Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YUAN Mengqi(袁梦琪), WANG You(王悠), ZHOU Bin(周斌), JIAN Xiaoyang(菅潇扬), DONG Wenlong(董文隆), TANG Xuexi(唐学玺)
- An integrated biomarker response index for the mussel Mytilus edulis based on laboratory exposure to anthracene and field transplantation experiments
- Chinese Journal of Oceanology and Limnology, 35(5): 1165-1178
- http://dx.doi.org/10.1007/s00343-017-6092-7
Article History
- Received Mar. 29, 2016
- accepted in principle May. 11, 2016
- accepted for publication Jul. 11, 2016
Coupled with economic development and the escalation of energy consumption in China, polycyclic aromatic hydrocarbon (PAH) emissions have steadily increased in recent decades (Chen et al., 2015). PAHs are major organic components of crude oil and major byproducts of incomplete combustion of fossil fuels and other organic materials (Xu et al., 2006; Ramu et al., 2007). They may enter the marine environment via atmospheric deposition, agricultural and industrial discharge, runoff from the land, accidental spills, and chronic discharges from ships (Piccardo et al., 2001; Hung et al., 2014). Anthracene, a highly toxic PAH with low molecular weight, is a major contaminant in estuarine and coastal areas and is an extensively studied model of PAH pollution (Palanikumar et al., 2012; Gravato et al., 2014; Sellami et al., 2015). Anthracene is highly soluble in seawater and is phototoxic to aquatic organisms (Allred and Giesy, 1985; Oris and Giesy, 1985; Gomes et al., 2009). In coastal areas of China, the measured concentrations of anthracene range from 1.24 to 7.46 ng/L in seawater and 5.30 to 165.69 ng/g in sediments (Wang et al., 2010; Jin et al., 2014). Anthracene is bio-accumulated within mollusks, especially Mytilus spp., at levels ranging from 0.50 to 27.41 ng/g in Europe and 0.2 to 15.7 ng/g in China (Fung et al., 2004; Turja et al., 2014; Vidal-Liñán et al., 2014). However, the toxicology of anthracene has been poorly studied in bivalves (Birdsall et al., 2001). Sessile and filterfeeding bivalve mollusks bio-accumulate a range of pollutants and are considered sentinel organisms that reflect the status of environmental contamination (Ramu et al., 2007). Blue mussels (Mytilus edulis) were used in this study because the species is widely used for pollution monitoring (Fung et al., 2004; Tsangaris et al., 2011; Brenner et al., 2013), and because of its wide distribution and ecological relevance in the Shandong coastal ecosystem.
Biomarkers provide early-warning signals of environmental contamination. A multi-biomarker approach has been widely used in laboratory and field experiments for evaluating the eco-toxicological effects of pollutants (Richardson et al., 2008; Tsangaris et al., 2010; Brenner et al., 2013; Xie et al., 2014). In this study, biomarkers in mussels were used to estimate the toxic effects of anthracene and to identify the impacts of this environmental stressor. Biomarkers examined in mussel tissues included the activities of the glutathione-antioxidant-system enzymes, glutathione S-transferase (GST, EC 2.5.1.18), glutathione peroxidase (GPx, EC 1.11.1.9), and glutathione reductase (GR, EC 1.6.4.2), and the concentrations of reduced glutathione (GSH) and indicators of oxidative damage, malondialdehyde (MDA) and the superoxide anion radical (O2∙-).
GST is widely used as biomarker of detoxification of PAHs in bivalve mollusks because the phase Ⅱ enzyme acts as a catalyst in the reaction of GSH conjugates with contaminants containing electrophilic centers (Hoarau et al., 2004; Damiens et al., 2007). GPx is an enzymatic antioxidant providing cellular protection against reactive oxygen species (ROS) (Sureda et al., 2011). GR reduces the oxidized form of glutathione, which is an essential process for the maintenance of cellar homeostasis (Manduzio et al., 2005). Another biomarker, GSH, responds to tissue redox status and is an important antioxidant agent that scavenges oxygen radicals to yield glutathione disulfide, which, in turn, protects cell membranes from lipid peroxidation (DeLeve and Kaplowitz, 1991; Meister, 1991). As an oxidative-damage biomarker, MDA reflects the status of lipid peroxidation of cell membranes (Knight et al., 1988). The ROS, O2∙-, is a metabolite of PAHs that reacts with various molecular targets. Oxidative cellular damage may occur when the rate of O2∙- generation is greater than its rate of elimination by the antioxidant system (Matés, 2000; Dröge, 2002).
Transplantation of a sentinel species from a clean area to a polluted site is a useful approach for monitoring the effects of environmental pollutants on marine organisms in estuarine and coastal environments, either through detection of their bioaccumulation or through multi-biomarker analysis. Specifically, it has been shown that transplanted mussels accumulate PAH compounds and show sensitive biological responses in monitoring programs (Roméo et al., 2003; Smolders et al., 2005; Hylland et al., 2008; Brooks et al., 2012; Cappello et al., 2013; Lourenço et al., 2015). The transplantation method reduces bias caused by differences in age and reproductive status of mussels and allows experiments to be carried out at sites that lack a native species (Tsangaris et al., 2011). This paper describes a field experiment in which changes in the levels of biomarkers were measured in mussels transplanted to an anthracene-contaminated site. Biomarkers were selected on the basis of laboratory experiments and measured in gills and digestive glands. Comparison of laboratory exposure and field transplantation indicated a meaningful and feasible method for application of multiple biomarkers in marine pollution monitoring, which should improve knowledge of the potential and limitations of the selected biomarkers (Zorita et al., 2005; Sundt et al., 2011).
An integrated biomarker response (IBR) index was used to summarize a series of biomarkers with a single index. The index was first proposed by Beliaeff and Burgeot (2002), and was revised by Devin et al. (2014) to avoid problems of formula misuse and arbitrary choice of the biomarker sequence initially encountered in application of the method. The IBR method is an effective tool for visualization of the biological effects of pollutants, and for simplifying the interpretation of relationships among multiple biomarkers and contamination levels (Damiens et al., 2007). It is now one of the most commonly used integrative indices in environmental biomonitoring programs in the field (Broeg and Lehtonen, 2006; Arzate-Cárdenas and Martínez-Jerónimo, 2011; Tsangaris et al., 2011; Serafim et al., 2012), and in laboratory experiments (Kim et al., 2010; Li et al., 2011; Kim et al., 2013; Zheng et al., 2013; Xie et al., 2014; Lee and Lee, 2015). Star plots provide an efficient graphical aid for visualization of IBR values.
Therefore, the aims of the present work were: (1) to evaluate the responses of selected biomarkers, GST, GPx, GR, MDA and O2∙- in M. edulis exposed to sublethal concentrations of anthracene and to screen for sensitive biomarkers; (2) to develop an IBR index for interpretation of the biomarkers; and (3) to apply the sensitive biomarkers and the IBR in a field experiment.
2 MATERIAL AND METHOD 2.1 Laboratory exposure to anthraceneHealthy blue mussels, M. edulis of standard shell length (45±5 mm) were captured from a commercial shellfish farm in Taiping Bay, located in the coastal zone of the city of Qingdao, Shandong Province, an area relatively unaffected by organic pollution (Xiu et al., 2014). The shell surfaces of collected mussels were scraped clean and the mussels were acclimated to laboratory conditions in glass aquaria for 10 d prior to anthracene exposure. The filtered seawater was continuously aerated, changed daily and maintained at 20±1℃, salinity 35, and with a photoperiod of 12 h L: 12 h D. Mussels were fed daily with 2×105 cells/mL Platymonas helgolandica but were unfed for 24 h prior to transfer to the experimental tanks.
