hydrogen contents were determined. Linkage ratios of HML to CS in the derivatives were determined using an Agilent 1260 HPLC (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a UVdetector. Chromatography was performed on a C18 reversed-phase column.
2.3 Synthesis of HML linked chitosan derivativesSynthesis was carried out in two steps. For the first step, HML was modified by chloroacetyl chloride, chloropropionyl chloride, or 4-(chloromethyl) benzoyl chloride to achieve a series of intermediates for the following reaction. The second step was synthesis of HML-CS derivatives.
In the first step, HML (4 g) was dissolved in dichloromethane (180 mL) and twice the equivalent amount of chloroacetyl chloride by mol, chloropropionyl chloride, or 4-(chloromethyl) benzoyl chloride added to the dichloromethane dropwise in an ice bath. Then, 1.2 times of equivalent amount of potassium carbonate was also added to the reaction system and the end of reaction tested by thin layer chromatography (TLC). When the reaction was done, the reaction mixture was added to water (50 mL) to extract remaining acyl chloride and the reaction mixture finally poured into a separatory funnel. The lower organic phase was collected and evaporated to dryness to obtain the intermediates.
In the second step, purified CS (3 g) was dispersed in trichloromethane (50 mL). After 30 min of magnetic stirring at room temperature, 12 mL of aqueous NaOH (10 mol/L) was added to the suspension and mixed at 50℃ for 4 h. Then, the dried intermediates from step 1 were dissolved in trichloromethane and added to the reaction system dropwise. After 8 h, the reaction raw product was filtered, added into 150 mL of methanol, and neutralized with acetic acid (1 mol/L). The product was washed with 100% methanol and dried at temperature of 50℃. Finally, HML-CS derivatives were obtained.
2.4 Determination of linkage ratios of HMLchitosan derivativesA 0.1-g mass of HML-CS was added into a methanolic solution (100 mL, pH 4.0 regulated by acetic acid) and, after hydrolyzing for 2 h, HML was extracted from the mixture and the solution then diluted 10 times for quantification. The linkage ratios of HML-CS derivatives were measured using an Agilent 1260 HPLC (Agilent Technologies, Inc.) equipped with a UV-detector. Chromatography was performed on C18 reversed-phase column, using a H2O/methanol (15/85, v/v) solution as the mobile phase at 1.0 mL/min, column temperature at 30℃, and detection wavelength at 220 nm (Pu and Chiou, 1979).
2.5 Antifungal activity assayAntifungal assays against R. solani CGMCC 3.28 and G. zeae CGMCC 3.42 were evaluated in vitro according to an agar medium method described in the literature (Zhong et al., 2007). CS and HML-CS derivatives were prepared to an initial concentration of 1% (w/v), and dissolved in 0.5% acetic acid (by vol). Samples were then mixed with sterile molten potato dextrose agar (PDA) to final concentrations of 100, 200, and 400 mg of derivatives/L. The Scheffe method was employed to evaluate the antifungal index results. All tests were carried out in triplicate and considered statistically significant with a P < 0.05.
3 RESULT AND DISCUSSION 3.1 Preparation of reaction intermediates and HML-CS derivativesIn this study, HML was linked to CS using three kinds of acyl chloride linkers. The linking reaction was performed in two steps: intermediate synthesis and then HML-CS derivative synthesis. The reaction for conjugation of HML-CS derivatives is shown in Fig. 1. In the first step, three reaction intermediates were synthesized by mixing HML and an acyl chloride in dichloromethane. The acid-binding agent was potassium carbonate, by adding the reagent powder directly into the reaction system. Reaction completion was determined by TLC (silica gel plate) developed with methanol/ethyl acetate (v/v, 1/3). In the second step, HML-CS derivatives were produced. Conjugates with linkers from chloroacetyl, chloropropionyl, and 4-(chloromethyl) benzoyl chlorides were named HML-CS-1, HML-CS-2, and HML-CS-3, respectively. The linkage ratios measured by HPLC for the three derivatives were 8.67%, 7.63%, and 6.32%, respectively.
