Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Chunhui(张春辉), LIU Jianguo(刘建国), ZHANG Litao(张立涛)
- Cell cycles and proliferation patterns in Haematococcus pluvialis
- Chinese Journal of Oceanology and Limnology, 35(5): 1205-1211
- http://dx.doi.org/10.1007/s00343-017-6103-8
Article History
- Received Mar. 31, 2016
- accepted in principle May. 6, 2016
- accepted for publication Jul. 4, 2016
2 National-Local Joint Engineering Research Center forHaematococcus pluvialisand Astaxanthin Products, Yunnan Alphy Biotech Co., Ltd., Chuxiong 675012, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China
Astaxanthin (3, 3'-dihydroxy-β, β-carotene-4, 4'-dione), a liposoluble red ketocarotenoid, is widely used in the poultry industry and in aquaculture for its good coloration effect (Johnson and Schroeder, 1996; Giannelli et al., 2015). It has potential applications in medicine, and is also used as a nutraceutical and in cosmetics because of its powerful antioxidant capacity (Guerin et al., 2003; Goswami et al., 2010; Liu et al., 2016). Haematococcus pluvialis, a unicellular member of Chlorophyceae, is widely known as an important source of natural astaxanthin (Chen et al., 2015) and is being cultivated on a commercial scale worldwide (López et al., 2006; Ugwu et al., 2008).
Most research studies on H. pluvialis have been focused on cell growth and astaxanthin accumulation (Liu et al., 2002, 2016; Sun et al., 2008; Imamoglu et al., 2010). However, an improved understanding of cell cycle in H. pluvialis is still needed. A complete understanding of cell cycles in H. pluvialis is important, because it is conducive to the further studies on the environment parameters affecting cell proliferation, and it contributes towards achieving full control in large-scale industrial production. Although Elliot (1934) published a relatively comprehensive description of the cell morphology and life history of H. pluvialis, significant aspects were not completely clarified. In this context, the current study further characterizes the cell cycles in H. pluvialis and provides some new insights to help foster industry production of H. pluvialis. This paper builds on a previous article by Liu (2000) published in Oceanologia et Limnologia Sinica, while lacked photos of the cell morphologies at various cell cycles stages. Additional observations were also conducted to further clarify cell cycles and proliferation patterns in H. pluvialis. Moreover, we present the pictures and explanations of the cell cycles as well as proliferation patterns in this paper.
2 MATERIAL AND METHOD 2.1 StrainsThe alga Haematococcus pluvialis was obtained from the algal collection of the Institute of Oceanology, Chinese Academy of Sciences. So far, 42 wild strains of H. pluvialis and 10 UV-and chemically induced mutants derived from them have been obtained in our laboratory (Liu et al., 2016). The wild strains of H. pluvialis were isolated from different geographic areas and habitats. The cell cycles and proliferation patterns in H. pluvialis were described after repeated microscopic observations on different strains of H. pluvialis in our laboratory over almost 20 years. There was no significant difference in cell morphology and proliferation patterns among the different strains of H. pluvialis.
2.2 Culture conditions and observation methodThe algae were grown in modified MCM medium (Sun et al., 2008) at 25±1℃, under indoor artificial light and outdoor natural light. All experiments were done at the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences (Qingdao, China) and the Yunnan Alphy Biotech Co., Ltd. (Chuxiong, China).
Samples of different phases were taken and observed using a camera and video recorder system mounted on a microscope (37XB, Shanghai, China).
3 RESULT AND DISCUSSION 3.1 Definition of phases and termsHaematococcus pluvialis is a unicellular green alga with complex life history, which is commonly divided into two phases based on cell morphology and physiology: the motile phase and the non-motile phase (Fig. 1). The H. pluvialis cell in the motile phase has a thin wall, two flagella, and mainly grows by cell proliferation. In contrast, the H. pluvialis cell in the non-motile phase has a thick wall, no flagellum, and mainly conducts astaxanthin accumulation.
