Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YAN Taiming(严太明), HU Jiaxiang(胡佳祥), CAI Yueping(蔡跃平), XIONG Sen(熊森), YANG Shiyong(杨世勇), WANG Xiongyan(王雄延), HE Zhi(何智)
- Otolith development in larval and juvenile Schizothorax davidi:ontogeny and growth increment characteristics
- Chinese Journal of Oceanology and Limnology, 35(5): 1197-1204
- http://dx.doi.org/10.1007/s00343-017-6138-x
Article History
- Received May. 17, 2016
- accepted in principle Jun. 22, 2016
- accepted for publication Jul. 11, 2016
Since Pannella's (1971) discovery of daily increments in fish otoliths, which occur in numerous larvae and juveniles fish species (Humphrey et al., 2003; Bustos et al., 2015), otolith microstructure has become a powerful tool to study early life stages of fish (Campana, 2005; Joh et al., 2005; Pavlov et al., 2015). Based on the relationship between otolith size and somatic size, daily otolith growth increments have been widely used to estimate daily age and hatch dates, as well as conduct growth trajectory analyses (Tuset, 2003; Burke et al., 2008; Guo et al., 2010; Zeng and Zhang, 2012). Furthermore, daily growth of otoliths is also important for assessing survival and recruitment success in fish (Feet et al., 2002; Begg et al., 2005; Parkinson et al., 2012). However, otolith microstructural characteristics differ among species because of species-specific otolith formation patterns (Campana, 2001; Yamada et al., 2009; Huang et al., 2014). Therefore, it is necessary to select suitable otoliths to determine the age of wild-caught specimens and validate the periodicity of increment formation through rearing experiments prior to an otolith microstructural analysis.
David's schizothoracin, Schizothorax davidi, belongs to the subfamily Schizothoracinae in the family Cyprinidae. The schizothoracine fishes are considered the largest and most diverse ichthyofauna group in the Qinghai-Tibetan Plateau (Chen, 1998). Schizothoracine fish populations have declined dramatically in recent years as a result of overfishing, dam construction, water pollution, and other human interferences. David's schizothoracin is only distributed in mountain rivers of Southwest China (Ding, 1994). Because it is ecologically vulnerable and populations have declined sharply, this species has been listed as a key wild animal species for protection in Sichuan Province, China. At present, successful captive breeding and release of hatcheryreared fish into natural waters is an important means to protect and restore wild populations.
Suitable otoliths, the otolith increment formation pattern, and otolith deposition periodicity must be determined to best evaluate recovery of the S. davidi resource using an otolith microstructure analysis. In the present study, we describe the morphological features of otoliths in laboratory-reared specimens, establish the most suitable otoliths for aging, and validate otolith microstructural characteristics and growth patterns. These results should benefit resource protection and performance evaluations.
2 MATERIAL AND METHODIn the present study, fish age was defined as the number of days post hatching (dph). All larval and juvenile David's schizothoracin were artificially propagated by the Baojian Breeding Co. Ltd. (Ya'an, Sichuan, China). Fertilized eggs were incubated in a round steel tub (diameter=80.0 cm) at room temperature in the Department of Aquaculture, College of Animal Science and Technology, Sichuan Agricultural University, China. The newly hatched larvae were reared in a biochemical incubator under controlled conditions. Water temperature was maintained at 23.0±1.0℃ during the day and was allowed to drop to ambient temperature at night. The photoperiod matched natural conditions. Water temperature was measured at 8:00 and 20:00 during the experiment, and half of the water was exchanged at that time. The fish were fed small cladocerans before 7 dph, and then with finely chopped Limnodrilus sp.
Larvae and juveniles of different ages were sampled during the rearing period. Body length (BL, notochord length for larvae and standard length for juveniles) of all sampled fish was measured with vernier calipers (±0.02 mm), and the specimens were preserved in 75% ethanol until dissection. Lapilli, sagittae, and asterisci were extracted with a dissecting needle under an anatomical lens (MZ16a; Leica, Heidelberg, Germany). The otoliths were removed, cleaned, dried, and mounted on a microscope slide with resinene.
The digital images were used to measure otolith radius (OR) (from the core along the anterior, posterior, dorsal, and ventral directions) and incremental width (from the centronucleus along the longest axis of lapilli and sagittae). Lapilli and sagittae increments were examined directly under a light microscope in larvae < 42 dph. Older larvae were ground and polished according to a previous report (Song et al., 2008). OR was measured, and the increments were counted using an image analysis system (E800; Nikon, Tokyo, Japan). Incremental widths were measured with the image analysis system described by Zhu et al. (2002). The number of increments in lapilli and sagittae was counted at least three times. If the counts did not vary by >10% from the mean, the mean of the total counts was taken as the final number.