Anthracene (Sigma-Aldrich, St. Louis, MO) was dissolved in a small volume of acetone (Sinopharm, Beijing, China), which was added to seawater to obtain the final nominal concentrations (final acetone concentrations were < 0.002 5%. Concentrations of anthracene were analyzed using an Agilent 1100 HPLC system (Agilent Technologies, Waldbronn, Germany) (Valavanidis et al., 2008; Kookana et al., 2013). Although the actual concentrations of anthracene sometimes deviated slightly from their nominal concentrations, they provided a gradation of anthracene exposure related to the experimental design.
2.1.1 Acute toxicity testMussels were exposed to 0.25, 1.25, 2, and 2.5 μg/L (actual concentrations) anthracene under static conditions over a period of 96 h. Each treatment consisted of three replicates of 10 mussels in 10-L glass aquaria. The animals were not fed during the experimental period. Other experimental conditions were the same as during the acclimation period. No mortality of mussels was recorded in any group during the 4-d experiment.
2.1.2 Sub-acute toxicity testMussels were exposed to four environmental treatments: seawater control, acetone control (0.002 5% acetone), and two levels of anthracene (0.25 and 2.5 μg/L, actual concentrations), for 21 d. All experiments were conducted in triplicate. Three 10-L glass aquaria were used per treatment group with 24 mussels in each aquarium. The anthracene concentrations represented trace and the maximum (saturation) concentrations, which typify the range of anthracene concentrations in the coastal seawater of Shandong Province (Jin et al., 2014). During this period, the mussels were exposed using a semi-static regime with 100% of the media renewed daily. The mussels were fed twice daily before the exposure media were changed. No mortality occurred in the controls, or in the anthracene-exposed mussels. Mussels were randomly sampled on days 0, 3, 6, 9, 12, 15, 18 and 21. At each sampling time, gills and digestive glands were excised from nine individuals (three from each replicate), separated from adhering soft tissues, frozen in liquid nitrogen, and stored at -80℃ until use.
2.1.3 Biochemical analysesBiomarker activities were measured in gill and digestive-gland tissues of three mussels from each of the three replicate groups. All procedures were carried out at a constant temperature of 4℃. Gills and digestive glands were homogenized in cold physiological saline (NaCl 154 mmol/L, 1:9 w/v) using a glass homogenizer. Homogenates were maintained on ice and then centrifuged at 9 000×g for 30 min; the supernatants were stored at -80℃ until the determination of biomarkers. GST activity was measured using 1-chloro-2, 4-dinitrobezene (CDNB) as a substrate following conjugation with GSH (Habig and Jakoby, 1981) and spectrophotometrically assayed at 412 nm. GPx activity was determined by monitoring the production of oxidized glutathione (GSSG) with GR in a redox cycle, according to Lawrence and Burk (2012), at 412 nm. GR activity was measured according to Carlberg and Mannervik (1975), by monitoring the depletion of NADPH used to reduce GSSG, at 340 nm. GSH concentration was measured as described by Ellman (1959), using 5, 5'-dithiobis-2-nitrobenzoic acid (DTNB) as a substrate; the absorbance change was measured at 420 nm. Malondialdehyde (MDA) was quantified by the formation of thiobarbituric acid-reactive species, and measured at 532 nm (Wills, 1987). O2∙- concentration was measured using xanthine/xanthine oxidase as the source of O2∙-. O2∙- production was calculated by the addition of Gress chromogenic agent and an electron acceptor, measuring absorbance at 550 nm (Li et al., 2011). Total protein concentrations in the supernatants were determined according to Bradford (1976), measuring the absorbance of Coomassie blue at 595 nm with bovine serum albumin (BSA) solution as a standard.
2.2 Field transplant experimentThe study area was located in the northern Yellow Sea. The reference site (S1) and the contaminated site (S2) were selected in different bays along the coast of the city of Rongcheng, Shandong Province (Fig. 1). Yangyuchi Bay (S2) is a small semi-enclosed bay chosen for mussel transplantation because of its characteristics of severe contamination by urban farming and domestic sewage. The oil concentration of seawater in the bay was 41.6–94.7 μg/L in autumn according to our previous measurements. The transplanted mussels originated from a relatively clean aquaculture farm in Sanggou Bay (S1), an open bay 4 km from the coast and distant from known sources of pollution. Previous studies have reported very low oil concentrations in this area (Hao et al., 2011; Qiao et al., 2011).

|
| Figure 1 Map showing the mussel transplantation sites on the coast of Rongcheng, Shandong Province S1 (reference site) located in Sanggou Bay (122°30′21.78″E, 37°6′53.85″N), S2 (contaminated site) located in Yangyuchi Bay (122°33′56.26″E, 37°18′53.40″N). |
Mytilus edulis for the transplantation experiment were collected from a single population at site S1 and sorted to a mean length of 45±5 mm. After collection, the transplanted mussels were transported to site S2 in StyrofoamTM boxes with ice bags, taking 40 min. A cylindrical cage (30 cm diameter ×40 cm high) constructed from polyethylene netting was located at each of the two sites, each containing 200 mussels. The cages were immersed at a depth of 6 m and maintained for one month.
After one month at the end of autumn (November), the mussels in each cage were collected and transported to the laboratory in a Styrofoam boxesTM with ice bags. Water samples were taken for analysis. The surviving mussels were counted and compared with the original number of mussels. Dead mussels were discarded. Gills and digestive glands were excised from surviving mussels, separated the soft tissues, frozen in liquid nitrogen, and stored at -80℃ until use.
Biomarkers (GST, GPx and GR) were measured in gills and digestive glands, as described above. The measurements were performed on three replicates of six individuals from each site. Analyses of seawater samples from the two sites for Cu, Zn, Cd, Hg, As and oil concentrations were conducted according to the standards GB 17378.4-2007 and GB 3097-1997 of China.
2.3 IBR calculationAn IBR was calculated with biomarker data obtained in the laboratory and field transplantation experiments using the method proposed by Devin et al. (2014). However, in the laboratory experiment it was modified to use 'exposure group' instead of 'site' as a factor, to reflect changes in biomarkers as a whole. The calculation procedure was as follows. Data were first standardized: (1) for a biomarker (X), the mean value (m) and standard deviation (s) were calculated for all exposure groups and exposure days, based on the comparisons to be made during the whole experimental period. We then computed the value of Y, where Y=(X-m)/s; (2) Z was calculated as +Y in the case of activation and -Y in the case of inhibition according to prior knowledge of biological effects in the enzymatic system; (3) S was calculated, where S=Z+|Min|, Min is the minimum value identified from all exposure groups across the exposure period for each biomarker; (4) all Si values were plotted on a star plot and IBR was computed as the sum of the areas of the triangles defined by k standardized biomarkers. 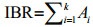
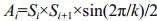
Statistical analyses were conducted using SPSS 20.0 for Windows (IBM Inc., Chicago, IL). Results were expressed as mean±standard deviation. Oneway ANOVA followed by Duncan's multiple range test were performed to identify significant differences among groups in the anthracene exposure experiment. Paired t-tests were used to identify differences in the biomarkers between sites in the transplantation experiment. The significance level was set at P < 0.05. Pearson correlation analysis was used to examine the relationship between the antioxidant biomarkers and oxidative damage biomarkers. All graphs were produced using Surfer 8.0 (Golden Software Inc., Golden, CO), SigmaPlot 12.5 (Systat Inc., Point Richmond, CA) and Excel 2013 (Microsoft Corp., Redmond, WA).