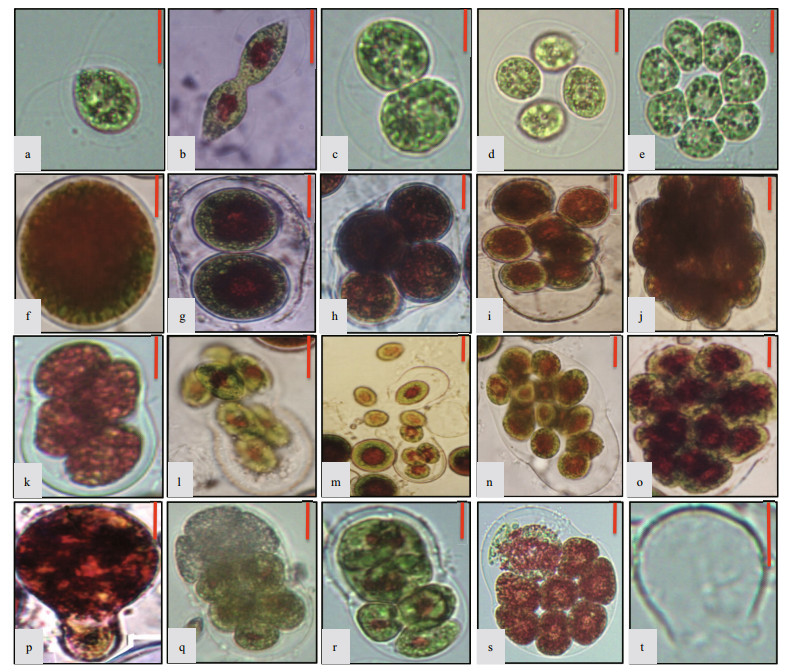
|
| Figure 1 The cell forms of Haematococcus pluvialis a. motile cell; b. vegetative reproduction by direct cell division in the motile phase; c. sporangium with two zoospores; d. sporangium with four zoospores; e. sporangium with eight zoospores; f. non-motile cell; g. sporangium with two aplanospores; h. sporangium with four aplanospores; i. sporangium with eight aplanospores being released; j. sporangium with >20 aplanospores; k. sporangium with 4 zoospores; l. sporangium with 8 zoospores being released; m. the moment of zoospores release; n. sporangium with 16 zoospores; o. sporangium with >20 zoospores; p. vegetative reproduction by cell budding in the nonmotile phase; q–r. unsynchronized cell division in sporangia during the process of zoospores formation; s. autolysis of some zoospores within the sporangium; t. theca after spores release. The length of each bar represents 10 μm. |
Many terms such as zoospore, flagellated cell, motile cell, red cell, green cell, palmella, non-motile cell, aplanospore and akinete have appeared in many articles and may result in confusion (Kobayashi et al., 1997; Hagen et al., 2002). Some of these terms are misused. Therefore, it is necessary to re-define the terms and concepts before describing the cell cycles of H. pluvialis.
3.1.1 Motile cell (Fig. 1a)Motile cell refers to the swimming H. pluvialis cells driven by two flagella. The cell is ovate or ellipsoidal, with a cell diameter of 5 μm or 20-30 μm, sometimes even bigger. The periplasmic space, filled with crystalline gelatinous matrix, exists between protoplast and cell wall, which are connected by plasmodesmata. No plasmodesma were found in the front part of motile cell, and the tip of the protoplast is often papillary. The motile cell has two isometric flagella, which emerge from the cell wall through the front fork gelatinous tube, with a length similar to the cell body in vitro. The nucleus is located in the center, with an eyespot localizing near the nucleus. The chloroplast, with a typical green algal pyrenoid, is goblet shaped, appearing granular or exhibiting complex reticulation.The motile cell is usually green, sometimes red.
3.1.2 Zoospore (Fig. 1c–e, k–o)Zoospores come from two sources, one source is asexual reproduction of motile cells under normal growth conditions, and the other is asexual reproduction of non-motile cells exposed to favorable conditions. During the process of asexual reproduction, a mother cell first gradually develops into a sporangium that divides into a number of cells. Then the sporangium swells and the wall breaks on one side to release some swimming daughter cells. The flagellated cells released from the sporangium are termed "zoospores".
The zoospore shape resembles the motile cell. There is not much space between the protoplast and the cell wall in zoospores. Moreover, a zoospore is generally much smaller than a motile cell; however, the two do not have significant boundaries, as the cell volume of a motile cell (as well as the volume of a non-motile cell) varies greatly, and the number of daughter cells produced by a mother cell is not consistent.