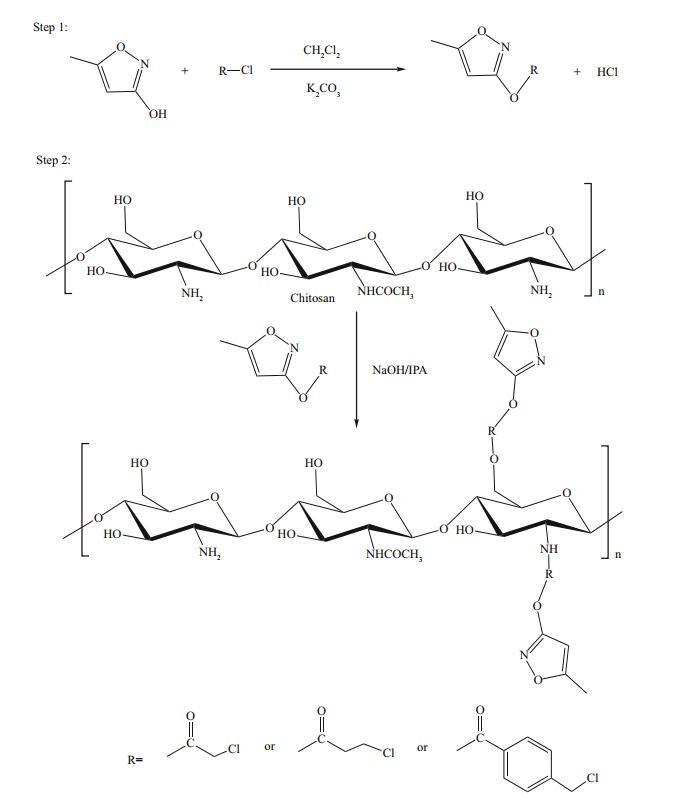
|
| Figure 1 The reaction sequence for HML-CS derivatives |
Figure 2 shows FT-IR spectra of CS and HMLCS-1, 2, and 3. For CS, the broad band around 3 384/ cm was attributed to OH and NH stretching vibrations. The weak band at 2 879/cm was the characteristic absorbance peak of C-H. The band at 1 595/cm was assigned to N-H bending of primary amine. The bands at 1 650, 1 550, and 1 320/cm were attributed to C-O stretching, N-H bending, and C-N stretching, respectively, of the residual N-acetyl groups. For HML, typical absorption peaks around 1 480/cm were caused by symmetric deformation of methyl groups (Yang et al., 2014) and not easily influenced by other substituent groups. For HMLCS-1 and 2, the obvious absorption peak at 1 750/cm was attributed to bending vibrations of C-H belonging to the previous acyl chloride structure. For HMLCS-3, new bands at 700 and 800/cm were observed and attributed to C-H, indicating a monosubstituted and a p-substituted benzene ring, which fitted the theoretical structure of HML-CS-3. All the above results confirmed that HML-CS derivatives had been successfully prepared.
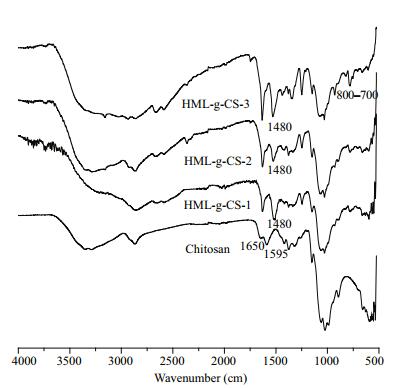
|
| Figure 2 FT-IR spectra of chitosan and HML-CS derivatives |
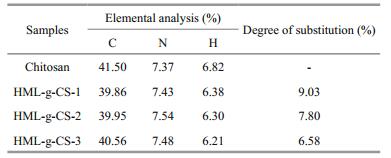
Elemental analysis results of HML-CS derivatives are shown in Table 1. The degree of substitution of HML-CS derivatives was also calculated from elemental compositions, using the formula shown below. As shown in Table 1, the degree of substitution of HML-CS derivatives were 9.03%, 7.80%, and 6.58%, which matched the results calculated from HPLC results.