3 RESULT 3.1 Laboratory exposure to anthraceneThe activities of GST, GPx, GR and concentrations of GSH were measured in the gills and digestive glands in response to anthracene exposure (Fig. 2). During the sub-acute toxicity test period (21 days), there were no significant differences in the biomarkers, measured in either tissue, between the mussels in the seawater control and the acetone control (P>0.05), suggesting acetone had no effect on the biomarkers. On day 3, the GST activities in both tissues decreased relative to the controls (P < 0.05) in response to the higher anthracene concentration (2.5 μg/L). On day 6, the GST activity in gills increased (P < 0.05) in response to the lower level of anthracene (0.25 μg/L). After 6 d of exposure, a conspicuous increase of GST activity (P < 0.05) was observed in the digestive glands of mussels in response to the lower level of anthracene.
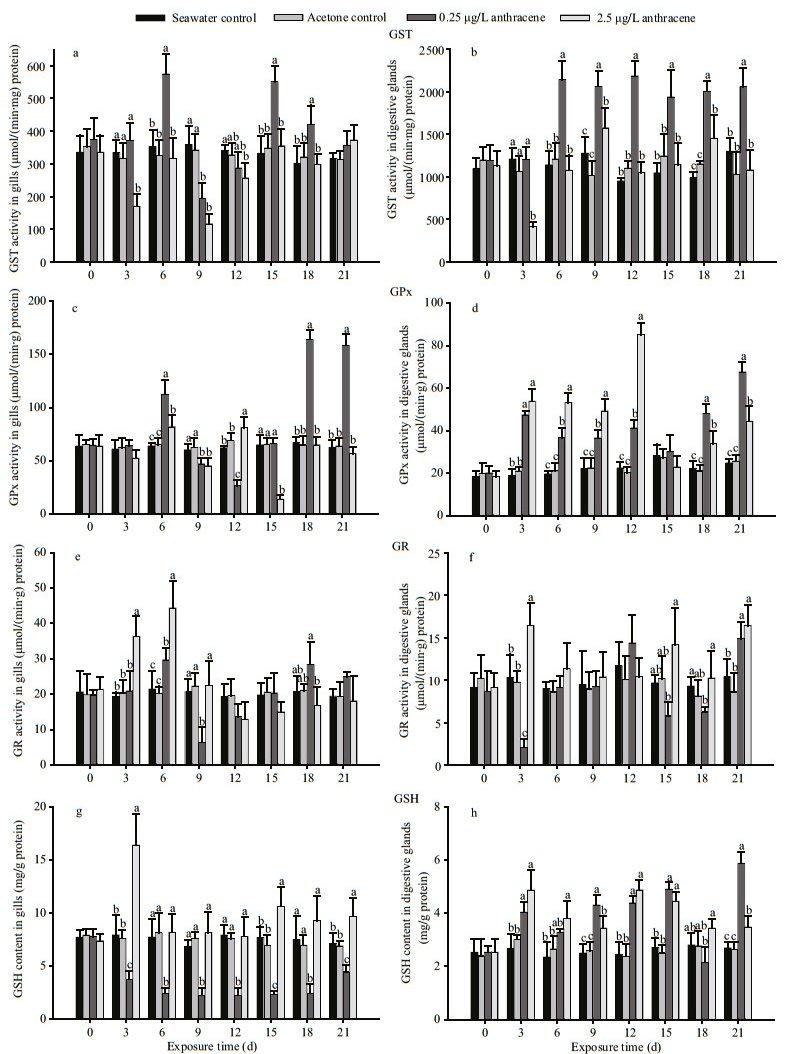
|
| Figure 2 GST, GPx and GR activities and GSH concentrations in gills (a, c, e, g) and digestive glands (b, d, f, h) of mussels exposed to control conditions and two anthracene concentrations for 21 d. Bars with different superscripts within each exposure day are significantly different (P < 0.05) |
As illustrated in Fig. 2c, the GPx activity in gills increased (P < 0.05) on day 6 in response to anthracene exposure, while a significant decrease was observed on day 9 in both treatments (P < 0.05). On days 18 and 21, the GPx activity in gills was conspicuously increased in response to 0.25 μg/L anthracene (P < 0.05). A significant increase in GPx activity relative to the controls was detected in the digestive glands of mussels subjected to both concentrations of anthracene for 3 d, but no significant difference was observed on day 15 (Fig. 2d). There was a positive relationship between the GPx activity of the digestive glands and anthracene concentration during the first half of the experimental period.
On day 3, the GR activities in gills and digestive glands increased relative to the controls (P < 0.05) in response to the higher anthracene concentration. On day 6, the GR activity in the gills was also higher than in control mussels (P < 0.05). After exposure for 9 d, the GR activity in gills was notably lower in the 0.25 μg/L anthracene group than in the control groups (P < 0.05). Interestingly, the GR activity in the digestive glands was higher (P < 0.05) in both treatments on day 21. At other times, the GR activity was not significantly different from the controls (P < 0.05) in either exposure group.
The GSH concentration in gills of mussels exposed to the lower concentration of anthracene were conspicuously lower than in the controls (P < 0.05) during the entire experimental period. Interestingly, on day 3, the GSH concentration in gills increased enormously in the high anthracene treatment group relative to the controls (P < 0.05). GSH was significantly higher in the digestive glands of all mussels exposed to anthracene relative to the controls, throughout the experimental period, except for statistically similar concentrations on day 18. In general, anthracene exposure induced increased activities of GST, GPx and GSH in the digestive glands and of GPx and GR in the gills.
Figure 3 shows the changes in MDA and O2∙- concentrations in M. edulis exposed to anthracene. On days 3 and 6, the MDA concentrations in gills had increased relative to the controls (P < 0.05) in response to 2.5 μg/L anthracene. However, the concentrations of MDA returned to control levels after day 9. Toward the end of the experiment (days 18 and 21), the MDA concentrations in the gills increased again (P < 0.05), in response to the lower anthracene concentration. The MDA concentrations in the digestive glands increased (P < 0.05) at the beginning of the experiment (days 3, 6 and 9) but did not increase again at the end of the exposure period.