The term "tetraspore" is applied to the four zoospores released by a 4-spore sporangium. The utility of this term should be questioned as the zoospore number released from mother cell sporangia is variable. Moreover, In the Rhodophyta, the "tetraspores" are usually associated with meiosis. In H. pluvialis the term "tetraspores" should be avoided, as it could cause confusion.
3.1.3 Flagellated cellFlagellated cell is the general term used for motile cells and zoospores, which can swim with flagella. The term "flagellated cell" appears in many papers and is widely used by some researchers. The term should not be used in describing the life cycle of H. pluvialis, because it is easy to confuse motile cell with zoospore.
3.1.4 Non-motile cell (Fig. 1f)Non-motile cell refers to the cellular morphology of non-motile vegetative cells in addition to the non motile aplanospores, in contrast to the motile cells of the motile phase. Under adverse environmental conditions, motile cells lose their flagella and develop into spherical non-motile cells. The cell size varies greatly, most commonly around 20–30 μm, but as small as 10 μm, and as large as 50 μm. The specific reasons for such a large variation in cell volume have not been determined, but might be due to differences in the sizes of motile cells from which they are derived and the timing of cell division. Non-motile cells transformed from motile cells are mostly green at first. Over time, astaxanthin starts to accumulate in the mid-region of the cell, around the cell nucleus, forming an obvious red centre and green periphery. Finally, when the astaxanthin has spread, the cell transforms to a completely red, non-mobile cell.
3.1.5 Aplanospore (Fig. 1g–j)After losing its motility, the non-motile cell appears to be in a resting stage, however, it still has proliferative ability. In fact, a non-motile cell can transform into a sporangium and divide into non-motile aplanospores. Basically, an aplanospore is a daughter cell originating by asexual reproduction of a non-motile cell.
3.1.6 PalmellaPalmella is a cell colony with variable numbers of small cells covered by colloids (Elliot, 1934). When applied in H. pluvialis, it is a misuse of the term, confusing sporangia containing aplanospores with a cell colony, and thus should not be used.
3.1.7 AkineteAkinete usually refers to the aplanospore or nonmotile cell with a thick cell wall. There is no significant difference of cell wall thickness between an aplanospore and a non-motile cell. It is not appropriate to add the term "akinete" to the cell cycles of H. pluvialis because it confuses the non-motile cell with the aplanospore.
It is also inappropriate to use "red cell" and "green cell" as professional terms. The astaxanthin accummulates gradually during the transformation making it impossible to define a boundary between the two.
In summary, the complex life history of H. pluvialis can be divided into the motile phase and the nonmotile phase. The cells in these stages of the life history can be classified under the following terms: motile cells, non-motile cells, zoospores and aplanospores. These four cell types can interconvert often through the formation of sporangia, and together constitute the life history of H. pluvialis. The cell cycles in H. pluvialis have been studied by one of us (Liu) since the mid-1990s. Moreover, this life history of H. pluvialis was previously proposed by Yin et al. (1998) and Liu et al. (2000), and has been widely used in studies of H. pluvialis (Zhuang et al., 2001; Dong et al., 2008; Gao et al., 2014 and etc.).
3.2 Proliferation patterns in life cycleUnder normal growth conditions in culture and during commercial cultivation (e.g., when motile cells are inoculated into fresh medium), the motile cell (Fig. 1a) usually produces two (Fig. 1c), sometimes four (Fig. 1d), and exceptionally eight (Fig. 1e) spores by asexual reproduction through formation of a sporangium (Fig. 2-1). All these spores are zoospores as they have two flagella and are motile. Over time, in the course of development, a zoospore transforms into a motile cell (Fig. 2-2).