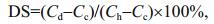
Cd is the HML-CS derivative nitrogen content, Cc the CS nitrogen content, and Ch the HML nitrogen content.
3.3 Antifungal activity of HML-CS derivativesAntifungal properties of HML-CS derivatives against the plant pathogenic fungi Rhizoctonia solani CGMCC 3.28 and Gibberella zeae CGMCC 3.42 were investigated at derivative concentrations of 100, 200, and 400 mg/L.
The antifungal activity of CS and CS-derivatives are influenced by many conditions, such as sample concentration, pH, molecular weight, and degree of deacetylation (Rabea et al., 2003). In this study, CS and HML-CS derivatives were prepared to an initial concentration of 1% (w/v) in 0.5% acetic acid (by vol). The samples were then mixed with sterile molten PDA to final concentrations of 100, 200, and 400 mg/L. Considering that acetic acid might contribute to antifungal activity, the inhibitory activity of the aqueous acetic acid against R. solani and G. zeae was evaluated. Acetic acid antifungal activity against R. solani and G. zeae increased with rising concentrations (Fig. 3). At concentrations < 0.02% (corresponding to the 400 mg/L sample), the inhibitory index of aqueous acetic acid was 0%. At sample concentrations from 100 to 400 mg/L, acetic acid disturbed antifungal activity, such that acetic acid >0.02%, the growth of the R. solani and G. zeae would be inhibited. A 0.05% acetic acid solution inhibited growth of R. solani at 10.5%.

|
| Figure 3 Inhibitory effect of different acetic acid concentrations |
Rhizoctonia solani is a pathogen that causes diseases on rice paddies, peanuts, and sesames (Brogue et al., 1991). CS antifungal activity was clearly enhanced by conjugation with HML (Fig. 4). All three HML-CS derivatives showed good antifungal activity against R. solani. At sample concentrations of 400 mg/L, compared with the CS antifungal index 26.68%, the antifungal indices of HML-CS derivatives were 52.70%, 50.42%, and 42.61%, respectively. The different linkers also impacted the antifungal activity, such that antifungal activities ranked as HML-CS-1>2>3. The chloroacetyl chloride as linker was better than the other two chlorides, which might have been because of two reasons. First, the smaller linker led to easier access to the antifungal activity sites, and second, the linkage ratio was the highest with chloroacetyl chloride as linker. Thus, more HML was on the CS polymer chain.
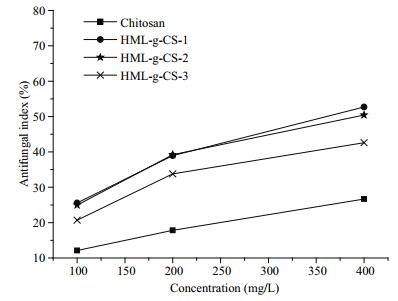
|
| Figure 4 Antifungal activity of HML-CS derivatives against R. solani CGMCC 3.28 |
Gibberella zeae is a pathogen mainly infecting gramineous plants, such as corn and wheat (Bai and Shaner, 1994). The antifungal activities of HML-CS derivatives against G. zeae, compared with CS, were enhanced (Table 2), but the degree of improvement was not as great as effects against R. solani. The antifungal activity of CS alone against G. zeae was stronger than R. solani, while the antifungal activity of HML-CS derivatives against G. zeae were weaker than R. solani. HML-CS-1 at 400 mg/L exhibited the best antifungal activity of the three and the antifungal indices increased with increasing concentration and decreased with linker length. Compared with the antifungal effect on R. solani, G. zeae was more sensitive to these new compounds.