Data are presented as mean±standard deviation. A P-value < 0.05 was considered significant for all statistical analyses. The paired t-test and Student's t-test were carried out using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
3 RESULT 3.1 Otolith developmentLapilli and sagittae appeared before larvae hatched, and the asterisci first developed at approximately 19 dph. Otolith morphology changed as the larvae and juveniles grew, and lapilli and sagittae were almost round at hatching. The lapilli became ovoid at 19 dph, changed to a fan shape at 37 dph, and then changed into the shape of a mussel at 52 dph (Fig. 1). Unlike the lapilli, changes in the shape of sagittae were significant during development; they were spindle shaped at 25 dph, irregularly shaped with a rounded anterior and pointed posterior at 37 dph, and finally changed to a long ellipse with a long pointed posterior at 87 dph (Fig. 1). Furthermore, the asterisci became quadrilateral at 25 dph, were irregular with some cristae at 37 dph, but remained stellate throughout development, except for some minor differences in the number of cristae (Fig. 1).

|
| Figure 1 Otolith development in S. davidi larvae and juveniles Scale bar=100 μm, except where otherwise stated |
No increments were detected on lapilli or sagittae of newly hatched larvae, but a diffuse and obscure increment first appeared at 2 dph (Fig. 2a). Thereafter, one increment was deposited per day. Five increments were found on 6-dph-old larvae otoliths (Fig. 2b), and otoliths of 20-dph-old larvae had 19 increments (Fig. 2c).

|
| Figure 2 S. davidi larvae and juvenile otoliths a. two-day post hatching (dph) larva lapillus showing the first increment (black arrow); b. six-dph larva sagitta showing five daily increments (black dots); c. twenty-dph larva lapillus showing 19 daily increments (black dots) |
Lapilli (IL) or sagittae (IS) incremental counts were equal to the number of dph (D), as expressed by the following linear regressions (Fig. 3): IL=0.991 7D-0.945 8 (R2=0.999 8, n=158) and IS =0.992 8D-0.9584 (R2=0.999 6, n=124), respectively. No differences were detected between the slopes of the equations and 1.0 (P>0.05), suggesting that the increments were deposited daily after hatching.

|
| Figure 3 Relationship between incremental numbers in lapilli (a) and sagittae (b) and age of S. davidi |
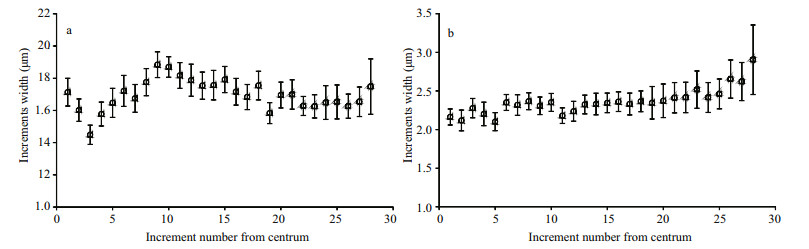
Increment widths were 1.45–1.88 μm in lapilli (Fig. 4a) and 2.10–2.90 μm in sagittae (Fig. 4b) from samples preserved at 28 dph. Lapilli increment widths were constant down to the third increment at 1.45±0.069 0 μm, and increased thereafter to a maximum of 1.88±0.080 0 μm for the ninth increment. Thereafter, increment widths dropped gradually almost to the otolith edge. However, sagittae increment widths increased gradually during growth.