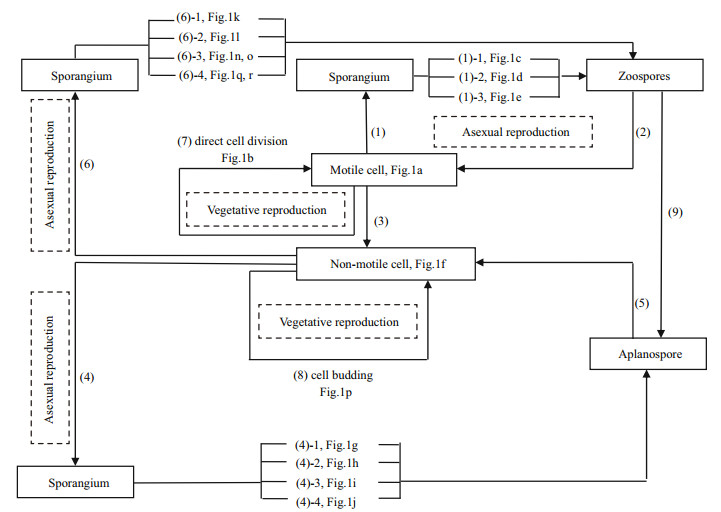
|
| Figure 2 Proliferation patterns and cell cycles in Haematococcus pluvialis Under normal growth conditions, the motile cells usually produce two (1-1), sometimes four (1-2), and exceptionally eight (1-3) zoospores by asexual reproduction through formation of sporangia (1), and released zoospores are then transformed into motile cells (2). Under unfavorable conditions, motile cells lose their flagella and transform into non-motile cells directly (3), and the non-motile cells usually produce 2 (4-1), 4 (4-2) or 8 aplanospores (4-3), and occasionally 20-32 aplanospores (4-4) through formation of sporangia (4) by asexual reproduction, and further develop into non-motile cells (5). Under suitable conditions, non-motile cells are also able to release zoospores through formation of sporangia (6), in which the bigger non-motile cell produces more than 16 zoospores (6-3), and the smaller one produces 8 (6-2) or 4 zoospores (6-1), occasionally with unsynchronized cell division in some sporangia (6-4). Vegetative reproduction occasionally happens by direct cell division in the motile stage (7) and by cell budding in the non-motile stage (8). (9) Under stressed conditions, zoospores transform into aplanospores in sporangia or undergo autolysis. |
Under unfavorable conditions, a motile cell or a zoospore released from a sporangium loses its flagella and transforms into a non-motile cell (Fig. 2-3) or an aplanospore (Fig. 2-9) directly. A non-motile cell remains able to proliferate by asexual reproduction, producing numbers of aplanospores through the formation of a sporangium (Fig. 2-4). The division rate of a non-motile cell is significantly lower than that of a motile cell. A non-motile cell usually produces 2 (Fig. 1g), 4 (Fig. 1h) or 8 aplanospores (Fig. 1i), and occasionally as many as 20-32 aplanospores (Fig. 1j) in a sporangium. A non-motile cell can also produce 3, 5, 6, or 7 aplanospores through unsynchronized cell division (Fig. 1q, r). All these aplanospores lack flagella and can develop further into non-motile cells after release from a sporangium (Fig. 2-5).
Once growth conditions became suitable again (e.g., by rainfall, or when non-motile cells are inoculated into fresh medium), a non-motile cell is also able to produce zoospores (spores with flagella) through formation of a sporangium (Fig. 2-6). The zoospores further develop into new motile cells after being released from a sporangium, leaving behind a transparent theca (Fig. 1t). Generally, a large, nonmotile cell produces more than 16 zoospores (Fig. 1n, o), and a smaller one produces 4 (Fig. 1k) or 8 zoospores (Fig. 1l).
The main cell proliferation of this unicellular green alga is asexual reproduction through formation of sporangia both in the motile stage and the non-motile stage (Mesquita and Santos, 1984). In fact, it is only in a few cases that cells carry out vegetative reproduction. Vegetative reproduction is by direct cell division (Fig. 1b) in the motile stage (Fig. 2-7) and by cell budding (Fig. 1p) in the non-motile stage (Fig. 2-8) (Liu et al., 2000). The cell division process can produce 2n+1 zoospores or aplanospores when sporangia produce cells with unequal division speeds (Fig. 1q, r). The daughter cells may be released before finishing their division, and may finish the division process by direct cell division or by cell budding.
The signals that trigger the transition between asexual reproduction via sporangia and direct vegetative cell division are currently unknown. Additionally, all proliferation patterns in the life cycle of H. pluvialis may co-occur in culture, depending on the conditions.