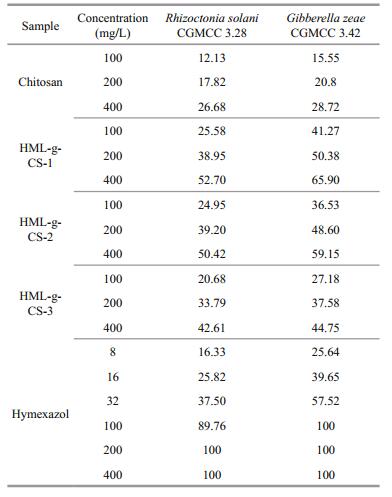
|
HML antifungal activity against R. solani and G. zeae was much stronger than the antifungal activities of the present HML-CS derivatives (Table 2). At concentrations >100 mg/L, the inhibition indices of HML against R. solani and G. zeae were both 100%, while the highest of the HML-CS derivative inhibition effects were 58.32% and 53.48%. When HML-CS derivative concentrations were 100, 200, and 400 mg/L and considering linkage ratios, the HML content was ~8, 16, and 32 mg/L, respectively. At the equivalent concentrations, HML-CS derivative antifungal activities were stronger than HML alone, which meant that HML-CS conjugation showed synergistic effects, suggesting an efficient method for enhancing CS's antifungal activity.
According to the present experimental results and data analysis, HML-CS derivatives showed much stronger antifungal activity than CS alone, indicating that HML-CS conjugation significantly improved antifungal activity relative to CS. Further research might reveal even greater increases in antifungal activity that might lead to an economical product.
4 CONCLUSIONThe preparation, characterization and antifungal activity of HML-CS derivatives were investigated. Results suggested that the HML could be linked to CS using different acyl chlorides as linkers. The intended conjugations were confirmed by FT-IR and elemental analysis. Antifungal assays of CS and HML-CS derivatives against R. solani and G. zeae were evaluated in vitro. Data analysis also indicated that CS antifungal activity was enhanced by this modification. This study provided a new approach for the development of antifungal-CS derivatives. These HML-CS derivatives showed great potential that requires further investigation for the development of novel fungicides.
| Allan C R, Hadwiger L A, 1979. The fungicidal effect of chitosan on fungi of varying cell wall composition. Experimental Mycology, 3(3): 285–287. Doi: 10.1016/S0147-5975(79)80054-7 |
| Alves N M, Mano J F, 2008. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. International Journal of Biological Macromolecules, 43(5): 401–414. Doi: 10.1016/j.ijbiomac.2008.09.007 |
| Bai G, Shaner G, 1994. Scab of wheat:prospects for control. Plant Disease, 78(8): 760–766. Doi: 10.1094/PD-78-0760 |
| Bautista-Baños S, Hernández-Lauzardo A N, Velázquez-del Valle M G, Hernández-López M, Ait Barka E, BosquezMolina E, Wilson C L, 2006. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Protection, 25(2): 108–118. Doi: 10.1016/j.cropro.2005.03.010 |
| Brogue K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais C J, Broglie R, 1991. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science, 254(5035): 1194–1197. Doi: 10.1126/science.254.5035.1194 |
| Du H L, Yang X Y, Pang X, Zhai G X, 2014. The synthesis, self-assembling, and biocompatibility of a novel Ocarboxymethyl chitosan cholate decorated with glycyrrhetinic acid. Carbohydrate Polymers, 111: 753–761. Doi: 10.1016/j.carbpol.2014.04.095 |
| Harveson R M, Windels C E, Smith J A, Brantner J R, Cattnach A W, Giles J F, Hubbell L, Cattnach N R, 2007. Fungicide registration and a small niche market:a case history of hymexazol seed treatment and the U.S. sugar beet industry. Plant Disease, 91(7): 780–790. Doi: 10.1094/PDIS-91-7-0780 |
| Higazy A, Hashem M, Elshafei A, Shaker N, Hady M A, 2010. Development of antimicrobial jute packaging using chitosan and chitosan-metal complex. Carbohydrate Polymers, 79(4): 867–874. Doi: 10.1016/j.carbpol.2009.10.011 |