|
| Figure 4 Relationships between mean increment width and increment number from the centrum along the longest axis in S. davidi lapilli (a) and sagittae (b) Larvae at 28 dph (lapillus, n=43; sagitta, n=28) were used for analysis |
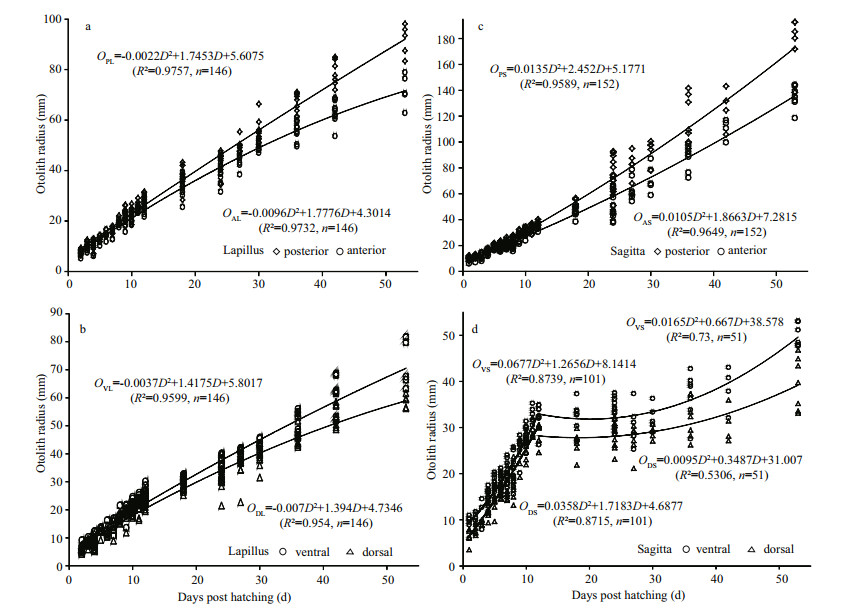
A quadratic functional relationship existed between growth of the posterior and anterior areas of lapilli (Fig. 5a) and sagittae (Fig. 5c), and growth rate of the posterior area was faster than that of the anterior area. Similarly, growth rate of the ventral area was faster than that of the dorsal area in lapilli (Fig. 5b) and sagittae (Fig. 5d). Furthermore, a clear inflexion point (approximately 12 dph) was detected in the relationship between OR and daily-age of sagittae (Fig. 5d); however, the growth trend (quadratic function) did not change before or after that time point.
|
| Figure 5 Relationship between otolith radius in different directions on lapilli (a, b) and sagittae (c, d) and S. davidi age |
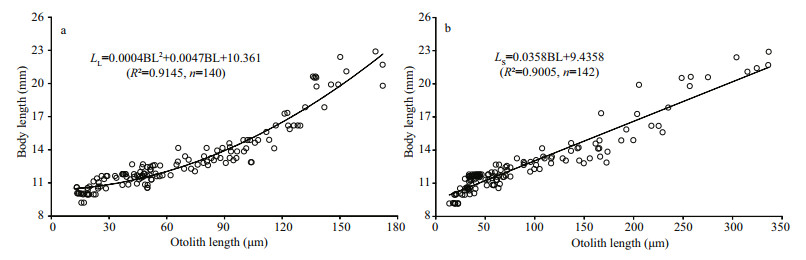
OR and BL of lapilli and sagittae were binomially and linearly related, respectively (Fig. 6). The regression equations between otolith length (sum length of radius anterior and posterior areas; LL, length of lapillus; LS, length of sagittae) and BL were: LL=0.000 4BL2+0.004 7BL+10.361 (R2=0.914 5, n=140) (Fig. 6a), and LS=0.035 8BL+9.435 8 (R2=0.900 5, n=142) (Fig. 6b).
|
| Figure 6 Relationships between otolith (a, lapillus; b, sagitta) radius and body length of Schizothorax davidi larvae and juveniles |
The morphology of sagittae changed significantly during development, which agrees with previous reports on Cyprinidae (Huang and Chiu, 1997; Song et al., 2008; Yan et al., 2014). The development of lapilli was relatively stable, but the changes in morphology varied in four areas. Allometric growth of the OR in lapilli and sagittae was very clear; the growth rate of the posterior area was faster than that of the anterior area, and the growth rate of the ventral surface was faster than that of the dorsal area. The differences in growth rates among the four areas may be the main reason for the morphological differences. Moreover, OR may be useful to back-calculate the growth trajectory during the early life history using the relationship between growth and morphology.
In the present study, lapilli and sagittae appeared before hatching, and the daily increment first appeared at 2 dph. Larvae asterisci emerged at 19 dph, but their increments were unclear and irregular. In contrast, both lapilli and sagittae are useful for assessing age in this species (Morioka and Matsumoto, 2007; Song et al., 2008). However, sagittae are easily damaged during extraction and grinding because of elongation along the anterior-posterior area. The increments along the anterior-posterior direction were obscure, and the peripheral increments of the dorsal-ventral area were pressed together because of the slow growth and were unable to be counted. The usefulness of lapilli rather than sagittae for determining the daily increment has been reported for other Schizothoracinae species in our laboratory (unpublished results), as well as for other cyprinids (Mugiya and Tanaka, 1992; Song et al., 2008; Yan et al., 2014), but the lapilli were more suitable for determining age of S. davidi.