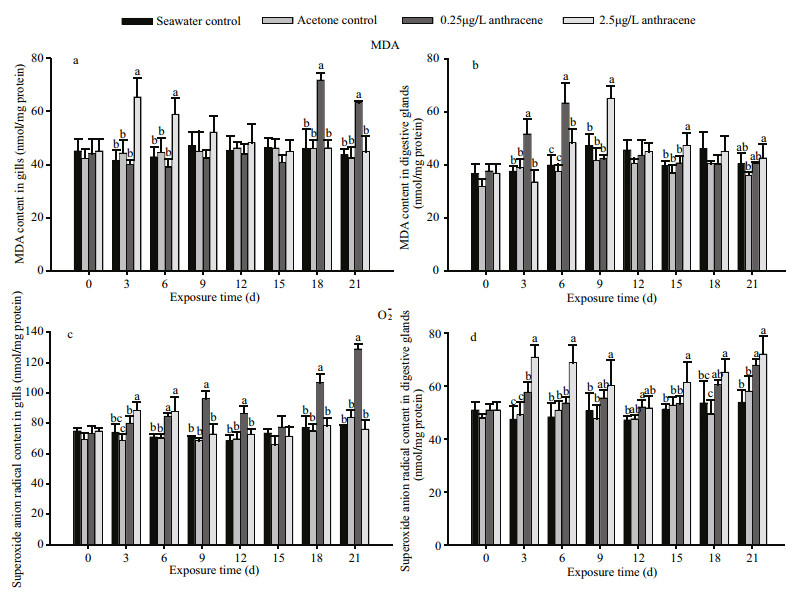
|
| Figure 3 MDA and O2∙- concentrations in gills (a, c) and digestive glands (b, d) of mussels exposed to control conditions and two anthracene concentrations for 21 d Bars with different superscripts within each exposure day are significantly different (P < 0.05) |
The O2∙- concentration was markedly higher in the gills than in the digestive glands in the controls and the anthracene-treated mussels (Fig. 3c, d). Compared with the control groups, anthracene exposure significantly induced generation of O2∙- (P < 0.05) at most of the measurement times. In addition, the concentrations of O2∙- in the digestive glands were higher at the higher anthracene concentration on each day of the exposure period.
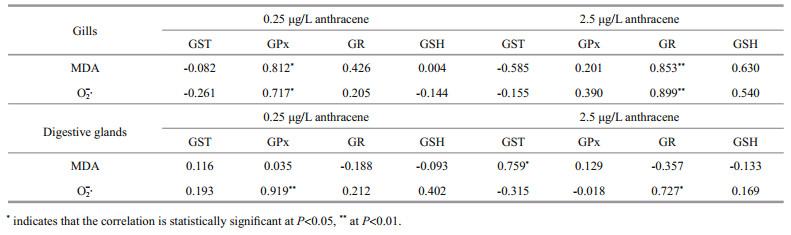
Correlations between the antioxidant biomarkers (GST, GPx, GR and GSH) and oxidative damage biomarkers (MDA and O2∙-) in gills and digestive glands of the mussels are summarized in Table 1. For mussels exposed to 0.25 μg/L anthracene, GPx activities were significantly positively correlated (P=0.014 and P=0.001, respectively) with O2∙- concentrations, in both tissues. In addition, GPx activities in gills were positively correlated (P=0.014) with MDA concentrations. At the higher anthracene concentration (2.5 μg/L), GR activities in both tissues were positively correlated (P=0.002 and P=0.041, respectively) with O2∙- concentrations; GR activities in gills and GST activities in digestive glands were positively correlated with MDA concentrations (P=0.007 and P=0.029, respectively).
|
At the end of the transplantation period, four dead mussels were recorded at site S2 out of 200 transplanted, and none at S1, indicating negligible mortality in the experiment. Seawater analyses for heavy metals (Cu, Zn, Pb, Cd, Hg and As) and oil concentrations at sites S1 and S2 are summarized in Table 2. In relation to the seawater quality standard of China (GB 3097-1997), seawater at S1 was considered of good quality whereas, at S2, arsenic and oil concentrations exceeded the upper limit of Grade-Ⅱ, suggesting that S2 is probably contaminated with PAHs (Bocquené et al., 2004).

|
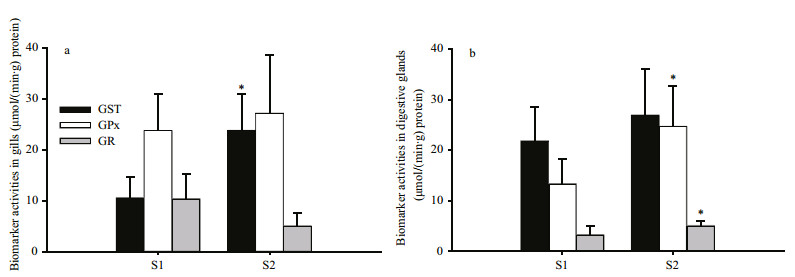
Biomarkers, measured in the gills and digestive glands of the transplanted mussels, are shown in Fig. 4. GST and GPx activities in both tissues, and GR activities in digestive glands, were considerably higher in the mussels transplanted to S2 than those at S1. GST activities in gills, and GPx and GR activities in digestive glands at site S2 differed significantly (P=0.034, P=0.027 and P=0.046, respectively) from the corresponding values at S1.
|
| Figure 4 GST, GPx and GR activities in the gills (a) and digestive glands (b) of mussels transplanted to the control site (S1) and to the contaminated site (S2) * indicates a significant difference from the control at P < 0.05 |
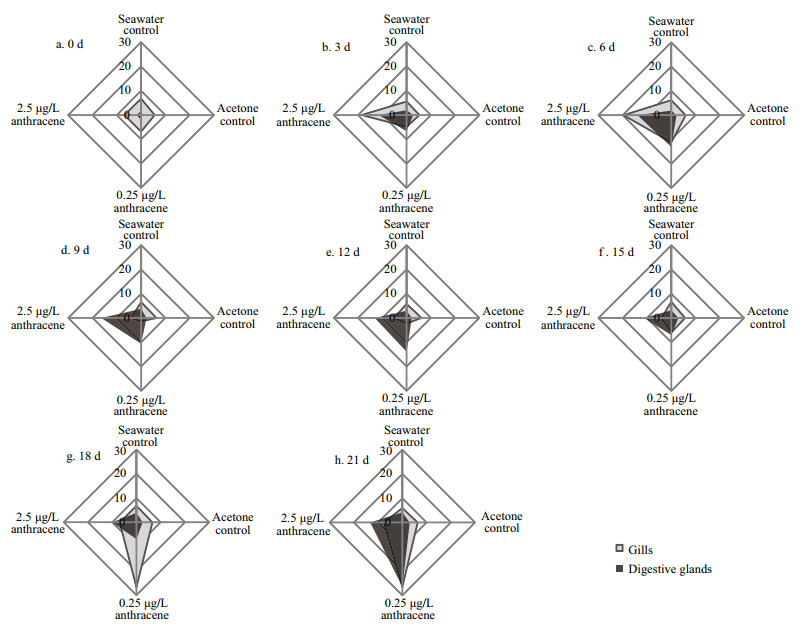
Figure 5 shows the IBR values calculated from standardized data of all six biomarkers in the control and anthracene-treated mussels at different exposure days. The IBR values were highly variable, ranging from 1.38 to 26.75 in gills and 0.60 to 26.56 in digestive glands. The lowest IBR value was observed in the digestive glands of mussels in the acetone control group sampled at 0 d (0.60) and the highest value was found in the gills of mussels exposed to 0.25 μg/L anthracene at 18 d (26.75). The lowest IBR indices were observed in the seawater and acetone control groups and the IBR in the anthracene treatments were generally higher than in the controls. A significant increase of the IBR in gills was observed in response to anthracene exposure on days 3 and 6. As the exposure time increased, the IBR decreased in all treatments relative to controls. However, at the end of the experimental period, a markedly higher IBR was detected in the gills of mussels exposed to the lower anthracene concentration. The IBR in digestive glands of anthracene-exposed mussels exhibited a marked increase relative to the controls, and reached a peak value in the lower anthracene concentration on day 21.