| Jia X, Sheng W B, Li W, Tong Y B, Liu Z Y, Zhou F, 2014. Adhesive polydopamine coated avermectin microcapsules for prolonging foliar pesticide retention. ACS Appl.Mater. Interfaces, 6(22): 19552–19558. Doi: 10.1021/am506458t |
| Kumar S, Deepak V, Kumari M, Dutta P K, 2016. Antibacterial activity of diisocyanate-modified chitosan for biomedical applications. International Journal of Biological Macromolecules, 84: 349–353. Doi: 10.1016/j.ijbiomac.2015.12.027 |
| Li S D, Li P W, Yang Z M, Peng Z, Quan W Y, Yang X H, Yang L, Dong J J, 2014. Synthesis and characterization of chitosan quaternary ammonium salt and its application as drug carrier for ribavirin. Drug Delivery, 21(7): 548–552. Doi: 10.3109/10717544.2013.853708 |
| Liu J, Lu J F, Kan J, Tang Y Q, Jin C H, 2013. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. International Journal of Biological Macromolecules, 62: 85–93. Doi: 10.1016/j.ijbiomac.2013.08.040 |
| Liu X F, Guan Y L, Yang D Z, Li Z, Yao K D, 2001. Antibacterial action of chitosan and carboxymethylated chitosan. Journal of Applied Polymer Science, 79(7): 1324–1335. Doi: 10.1002/(ISSN)1097-4628 |
| Muzzarelli R A A, 1983. Chitin and its derivatives:new trends of applied research. Carbohydrate Polymers, 3(1): 53–75. Doi: 10.1016/0144-8617(83)90012-7 |
| Nakanishi T, Sisler H D, 1983. Mode of Action of Hymexazol in Pythium aphanidermatum. Journal of Pesticide Science, 8(2): 173–181. Doi: 10.1584/jpestics.8.173 |
| Peng Y F, Han B Q, Liu W S, Xu X J, 2005. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohydrate Research, 340(11): 1846–1851. Doi: 10.1016/j.carres.2005.05.009 |
| Pu F S, Chiou W L, 1979. Creatinine Ⅶ:determination of saliva creatinine by high-performance liquid chromatography. Journal of Pharmaceutical Sciences, 68(4): 534–535. Doi: 10.1002/jps.2600680445 |
| Qin Y K, Liu S, Xing R E, Yu H H, Li K C, Meng X T, Li R F, Li P C, 2012. Synthesis and characterization of dithiocarbamate chitosan derivatives with enhanced antifungal activity. Carbohydrate Polymers, 89(2): 388–393. Doi: 10.1016/j.carbpol.2012.03.018 |
| Qin Y K, Xing R E, Liu S, Yu H H, Li K C, Hu L F, Li P C, 2014. Synthesis and antifungal properties of (4-tolyloxy)-pyrimidyl-α-aminophosphonates chitosan derivatives. International Journal of Biological Macromolecules, 63: 83–91. Doi: 10.1016/j.ijbiomac.2013.10.023 |
| Rabea E I, Badawy M E T, Stevens C V, Smagghe G, Steurbaut W, 2003. Chitosan as antimicrobial agent:applications and mode of action. Biomacromolecules, 4(6): 1457–1465. Doi: 10.1021/bm034130m |
| Rinaudo M, 2006. Chitin and chitosan:properties and applications. Progress in Polymer Science, 31(7): 603–632. Doi: 10.1016/j.progpolymsci.2006.06.001 |
| Varma A J, Deshpande S V, Kennedy J F, 2004. Metal complexation by chitosan and its derivatives:a review. Carbohydrate Polymers, 55(1): 77–93. Doi: 10.1016/j.carbpol.2003.08.005 |
| Xing R E, Yu H H, Song L, Zhang W W, Zhang Q B, Li Z E, Li P C, 2005. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorganic & Medicinal Chemistry, 13(4): 1387–1392. |
| Yang C Q, Xu L, Zeng H Y, Tang Z F, Zhong L, Wu G Z, 2014. Water dispersible polytetrafluoroethylene microparticles prepared by grafting of poly (acrylic acid). Radiation Physics & Chemistry, 103: 103–107. |
| Zhong Z M, Chen R, Xing R E, Chen X L, Liu S, Guo Z Y, Ji X, Wang L, Li P C, 2007. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydrate Research, 342(16): 2390–2395. Doi: 10.1016/j.carres.2007.07.015 |
 2017, Vol. 35
2017, Vol. 35