Otolith microstructural analyses are very valuable for relating biotic and abiotic factors to life-history traits. However, periodicity of increment formation must be validated when studying otolith microstructure in different species (Humphrey et al., 2003). Validation methods include monitoring known-age fish under laboratory conditions (Song et al., 2008; Yamada et al., 2009; Pavlov et al., 2012; Yan et al., 2014), using fluorescent markers (Sugeha et al., 2001; Joh et al., 2005), performing a marginal incremental analysis (Moku et al., 2005), and using statistical inferences (Morley et al., 2005). Of these, the most direct method to validate increment deposition periodicity is to hatch eggs and rear the larvae (Campana, 2001; Sponaugle, 2009), and this method has been used to validate daily increment deposition in many species (Mendiola and Álvarez, 2008). By analyzing larvae of known-age and counting the increments under laboratory conditions, we have demonstrated that the first regular increment formed the 2 dph, and that the number of increments was equal to the actual age in days−1. These findings suggest that increments were deposited daily, which agrees with previous results obtained with a fluorescent marker in fish reared under different temperatures (He et al., 2008). However, the method of monitoring known-age larvae used in this study was easier and more direct for validating daily increments compared to the aforementioned. Additionally, the fish were less affected by endocrine-driven endogenous rhythms and environmental conditions.
The relationship between fish length and OR helps with the back-calculation of the growth trajectory during early life history, but this can be limited by many factors. Although fish body length and OR are closely related, the relationship varies with growth, and the slope of this relationship is affected by temperature (Aldanondo et al., 2008). Additionally, starvation, infrequent feeding, and other environmental conditions may cause similar problems (Folkvord et al., 2000; Fox et al., 2003). Furthermore, ontogenetic shifts in growth patterns, which are controlled by endogenous factors, can also affect the OR-body size relationship, as demonstrated in perch when they begin exogenous feeding (Kristensen et al., 2008). The relationship between fish length and OR of clupeoids also changes from an allometric relationship during the larval stage to a linear relationship during the juvenile stage (Watanabe and Kuroki, 1997; Takahashi et al., 2001). However, the relationship between OR and body length in S. davidi was either linear or binomial, and the effects of environmental conditions and ontogenetic shifts had no obvious impact under laboratory-rearing conditions. Therefore, these species-specific differences should be considered during early life stages when backcalculating growth using the OR-body size relationship.
Notably, the incremental changes in width differed between lapilli and sagittae. The first lapilli increment width was less than that in sagittae, and peaks and troughs were observed in the increment widths of lapilli, whereas those of sagittae tended to increase slowly during growth. This result may be closely related to otolith and fish growth (Ivarjorda et al., 2008; Yamada et al., 2009). In this study, lapilli were more regular in shape than sagittae, and growth was relatively consistent between different areas. In contrast, sagittae changed shape significantly, particularly in the ventral and dorsal areas, and growth in the posterior area also differed significantly from that in other areas. Furthermore, the maximum lapilli increment width appeared at 9 dph, and then decreased, which has been associated with exogenous food intake during the feed transition. Therefore, the change in lapillus increment microstructure may be a true reflection of fish growth when compared to the steady increase in sagitta growth.
5 CONCLUSIONIn summary, the morphological features and developmental characteristics of S. davidi otoliths were species-specific; the first regular increment formed at 2 dph, and deposition was clearly periodic. Lapilli and sagittae development and growth were closely related to age and body length of fish. These results will advance the research and protection of wild resources and fisheries management of this species.
6 ACKNOWLEDGMENTWe thank LIU Xiaoshuai, WANG Yufeng, and YANG Ting for their help with sample collection. We are grateful to Professor SONG Zhaobin for evaluating the manuscript.