|
| Figure 5 Star plots of the IBR of gills and digestive glands of mussels in the control and anthracene-exposed groups after different exposure times (days) |
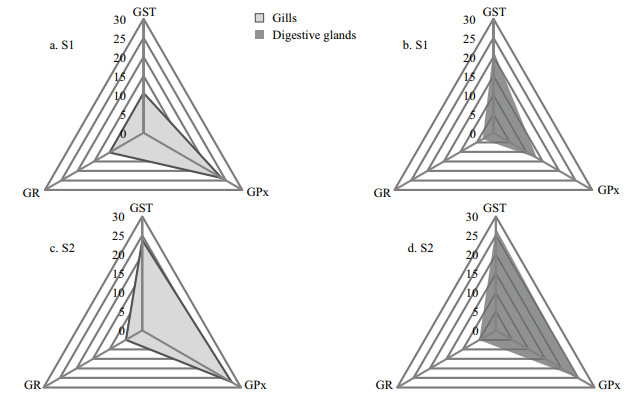
The three biomarkers selected from the transplantation experiment are shown graphically in Fig. 6. The areas corresponding to the IBR values for sites S1 and S2 were 522.5 and 777.5, respectively in gills, and 350.5 and 800.9, respectively in digestive glands. For mussels at S2, star plots of both tissues showed similar patterns for the three biomarkers. GST and GPx contributed almost equally to the IBR.
|
| Figure 6 Star plots of the IBR in gills and digestive glands of mussels at the control site (S1) and in those transplanted to the contaminated site (S2) The shaded areas represent the IBR values |
According to Livingstone (2003) and Baussant et al. (2009), the toxicity mechanisms of organic pollutants in bivalve mollusks are complex. ROS production causes oxidative damage to DNA, proteins and membrane lipids inducing changes in antioxidant enzymes. Our results verified that anthracene treatment was associated with the formation of ROS in M. edulis leading to significant alterations of antioxidant enzymes and lipid peroxidation, which is in agreement with other studies of PAH exposure conducted with bivalve mollusks (Cheung et al., 2001; Frouin et al., 2007; Richardson et al., 2008; Qiu et al., 2013).
Superoxide (O2∙-), is a toxic by-product of oxidative metabolism that interacts with hydrogen peroxide (H2O2) to form the hydroxyl radical (∙OH) (Matés, 2000; Dröge, 2002). Figure 3c, d shows that O2∙- accumulation in blue mussels was significantly enhanced during exposure to anthracene. Previous studies have suggested that PAHs stimulate ROS formation in gills and digestive glands of bivalve mollusks (Frouin et al., 2007; Baussant et al., 2009; Bebianno and Barreira, 2009).
Cells in bivalve mollusks have a complex defense system for scavenging ROS, which comprises antioxidant enzymes and non-enzymatic scavengers (Richardson et al., 2008). Oxidative stress may induce the activities of antioxidants but severe oxidative stress may suppress these activities, leading to oxidative damage (Yin et al., 2010). After 3 d of exposure, anthracene induced increased activities of GST, GPx and GSH in digestive glands and GPx and GR in gills, consistent with strengthening of antioxidant defense.
In the glutathione antioxidant system, GSH, GPx, GST and GR form a closed system (Fig. 7). Our data agree with former studies that consider GSH to be the first line of antioxidant defense (Meister, 1991; Oliveira et al., 2008). In this study, the MDA and O2∙- concentrations in gills were not markedly altered by 2.5 μg/L anthracene, which might reflect the extra protection provided by the increased GSH levels. GST and GPx, as GSH-dependent enzymes, are suggested as a second line of antioxidant defense. GSH is required as a substrate for GST and is consumed by GPx during peroxide neutralization, these reactions lead to the loss of GSH (Richardson et al., 2008). In this study, GSH activity decreased whereas GST and GPx activities increased in gills of mussels exposed to 0.25 μg/L anthracene, which implies metabolic adaptation to the anthracene stress. The oxidized form of glutathione (GSSG) is reduced back to GSH by GR at the expense of NADPH. As a result of GSH consumption, GR activity is expected to increase (Oliveira et al., 2008; Sureda et al., 2011). In this work, a rise in GSH activities in both tissues was concomitant with a rise in GR on day 3. As the GSH and GPx activities both increased in digestive glands, the combination of these two lines of antioxidant defense may be responsible for preventing the increase in MDA. Correlation analysis showed that GST, GPx and GR played essential roles in scavenging free radicals and alleviating oxidative stress in gills and digestive glands of blue mussel, and were considered effective biomarkers in the field experiment.

|
| Figure 7 Redox cycle of the glutathione antioxidant system, consisting of GSH, GPx, GST and GR |
MDA concentration is an important biomarker of lipid peroxidation in aquatic organisms. Bocquené et al. (2004) reported that the MDA concentration in M. edulis was positively correlated with PAH concentrations in transplanted mussels, reflecting oil pollution. In this study, increased MDA concentrations revealed that gills and digestive glands of mussels were in oxidative stress from anthracene exposure.
Since the gills and digestive glands are the main target tissues for anthracene accumulation in bivalve mollusks (Fasulo et al., 2012), they were selected for study of the biochemical response to anthracene exposure. Gills represent the first line of defense against pollutants and are the principal regions of accumulation because they are directly exposed to the medium with a large surface area and high permeability. They are considered a good choice for early assessment of the biological effects of watersoluble chemicals (Oliveira et al., 2008). The digestive gland is an important organ of metabolism of organic pollutants and an important part of biotransformation activities in mollusks (Livingstone, 1998). In this study, the digestive gland showed a metabolic response to anthracene and consequent antioxidant activation. GPx and GST activities and the GSH concentration increased during the exposure period. The antioxidant biomarkers in gills were generally activated by day 6, whereas the biomarkers in the digestive glands showed a response at the first sample time. Therefore, digestive glands seem to be more sensitive than the gills to sub-lethal doses of anthracene. However, this contrasts with other studies, which report the gills as the more sensitive organ in bivalve mollusks (Bainy et al., 1996; Cheung et al., 2001).
In the field transplantation experiment, environmental pollutants induced increased activities of biomarkers in the gills and digestive glands of mussels transplanted to the contaminated site (S2). The digestive gland appeared to be more sensitive than the gills. Transplantation of mussels from a clean site (Sanggou Bay) to a contaminated site (Yangyuchi Bay) proved to be an effective strategy for measurement of responses to water pollution. Water quality assessment showed that the site located in Yangyuchi Bay was polluted with oil. Yangyuchi Bay is an enclosed bay with poor self-purification capacity. Petrochemical products enter the bay as a result of harbor activities, shipping transport, domestic sewage discharge, and other anthropogenic activities. Protection of the natural environment of Yangyuchi Bay requires strengthening of the management of oil emissions.
The present study validates the use of biomarkers for contamination assessment under laboratory and in field conditions. Biomarker analyses were useful to identify mussels exposed to contaminated environment in both experiments. The combination of laboratory and field studies allowed assessment and selection of appropriate biomarkers. GST, GPx and GR were considered to be sensitive biomarkers of stress in mussels and are recommended for detecting pollutants in marine pollution monitoring.
The star plots of IBR values used in this study facilitated visualization of between-survey differences. Differences in the biomarkers were observed among mussels exposed to different anthracene levels, and were reflected in IBR values. At most of the exposure times, the IBR values were dose-dependent; as the exposure concentrations increased, the IBR values tended to increase. The increased IBR values of anthracene-exposed mussels compared with controls, particularly in the digestive glands, were consistent with previous reports (Serafim et al., 2012; Bellas et al., 2014). Therefore, it is suggested that the IBR could serve as a useful tool for quantitative monitoring of toxin-induced stress levels in mussels. In the transplantation experiment, IBR values successfully distinguished between sites S1 and S2. The index indicated that the transplantation site was impacted by contaminants. Thus, mussels from S2 were inferred to be under great stress from the commercial harbor, which was consistent with the higher arsenic and oil levels in seawater samples. Nevertheless, it is possible that the present multibiomarker approach will not fully respond to all contaminants. In future, other biomarkers could be combined with those already taken into account in computing IBR values.