There is no direct evidence nor a convincing report about sexual reproduction in H. pluvialis. Lee and Ding (1994) thought non-motile cells transformed from motile cells by cell fusion. They reported that cells fused after approximately five doublings, and that the DNA content of most cells doubled. Thereafter, motile cells decreased whereas non-motile cells increased. DNA doubling would result from cell fusion; however it may also be caused by other processes. Thus the data they presented are not sufficient to claim the existence of sexual reproduction.
Sexual reproduction has not been observed in experimental cultivation or wild natural conditions. Only once in almost 20 years of repeated observations did Liu observe a pair of small motile cells (looked like gametes) paired head to head, but after further monitoring, the two cells separated again without fusing. Whether sexual reproduction exists in H. pluvialis is suspect. The small cells with a diameter below 5 μm are not often present in experimental cultivation or wild natural conditions. Small cells with a cell diameter below 5 μm have been observed in winter at low temperature, but these were difficult to collect. Also, when clonal isolates of small cells have been established, microscopic observation found that they were not H. pluvialis cells. Recently, Strittmatter et al. (2016) found that the "microzoid" observed in the H. pluvialis cultures were more likely a pathogen of H. pluvialis. Cells of this new pathogen, Paraphysoderma sedebokerense settle, attach and encyst on the H. pluvialis host cells under favorable conditions. During the course of infection, the cyst develops into a vegetative sporangium, and then amoeboid swarmers, which are able to move via pseudopodia, are released from these sporangia (Hoffman et al., 2008).
Cell autolysis exists in every stage of the cell cycles in H. pluvialis (Fig. 1s). Cells become colorless with loss of pigments and decomposition of the cytoplasm, vacuole, mitochondrion, chloroplast, nucleus and so on, until only the theca remains (Fig. 1t).
The cell cycles and proliferation patterns in H. pluvialis were described based on repeated microscopic observation of various strains of H. pluvialis in our laboratory over almost 20 years. Additionally, all the proliferation patterns in the life cycle of H. pluvialis may co-occur in culture depending on the conditions. Growth conditions are difficult to quantify, as they encompass nutrient conditions, photoperiod, aeration, temperature, pH and so on. Overall, we propose an updated and revised life cycle for H. pluvialis that is coherent with earlier observations of Elliot (1934), and integrate available pictures on cell cycles and proliferation patterns with the previous article by Liu et al. (2000). Based on the above observations, the cell cycles/life history of H. pluvialis can be described as depicted in Fig. 2.
4 ACKNOWLEDGEMENTWe thank Dr. John van der Meer (Pan-American Marine Biotechnology Association) for his assistance with proofreading.
| Chen G Q, Wang B B, Han D X, Sommerfeld M, Lu Y H, Chen F, Qiang H, 2015. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis inHaematococcuspluvialis(Chlorophyceae). The Plant Journal, 81(1): 95–107. Doi: 10.1111/tpj.12713 |
| Dong Q L, Xing X Y, Cai Y, Wu H X, Lv M J, 2008. Phycocyanin synthesis in green alga Haematococcus pluvialis during a novel reproduction process. Chemical Engineering (China), 36(10): 55–57, 61. |
| Elliot A M, 1934. Morphology and life history of Haematococcus pluvialis. Arch. Protistenk., 82: 250–272. |
| Gao G L, Cheng J Y, Ma J, 2014. Research review of Haematococcus pluvialis and astaxathin. Journal of Fisheries of China, 38(2): 308–315. |
| Giannelli L, Yamada H, Katsuda T, Katsuada T, 2015. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng., 119(3): 345–350. Doi: 10.1016/j.jbiosc.2014.09.002 |