| Aldanondo N, Cotano U, Etxebeste E, Irigoien X, Álvarez P, De Murguía A M, Herrero D L, 2008. Validation of daily increments deposition in the otoliths of European anchovy larvae (Engraulis encrasicolus L.) reared under different temperature conditions. Fisheries Research, 93(3): 257–264. Doi: 10.1016/j.fishres.2008.04.012 |
| Begg G A, Campana S E, Fowler A J, Suthers I M, 2005. Otolith research and application:current directions in innovation and implementation. Marine and Freshwater Research, 56(5): 477–483. Doi: 10.1071/MF05111 |
| Burke N, Brophy D, King P A, 2008. Otolith shape analysis:its application for discriminating between stocks of Irish Sea and Celtic Sea herring (Clupea harengus) in the Irish Sea. ICES Journal of Marine Science, 65(9): 1670–1675. Doi: 10.1093/icesjms/fsn177 |
| Bustos C A, Landaeta M F, Palacios-Fuentes P, JahnsenGuzmán N, Balbontín F, 2015. Comparing early life traits of hakes from Chilean Patagonian fjords inferred by otolith microstructure analysis. Fisheries Research, 164: 35–44. Doi: 10.1016/j.fishres.2014.10.016 |
| Campana S E, 2001. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology, 59(2): 197–242. Doi: 10.1111/jfb.2001.59.issue-2 |
| Campana S E, 2005. Otolith science entering the 21st century. Marine and Freshwater Research, 56(5): 485–495. Doi: 10.1071/MF04147 |
| Chen Y Y. 1998. Fauna Sinica, Osteichthyes, Cypriniformes Ⅱ. Science Press Ltd, Beijing. (in Chinese) |
| Ding R H. 1994. The Fishes of Sichuan, China. Sichuan Publishing House of Science and Technology, Chengdu. (in Chinese) |
| Feet P O, Ugland K I, Moksness E, 2002. Accuracy of age estimates in spring spawning herring (Clupea harengus L.) reared under different prey densities. Fisheries Research, 56(1): 59–67. Doi: 10.1016/S0165-7836(01)00313-7 |
| Folkvord A, Blom G, Johannessen A, Moksness E, 2000. Growth-dependent age estimation in herring (Clupea harengus L.) larvae. Fisheries Research, 46(1-3): 91–103. Doi: 10.1016/S0165-7836(00)00136-3 |
| Fox C J, Folkvord A, Geffen A J, 2003. Otolith micro-increment formation in herring Clupea harengus larvae in relation to growth rate. Marine Ecology Progress Series, 264: 83–94. Doi: 10.3354/meps264083 |
| Guo H Y, Wei K, Tang W Q, Wu J M, Chen W Y, 2010. Sibling species discrimination for chinese genus of coilia fishes based on sagittal otolith morphology. Acta Zootaxonornica Sinica, 35: 127–134. |
| He C L, Fu Z D, Yan T M, Song Z B, 2008. Otolith marking of laerval schizothorax davidi with fluorescent substances. Sichuan Journal of Zoology, 27(3): 331–334. |
| Huang W B, Chiu T S, 1997. Daily increments in otoliths and growth equation of black porgy, Acanthopagrus schlegeli, larvae. Acta Zoologica Taiwanica, 8(2): 121–131. |
| Huang Y F, Cheng F, Murphy B R, Xie S G, 2014. Sagittal otolith microstructure, early growth and development of Coilia ectenes in the Yangtze Estuary, China. Fisheries Science, 80(3): 435–443. Doi: 10.1007/s12562-014-0701-6 |
| Humphrey C, Klumpp D W, Pearson R G, 2003. Early development and growth of the eastern rainbowfish, Melanotaenia splendida (Peters). Ⅱ. Otolith development, increment validation and larval growth. Marine and Freshwater Research, 54(2): 105–111. |
| Ivarjord T, Pedersen T, Moksness E, 2008. Effects of growth rates on the otolith increments deposition rate in capelin larvae (Mallotus villosus). Journal of Experimental Marine Biology and Ecology, 358(2): 170–177. Doi: 10.1016/j.jembe.2008.02.011 |
| Joh M, Takatsu T, Nakaya M, Higashitani T, Takahashi T, 2005. Otolith microstructure and daily increment validation of marbled sole (Pseudopleuronectes yokohamae). Marine Biology, 147(1): 59–69. Doi: 10.1007/s00227-004-1545-x |
| Kristensen P B, Closs G P, Lokman P M, Grønkjær P, 2008. Otolith formation, microstructure and daily increment validation in juvenile perch Perca fluviatilis. Journal of Fish Biology, 73(6): 1478–1483. Doi: 10.1111/jfb.2008.73.issue-6 |