5 CONCLUSIONAnthracene induced the production of O2∙- in M. edulis, resulting in elevated oxidative stress and damage to the tissues of mussels. Associated changes in anti-oxidative responses and lipid peroxidation were observed. Among these biomarkers, the activities of GST, GPx and GR were most sensitive to anthracene and were considered suitable biomarkers for pollution monitoring in field transplantation. The digestive glands were more sensitive to anthracene stress than the gills were. IBR values based on selected biomarkers help to explain seemingly conflicting biological effects and facilitate the implementation of these biomarkers for monitoring of PAHs in coastal and estuarine environments.
6 ACKNOWLEDGEMENTWe thank ZHANG Yongsheng from Rongcheng Municipal Bureau of Ocean and Fisheries for assistance with the field transplantation experiment.
| Allred P M, Giesy J P, 1985. Solar radiation-induced toxicity of anthracene to Daphnia pulex. Environmental Toxicology and Chemistry, 4(2): 219–226. Doi: 10.1002/etc.v4:2 |
| Arzate-Cárdenas M A, Martínez-Jerónimo F, 2011. Agealtered susceptibility in hexavalent chromium-exposed Daphnia schodleri (Anomopoda:Daphniidae):integrated biomarker response implementation. Aquatic Toxicology, 105(3-4): 528–534. Doi: 10.1016/j.aquatox.2011.08.006 |
| Bainy A C D, Saito E, Carvalho P S M, Junqueira V B C, 1996. Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquatic Toxicology, 34(2): 151–162. Doi: 10.1016/0166-445X(95)00036-4 |
| Baussant T, Bechmann R K, Taban I C, Larsen B K, Tandberg A H, Bjørnstad A, Torgrimsen S, Nævdal A, Øysæd K B, Jonsson G, Sanni S, 2009. Enzymatic and cellular responses in relation to body burden of PAHs in bivalve molluscs:a case study with chronic levels of North Sea and Barents Sea dispersed oil. Marine Pollution Bulletin, 58(12): 1796–1807. Doi: 10.1016/j.marpolbul.2009.08.007 |
| Bebianno M J, Barreira L A, 2009. Polycyclic aromatic hydrocarbons concentrations and biomarker responses in the clam Ruditapes decussatus transplanted in the Ria Formosa lagoon. Ecotoxicology and Environmental Safety, 72(7): 1849–1860. Doi: 10.1016/j.ecoenv.2009.03.016 |
| Beliaeff B, Burgeot T, 2002. Integrated biomarker response:a useful tool for ecological risk assessment. Environmental Toxicology and Chemistry, 21(6): 1316–1322. Doi: 10.1002/etc.v21:6 |
| Bellas J, Albentosa M, Vidal-Liñán L, Besada V, Franco M Á, Fumega J, González-Quijano A, Viñas L, Beiras R, 2014. Combined use of chemical, biochemical and physiological variables in mussels for the assessment of marine pollution along the N-NW Spanish coast. Marine Environmental Research, 96: 105–117. Doi: 10.1016/j.marenvres.2013.09.015 |
| Birdsall K, Kukor J J, Cheney M A, 2001. Uptake of polycyclic aromatic hydrocarbon compounds by the gills of the bivalve mollusk Elliptio complanata. Environmental Toxicology and Chemistry, 20(2): 309–316. Doi: 10.1002/etc.v20:2 |
| Bocquené G, Chantereau S, Clérendeau C, Beausir E, Ménard D, Raffin B, Minier C, Burgeot T, Leszkowicz A P, Narbonne J F, 2004. Biological effects of the "Erika" oil spill on the common mussel (Mytilus edulis). Aquatic Living Resources, 17(3): 309–316. Doi: 10.1051/alr:2004033 |
| Bradford M M, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248–254. Doi: 10.1016/0003-2697(76)90527-3 |
| Brenner M, Broeg K, Frickenhaus S, Buck B H, Koehler A, 2013. Multi-biomarker approach using the blue mussel(Mytilus edulis L.) to assess the quality of marine environments:season and habitat-related impacts. Marine Environmental Research, 95: 13–27. |
| Broeg K, Lehtonen K K, 2006. Indices for the assessment of environmental pollution of the Baltic Sea coasts:integrated assessment of a multi-biomarker approach. Marine Pollution Bulletin, 53(8-9): 508–522. Doi: 10.1016/j.marpolbul.2006.02.004 |
| Brooks S, Harman C, Soto M, Cancio I, Glette T, Marigómez I, 2012. Integrated coastal monitoring of a gas processing plant using native and caged mussels. Science of the Total Environment, 426: 375–386. Doi: 10.1016/j.scitotenv.2012.03.059 |
| Cappello T, Maisano M, D'Agata A, Natalotto A, Mauceri A, Fasulo S, 2013. Effects of environmental pollution in caged mussels (Mytilus galloprovincialis). Marine Environmental Research, 91: 52–60. Doi: 10.1016/j.marenvres.2012.12.010 |
| Carlberg I, Mannervik B, 1975. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. The Journal of Biological Chemistry, 250(14): 5475–5480. |
| Chen Y J, Zhi G R, Feng Y L, Tian C G, Bi X H, Li J, Zhang G, 2015. Increase in polycyclic aromatic hydrocarbon(PAH) emissions due to briquetting:a challenge to the coal briquetting policy. Environmental Pollution, 204: 58–63. Doi: 10.1016/j.envpol.2015.04.012 |
| Cheung C C C, Zheng G J, Li A M Y, Richardson B J, Lam P K S, 2001. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquatic Toxicology, 52(3-4): 189–203. Doi: 10.1016/S0166-445X(00)00145-4 |
| Damiens G, Gnassia-Barelli M, Loquès F, Roméo M, Salbert V, 2007. Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere, 66(3): 574–583. Doi: 10.1016/j.chemosphere.2006.05.032 |
| DeLeve L D, Kaplowitz N, 1991. Glutathione metabolism and its role in hepatotoxicity. Pharmacology & Therapeutics, 52(3): 287–305. |
| Devin S, Burgeot T, Giambérini L, Minguez L, Pain-Devin S, 2014. The integrated biomarker response revisited:optimization to avoid misuse. Environmental Science and Pollution Research, 21(4): 2448–2454. Doi: 10.1007/s11356-013-2169-9 |
| Dröge W, 2002. Free radicals in the physiological control of cell function. Physiological Reviews, 82(1): 47–95. Doi: 10.1152/physrev.00018.2001 |
| Ellman G L, 1959. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1): 70–77. Doi: 10.1016/0003-9861(59)90090-6 |
| Fasulo S, Iacono F, Cappello T, Corsaro C, Maisano M, D'Agata A, Giannetto A, De Domenico E, Parrino V, Paro G L, Mauceri A, 2012. Metabolomic investigation of Mytilus galloprovincialis (Lamarck 1819) caged in aquatic environments. Ecotoxicology and Environmental Safety, 84: 139–146. Doi: 10.1016/j.ecoenv.2012.07.001 |