| Goswami G, Chaudhuri S, Dutta D, 2010. The present perspective of astaxanthin with reference to biosynthesis and pharmacological importance. World J. Microbiol.Biotechnol., 26(11): 1925–1939. Doi: 10.1007/s11274-010-0373-z |
| Guerin M, Huntley M E, Olaizola M, 2003. Haematococcus astaxanthin:applications for human health and nutrition. Trends Biotechnol., 21(5): 210–216. Doi: 10.1016/S0167-7799(03)00078-7 |
| Hagen C, Siegmund S, Braune W, 2002. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. European Journal of Phycology, 37(2): 217–226. Doi: 10.1017/S0967026202003669 |
| Hoffman Y, Aflalo C, Zarka A, Gutman J, James T Y, Boussiba S, 2008. Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota), parasitic on the green alga Haematococcus. Mycological Research, 112(1): 70–81. Doi: 10.1016/j.mycres.2007.09.002 |
| Imamoglu E, Dalay M C, Sukan F V, 2010. Semi-continuous cultivation of Haematococcus pluvialis for commercial production. Applied Biochemistry and Biotechnology, 160(3): 764–772. Doi: 10.1007/s12010-009-8627-7 |
| Johnson E A, Schroeder W A. 1996. Biotechnology of astaxanthin production in Phaffia rhodozyma. In: Takeda G R, Teranishi R, Williams P J, Kobayashi A eds. |
| Biotechnology for Improved Foods and Flavors. American Chemical Society, Washington, DC. p. 39-50. |
| Kobayashi M, Kurimura Y, Kakizono T, Nishio N, Tsuji Y, 1997. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. Journal of Fermentation and Bioengineering, 84(1): 94–97. Doi: 10.1016/S0922-338X(97)82794-8 |
| Lee Y K, Ding S Y, 1994. Cell cycle and accumulation of astaxanthin in Haematococcus lacustris (ChloroPhyta). J.Phycol., 30(3): 445–449. Doi: 10.1111/j.0022-3646.1994.00445.x |
| Liu J G, van der Meer J, Zhang L T, Zhang Y. 2016. Cultivation of Haematococcus pluvialis for astaxanthin production. In: Slocombe S P, Benemann J R eds. Microalgal Production for Biomass and High-Value Products. CRC Press, New York, USA. p. 267-293. |
| Liu J G, Yin M Y, Zhang J P, Liu W, Meng Z C, 2002. Dynamic changes of inorganic nitrogen and astaxanthin accumulation in Haematococcus pluvialis. Chinese Journal of Oceanology and Limnology, 20(4): 358–364. Doi: 10.1007/BF02847927 |
| Liu J G, Yin M Y, Zhang J P, Meng Z C, Bourne W F, 2000. Studies of cell cycle in Haematococcus pluvialis. Oceanologia et Limnologia Sinica, 31(2): 145–150. |
| López M C G M, Sánchez E D R, López J L G, Fernández F G A, Sevilla J M F, Rivas J, Guerrero M G, Grima E M, 2006. Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. Journal of Biotechnology, 123(3): 329–342. Doi: 10.1016/j.jbiotec.2005.11.010 |
| Mesquita J F, Santos M F, 1984. Ultrastructural study of Haematococcus lacustris (Griod) Rostafinski(Volvocales). Ⅱ. Mitosis and cytokinesis. Cytologia, 49(1): 229–241. Doi: 10.1508/cytologia.49.229 |
| Strittmatter M, Guerra T, Silva J, Gachon C M M, 2016. A new flagellated dispersion stage in Paraphysoderma sedebokerense, a pathogen of Haematococcus pluvialis. Journal of Applied Phycology, 28(3): 1553–1558. Doi: 10.1007/s10811-015-0700-8 |
| Sun Y H, Liu J G, Zhang X L, Lin W, 2008. Strain H2-419-4 of Haematococcus pluvialis induced by ethyl methanesulphonate and ultraviolet radiation. Chinese Journal of Oceanology and Limnology, 26(2): 152–156. Doi: 10.1007/s00343-008-0152-y |
| Ugwu C U, Aoyagi H, Uchiyama H, 2008. Photobioreactors for mass cultivation of algae. Bioresource Technology, 99(10): 4021–4028. Doi: 10.1016/j.biortech.2007.01.046 |
| Yin M Y, Liu J G, Zhang J P, Meng Z C. 1998. Review of studies of Haematococcus pluvialis and its astaxanthin. Transaction of Oceanology and Limnology, (2): 53-62. (in Chinese with English abstract) |
| Zhuang H R, Chen W L, Lu H S, Zhong X R, Chen L Y, 2001. The research of ultrastructure for different morphological cells of Haematococcus pluvialis. Chinese Journal of Applied & Environmental Biology, 7(5): 428–433. |
 2017, Vol. 35
2017, Vol. 35