| Mendiola D, Álvarez P, 2008. Validation of daily increments in the otolith microstructure of Northeast Atlantic mackerel fish larvae. Fisheries Research, 89(3): 300–304. Doi: 10.1016/j.fishres.2007.10.018 |
| Moku M, Hayashi A, Mori K, Watanabe Y, 2005. Validation of daily otolith increment formation in the larval myctophid fish Diaphus slender-type spp. Journal of Fish Biology, 67(5): 1481–1485. Doi: 10.1111/jfb.2005.67.issue-5 |
| Morioka S, Matsumoto S, 2007. Otolith development and daily increment formation in larvae of the Kabyabya, a Malawian cyprinid, Opsaridium tweddleorum. Ichthyological Research, 54(1): 44–48. Doi: 10.1007/s10228-006-0372-0 |
| Morley S A, Belchier M, Dickson J, Mulvey T, 2005. Daily otolith increment validation in larval mackerel icefish, Champsocephalus gunnari. Fisheries Research, 75(1-3): 200–203. Doi: 10.1016/j.fishres.2005.04.008 |
| Mugiya Y, Tanaka S, 1992. Otolith development, increment formation, and an uncoupling of otolith to somatic growth rates in larval and juvenile goldfish. Nippon Suisan Gakkaishi, 58(5): 845–854. Doi: 10.2331/suisan.58.845 |
| Parkinson K L, Booth D J, Lee J E, 2012. Validation of otolith daily increment formation for two temperate syngnathid fishes:the pipefishes Stigmatopora argus and Stigmatopora nigra. Journal of Fish Biology, 80(3): 698–704. Doi: 10.1111/jfb.2012.80.issue-3 |
| Pavlov D A, Ha V T, Thuan L T B, 2012. Otolith morphology and periodicity of increment formation on the sagitta of manybar goatfish Parupeneus multifasciatus (Mullidae). Journal of Ichthyology, 52(7): 463–475. Doi: 10.1134/S003294521204008X |
| Pavlov D A, Ha V T, Thuan L T B, 2015. Otolith morphology, age, and growth of freckled goatfish Upeneus tragula(Mullidae) in the coastal zone of Vietnam. Journal of Ichthyology, 55(3): 363–372. Doi: 10.1134/S0032945215030108 |
| Song Z B, Fu Z D, Li J, Yue B S, 2008. Validation of daily otolith increments in larval and juvenile Chinese sucker, Myxocyprinus asiaticus. Environmental Biology of Fishes, 82(2): 165–171. Doi: 10.1007/s10641-007-9269-7 |
| Sponaugle S. 2009. Daily otolith increments in the early stages of tropical fish. In: Green B S, Mapstone B D, Carlos G, Begg G A eds. Tropical Fish Otoliths: Information for Assessment, Management and Ecology. Reviews: Methods and Technologies in Fish Biology and Fisheries. Springer, Netherlands. |
| Sugeha H Y, Shinoda A, Marui M, Arai T, Tsukamoto K, 2001. Validation of otolith daily increments in the tropical eel Anguilla marmorata. Marine Ecology Progress Series, 220: 291–294. Doi: 10.3354/meps220291 |
| Takahashi M, Watanabe Y, Kinoshita T, Watanabe C, 2001. Growth of larval and early juvenile Japanese anchovy, Engraulis japonicus, in the Kuroshio-Oyashio transition region. Fisheries Oceanography, 10(2): 235–247. Doi: 10.1046/j.1365-2419.2001.00160.x |
| Tuset V M, Lozano I J, González J A, Pertusa J F, García-Díaz M M, 2003. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). Journal of Applied Ichthyology, 19(2): 88–93. Doi: 10.1046/j.1439-0426.2003.00344.x |
| Watanabe Y, Kuroki T, 1997. Asymptotic growth trajectories of larval sardine (Sardinops melanostictus) in the coastal waters off western Japan. Marine Biology, 127(3): 369–378. Doi: 10.1007/s002270050023 |
| Yamada H, Chimura M, Asami K, Sato T, Kobayashi M, Nanami A, 2009. Otolith development and daily increment formation in laboratory-reared larval and juvenile blackspot tuskfish Choerodon schoenleinii. Fisheries Science, 75(5): 1141–1146. Doi: 10.1007/s12562-009-0146-5 |
| Yan T M, Hu J X, Yang T, Zhao L L, He Z, 2014. Study on the otolith development and the formation of increments in larvae and juvenile of Chuanchia labiosa. Acta Hydrobiologica Sinica, 38(4): 764–771. |
| Zeng X B, Zhang G H, 2012. Species identification at the larval and juvenile stages for several Chinese domestic fishes by elliptical Fourier analysis of otolith form. Journal of Fishery Sciences of China, 19(6): 970–977. |
| Zhu Q, Xia L Q, Chang J B, 2002. Computer identification on otolith microstructure of fish. Acta Hydrobiologica Sinica, 26(6): 600–604. |
 2017, Vol. 35
2017, Vol. 35