| Frouin H, Pellerin J, Fournier M, Pelletier E, Richard P, Pichaud N, Rouleau C, Garnerot F, 2007. Physiological effects of polycyclic aromatic hydrocarbons on softshell clam Mya arenaria. Aquatic Toxicology, 82(2): 120–134. Doi: 10.1016/j.aquatox.2007.02.005 |
| Fung C N, Lam J C W, Zheng G J, Connell D W, Monirith I, Tanabe S, Richardson B J, Lam P K S, 2004. Musselbased monitoring of trace metal and organic contaminants along the east coast of China using Perna viridis and Mytilus edulis. Environmental Pollution, 127(2): 203–216. Doi: 10.1016/j.envpol.2003.08.007 |
| Gomes V, Passos M J A C R, Leme N M P, Santos T C A, Campos D Y F, Hasue F M, van Ngan Phan, 2009. Photoinduced toxicity of anthracene in the Antarctic shallow water amphipod, Gondogeneia antarctica. Polar Biology, 32(7): 1009–1021. Doi: 10.1007/s00300-009-0600-y |
| Gravato C, Almeida J R, Silva C, Oliveira C, Soares A M V M, 2014. Using a multibiomarker approach and behavioural responses to assess the effects of anthracene in Palaemon serratus. Aquatic Toxicology, 149: 94–102. Doi: 10.1016/j.aquatox.2014.01.024 |
| Habig W H, Jakoby W B, 1981. Assays for differentiation of glutathione S-transferases. Methods in Enzymology, 77: 398–405. Doi: 10.1016/S0076-6879(81)77053-8 |
| Hao L H, Sun P X, Jiang M J, Zhang X J, Lian Y, Liu X J, Dai X X, 2011. Characteristics of Petroleum Hydrocarbon distribution and its relationship with environmental factors in Sanggou Bay. Advances in Marine Science, 29(3): 386–394. |
| Hoarau P, Garello G, Gnassia-Barelli M, Roméo M, Girard J P, 2004. Effect of three xenobiotic compounds on Glutathione S-transferase in the clam Ruditapes decussatus. Aquatic Toxicology, 68(1): 87–94. Doi: 10.1016/j.aquatox.2004.03.001 |
| Hung C C, Ko F C, Gong G C, Chen K S, Wu J M, Chiang H L, Peng S C, Santschi P H, 2014. Increased zooplankton PAH concentrations across hydrographic fronts in the East China Sea. Marine Pollution Bulletin, 83(1): 248–257. Doi: 10.1016/j.marpolbul.2014.03.045 |
| Hylland K, Tollefsen K E, Ruus A, Jonsson G, Sundt R C, Sanni S, Utvik T I R, Johnsen S, Nilssen I, Pinturier L, Balk L, Baršienė J, Marigòmez I, Feist S W, Børseth J F, 2008. Water column monitoring near oil installations in the North Sea 2001-2004. Marine Pollution Bulletin, 56(3): 414–429. Doi: 10.1016/j.marpolbul.2007.11.004 |
| Jin Q, Pan L Q, Liu D, Hu F X, Xiu M, 2014. Assessing PAHs pollution in Qingdao coastal area (China) by the combination of chemical and biochemical responses in scallops, Chlamys farreri. Marine Pollution Bulletin, 89(1-2): 473–480. Doi: 10.1016/j.marpolbul.2014.09.026 |
| Kim W K, Lee S K, Choi K, Jung J, 2013. Integrative assessment of biomarker responses in pale chub (Zacco platypus) exposed to copper and benzo. Ecotoxicology and Environmental Safety, 92: 71–78. Doi: 10.1016/j.ecoenv.2013.02.010 |
| Kim W K, Lee S K, Jung J, 2010. Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. Journal of Hazardous Materials, 180(1-3): 395–400. Doi: 10.1016/j.jhazmat.2010.04.044 |
| Knight J A, Pieper R K, McClellan L, 1988. Specificity of the thiobarbituric acid reaction:its use in studies of lipid peroxidation. Clinical Chemistry, 34(12): 2433–2438. |
| Kookana R S, Shareef A, Fernandes M B, Hoare S, Gaylard S, Kumar A, 2013. Bioconcentration of triclosan and methyltriclosan in marine mussels (Mytilus galloprovincialis) under laboratory conditions and in metropolitan waters of Gulf St Vincent, South Australia. Marine Pollution Bulletin, 74(1): 66–72. Doi: 10.1016/j.marpolbul.2013.07.030 |
| Lawrence R A, 2012. Reprint of "glutathione peroxidase activity in selenium-deficient rat liver". Biochemical & Biophysical Research Communications, 425(3): 503–509. |
| Lee W, Lee C C, 2015. Developmental toxicity of cigarette butts-an underdeveloped issue. Ecotoxicology and Environmental Safety, 113: 362–368. Doi: 10.1016/j.ecoenv.2014.12.018 |
| Li Z H, Velisek J, Zlabek V, Grabic R, Machova J, Kolarova J, Li P, Randak T, 2011. Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss):effects on morphological indices, hematological parameters and antioxidant responses. Journal of Hazardous Materials, 185(2-3): 870–880. Doi: 10.1016/j.jhazmat.2010.09.102 |
| Livingstone D R, 1998. The fate of organic xenobiotics in aquatic ecosystems:quantitative and qualitative differences in biotransformation by invertebrates and fish. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 120(1): 43–49. |
| Livingstone D R, 2003. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue de Médecine Vétérinaire, 154(6): 427–430. |
| Lourenço R A, De Oliveira F F, Nudi A H, De Luca Rebello Wagener Â, De Fátima Guadalupe Meniconi M, Francioni E, 2015. PAH assessment in the main Brazilian offshore oil and gas production area using semi-permeable membrane devices (SPMD) and transplanted bivalves. Continental Shelf Research, 101: 109–116. Doi: 10.1016/j.csr.2015.04.010 |
| Manduzio H, Cosette P, Gricourt L, Jouenne T, Lenz C, Andersen O K, Leboulenger F, Rocher B, 2005. Proteome modifications of blue mussel (Mytilus edulis L.) gills as an effect of water pollution. Proteomics, 5(18): 4958–4963. Doi: 10.1002/(ISSN)1615-9861 |
| Matés J M, 2000. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology, 153(1-3): 83–104. Doi: 10.1016/S0300-483X(00)00306-1 |
| Meister A, 1991. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacology & Therapeutics, 51(2): 155–194. |
| Oliveira M, Pacheco M, Santos M A, 2008. Organ specific antioxidant responses in golden grey mullet (Liza aurata) following a short-term exposure to phenanthrene. Science of the Total Environment, 396(1): 70–78. Doi: 10.1016/j.scitotenv.2008.02.012 |
| Oris J T, Giesy J P Jr, 1985. The photoenhanced toxicity of anthracene to juvenile sunfish (Lepomis spp.). Aquatic Toxicology, 6(2): 133–146. Doi: 10.1016/0166-445X(85)90012-8 |
| Palanikumar L, Kumaraguru A K, Ramakritinan C M, Anand M, 2012. Biochemical response of anthracene and benzo. Ecotoxicology and Environmental Safety, 75: 187–197. Doi: 10.1016/j.ecoenv.2011.08.028 |
| Piccardo M T, Coradeghini R, Valerio F, 2001. Polycyclic aromatic hydrocarbon pollution in native and caged mussels. Marine Pollution Bulletin, 42(10): 951–956. Doi: 10.1016/S0025-326X(01)00057-1 |
| Qiao X Y, Chen B J, Zhou M Y, Cui Z G, 2011. Petroleum hydrocarbon pollution status in shellfish culture area of Sanggou bay and effect on quality safety of shellfish. Environmental Science, 32(8): 2391–2396. |
| Qiu J B, Ma F F, Fan H, Li A F, 2013. Effects of feeding Alexandrium tamarense, a paralytic shellfish toxin producer, on antioxidant enzymes in scallops(Patinopecten yessoensis) and mussels (Mytilus galloprovincialis). Aquaculture, 396-399: 76–81. Doi: 10.1016/j.aquaculture.2013.02.040 |
| Ramu K, Kajiwara N, Sudaryanto A, Isobe T, Takahashi S, Subramanian A, Ueno D, Zheng G J, Lam P K S, Takada H, Zakaria M P, Viet P H, Prudente M, Tana T S, Tanabe S, 2007. Asian mussel watch program:contamination status of polybrominated diphenyl ethers and organochlorines in coastal waters of Asian countries. Environmental Science & Technology, 41(13): 4580–4586. |
| Richardson B J, Mak E, De Luca-Abbott S B, Martin M, McClellan K, Lam P K S, 2008. Antioxidant responses to polycyclic aromatic hydrocarbons and organochlorine pesticides in green-lipped mussels (Perna viridis):do mussels "integrate" biomarker responses?. Marine Pollution Bulletin, 6-12: 503–514. |
| Roméo M, Mourgaud Y, Geffard A, Gnassia-Barelli M, Amiard J C, Budzinski H, 2003. Multimarker approach in transplanted mussels for evaluating water quality in Charentes, France, coast areas exposed to different anthropogenic conditions. Environmental Toxicology, 18(5): 295–305. Doi: 10.1002/tox.v18:5 |
| Sellami B, Khazri A, Mezni A, Louati H, Dellali M, Aissa P, Mahmoudi E, Beyrem H, Sheehan D, 2015. Effect of permethrin, anthracene and mixture exposure on shell components, enzymatic activities and proteins status in the Mediterranean clam Venerupis decussata. Aquatic Toxicology, 158: 22–32. Doi: 10.1016/j.aquatox.2014.10.020 |
| Serafim A, Company R, Lopes B, Fonseca V F, França S, Vasconcelos R P, Bebianno M J, Cabral H N, 2012. Application of an integrated biomarker response index(IBR) to assess temporal variation of environmental quality in two Portuguese aquatic systems. Ecological Indicators, 19: 215–225. Doi: 10.1016/j.ecolind.2011.08.009 |
| Smolders R, Voets J, Bervoets L, Blust R, Wepener V. 2005. Active Biomonitoring (ABM) by Translocation of Bivalve Molluscs. John Wiley & Sons, Inc. , New York. |
| Sundt R C, Pampanin D M, Grung M, Baršienė J, Ruus A, 2011. PAH body burden and biomarker responses in mussels (Mytilus edulis) exposed to produced water from a North Sea oil field:laboratory and field assessments. Marine Pollution Bulletin, 62(7): 1498–1505. Doi: 10.1016/j.marpolbul.2011.04.009 |
| Sureda A, Box A, Tejada S, Blanco A, Caixach J, Deudero S, 2011. Biochemical responses of Mytilus galloprovincialis as biomarkers of acute environmental pollution caused by the Don Pedro oil spill (Eivissa Island, Spain). Aquatic Toxicology, 101(3-4): 540–549. Doi: 10.1016/j.aquatox.2010.12.011 |
| Tsangaris C, Hatzianestis I, Catsiki V A, Kormas K A, Strogyloudi E, Neofitou C, Andral B, Galgani F, 2011. Active biomonitoring in Greek coastal waters:application of the integrated biomarker response index in relation to contaminant levels in caged mussels. Science of the Total Environment, 412-413: 359–365. Doi: 10.1016/j.scitotenv.2011.10.028 |
| Tsangaris C, Kormas K, Strogyloudi E, Hatzianestis I, Neofitou C, Andral B, Galgani F, 2010. Multiple biomarkers of pollution effects in caged mussels on the Greek coastline. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 151(3): 369–378. |
| Turja R, Höher N, Snoeijs P, Baršienė J, Butrimavičienė L, Kuznetsova T, Kholodkevich S V, Devier M H, Budzinski H, Lehtonen K K, 2014. A multibiomarker approach to the assessment of pollution impacts in two Baltic Sea coastal areas in Sweden using caged mussels (Mytilus trossulus). Science of the Total Environment, 473-474: 398–409. Doi: 10.1016/j.scitotenv.2013.12.038 |
| Valavanidis A, Vlachogianni T, Triantafillaki S, Dassenakis M, Androutsos F, Scoullos M, 2008. Polycyclic aromatic hydrocarbons in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Saronikos Gulf (Greece). Estuarine, Coastal and Shelf Science, 79(4): 733–739. Doi: 10.1016/j.ecss.2008.06.018 |
| Vidal-Liñán L, Bellas J, Etxebarria N, Nieto O, Beiras R, 2014. Glutathione S-transferase, glutathione peroxidase and acetylcholinesterase activities in mussels transplanted to harbour areas. Science of the Total Environment, 470-471: 107–116. Doi: 10.1016/j.scitotenv.2013.09.073 |
| Wang J T, Tan L J, Zhang W H, Lian Z R, 2010. Concentrations and distribution characteristic of PAHs, PCBs and OCPs in the surface sediments of Qingtao coastal area. Environmental Science, 31(11): 2713–2722. |
| Wills E. 1987. Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullock B eds. Biochemical Toxicology, A Practical Approach. IRL Press, Oxford, Washington DC. 285p. |
| Xie Z X, Lu G H, Qi P D, 2014. Effects of BDE-209 and its mixtures with BDE-47 and BDE-99 on multiple biomarkers in Carassius auratus. Environmental Toxicology and Pharmacology, 38(2): 554–561. Doi: 10.1016/j.etap.2014.08.008 |
| Xiu M, Pan L Q, Jin Q, 2014. Bioaccumulation and oxidative damage in juvenile scallop Chlamys farreri exposed to benzo. Ecotoxicology and Environmental Safety, 107: 103–110. Doi: 10.1016/j.ecoenv.2014.05.016 |
| Xu S S, Liu W X, Tao S, 2006. Emission of polycyclic aromatic hydrocarbons in China. Environmental Science & Technology, 40(3): 702–708. |
| Yin Y, Wang X R, Yang L Y, Sun Y Y, Guo H Y, 2010. Bioaccumulation and ROS generation in Coontail Ceratophyllum demersum L. exposed to phenanthrene. Ecotoxicology, 19(6): 1102–1110. |
| Zheng Q, Feng M B, Dai Y, 2013. Comparative antioxidant responses in liver of Carassius auratus exposed to phthalates:an integrated biomarker approach. Environmental Toxicology and Pharmacology, 36(3): 741–749. Doi: 10.1016/j.etap.2013.07.008 |
| Zorita I, Strogyloudi E, Buxens A, Mazón L I, Papathanassiou E, Soto M, Cajaraville M P, 2005. Application of two SHbased methods for metallothionein determination in mussels and intercalibration of the spectrophotometric method:laboratory and field studies in the Mediterranean Sea. Biomarkers, 10(5): 342–359. Doi: 10.1080/13547500500264645 |
 2017, Vol. 35
2017, Vol. 35


