Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Rediat ABATE(RediatABATE), GAO Yahui(高亚辉), CHEN Changping(陈长平), LIANG Junrong(梁君荣), CHEN Weifang(陈蔚芳), SUN Lin(孙琳), Demeke KIFILE(DemekeKIFILE)
- Environmental change and its effects on inter-decadal variations of diatom production, species composition and frustule dissolution in a coastal marginal sea
- Chinese Journal of Oceanology and Limnology, 35(6): 1362-1373
- http://dx.doi.org/10.1007/s00343-017-0084-5
Article History
- Received Apr. 24, 2016
- accepted in principle Jun. 8, 2016
- accepted for publication Oct. 25, 2016
2 State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China;
3 College of Natural Science, Arba Minch University, Arba Minch PO Box 21, Ethiopia;
4 Department of Zoological Sciences, Addis Ababa University, Addis Ababa, PO Box 1176, Ethiopia
It has been decades since climate change and anthropogenic pressures on aquatic systems became a major issue in scientific communities (Lee and Olsen, 1985; Rosenberg et al., 1990; Trenberth and Hurrell, 1994; Treppke et al., 1996; Bianchi et al., 2002; Brush, 2009; Itoh et al., 2009; Menge et al., 2009; Giani et al., 2012). Aquatic ecologists have been trying to determine the effects of climate change and anthropogenic pressure and their impact on ecological stability and human livelihood; however, owing to their complexity and erratic species responses, it has not been easy to understand the behavior of ecosystems. To simplify this problem, aquatic ecologists commonly study year-to-year variability of representative biological and chemical variables over short-and long-term time intervals. However, limitations in logistics and the time required to analyze decadal, centurial and millennial samples has led to the study of time-series of biological and chemical variables from sediment cores becoming the preferred and common practice (Rühland et al., 2008; Perren et al., 2009). This kind of paleoceanographic study has become a research focus, mainly because sediment preserved biological remains such as microplankton can be used as a proxy for physicochemical conditions and are intimately correlated with the overlying water column conditions (Abrantes, 1988), which are in turn intrinsically connected to atmospheric and oceanic circulation patterns and human impacts.
Diatoms are eukaryotic and primarily microplanktonic algae that have commonly been used as environmental change indicator species because of the generally good preservation of their cell walls in the sediment and rapid reaction to environmental changes (Finkelstein and Gajewski, 2007; Miller et al., 2011). The abundance and compositions of diatom assemblages are intimately linked to the physicochemical dynamics of temperature, nutrients and light in the water column and habitat. Consequently, diatoms are often used to infer the ecological and chemical histories of water bodies, as well as to provide a possible means of checking whether the system has been subjected to substantial changes or remained stable throughout a given period of time. Related to this, diatoms have been used to analyze past environmental changes and future predictions. Although valve concentrations and species composition in the sediment samples are some of the most reliable paleoenvironmental proxy records, due to differential dissolution among diatom taxa, it is recommended that diatom dissolution data be considered during analyses.
The South China Sea (SCS) is one of the largest marginal seas in South East Asia, which is located in the western Pacific Ocean and experiences a great deal of climate change. The impacts of recent climate change and anthropogenic pressure have been recorded throughout the world (Bianchi et al., 2002; Weckström et al., 2007; Itoh et al., 2009; Menge et al., 2009; Montes-Hugo et al., 2009; Klais et al., 2011; Hinder et al., 2012). The Pacific Decadal Oscillation (PDO) is believed to play a major role in regime shifts and related long-term changes in the Pacific Ocean. The PDO is a long-lived El Niño-like pattern of Pacific climate variability (Lee et al., 2012) that manifests itself as warm or cool surface waters in the Pacific Ocean north of 20°N. The El Niño Southern Oscillation (ENSO) is a periodic change in the atmosphere and ocean of the tropical Pacific region that is associated with opposite extremes of convective rainfall, surface air pressure, and atmospheric circulation in the Tropics from Indonesia to South America. El Niño is the warm phase of the oscillation, while La Niña is the cold phase (Lee et al., 2012).
An anomaly consisting of a significantly detectable warming trend in summer (June-August) sea surface temperature has been observed over the SCS for the past ~60 years, which is further accompanied by an inter-decadal regime shift around the 1970s (Wang et al., 2009). Oey et al. (2013) reported a weakening trend of the East Asian Winter Monsoon since the 1970s. In addition to this, several papers have pointed out that the climatic conditions of the Pacific Ocean have changed since 1976 (Liu et al., 2008). Similarly, data from Sr/Ca coral records clearly showed declining winter monsoon wind velocity throughout the 20th Century in the SCS (Liu et al., 2008). Furthermore, Liu et al. (2008) illustrated a decline in average annual mean winter monsoon wind velocity values from 5.73 m/s to 4.5 m/s for 1958 to 1976 and 1977 to 1998, respectively. Wang et al. (2009) reported that the relationship between the SCS summer monsoon and El Niño has significantly strengthened since the late 1970s, and that 1993 marked a sudden inter-decadal change in precipitation and circulation in the SCS and surrounding regions.
Marginal seas are shallow, their large area lays on the continental shelf, and they are proximate to land; therefore, they are more prone to changes in sea surface temperature than the open ocean (Belkin, 2009). Moreover, since the SCS is a marginal sea, the observed climate shift would also result in a concomitant impact on the biogeochemical variables of the SCS. This scenario was likely reflected by diatom species composition, diatom production, diatom dissolution index and the percent planktonic abundance. However, this implication has rarely been reported in previous studies of the SCS (Jiang et al., 2004; Wu et al., 2013).
The South China Sea has been subjected to a vast array of anthropogenic impacts (Hu et al., 2009). It is surrounded by one of the world's most economically dynamic regions, notably the Zhujiang (Pearl) River Delta, and includes metropolises such as Hong Kong, Shenzhen and Guangzhou. Rapid urbanization and industrialization have occurred over the past 50 years in the lower reaches of the Zhujiang River, Zhujiang River Delta and Hong Kong. As a result, the total population in the Delta, Hong Kong and Macau is about 35 million people, and this is increasing (Harrison et al., 2008). Moreover, the Zhujiang River drainage area is characterized by intensive agricultural activities for production of commercial crops, which resulted in large nutrient inputs from artificial fertilizers that led to a rapid increase in nitrate concentration in the last few decades, while phosphate concentration is expected to become a limiting nutrient of phytoplankton growth in the estuary (Cai et al., 2004). Consequently, eutrophication and harmful algal blooms have generated a great deal of concern. In the last few decades, several species of dinoflagellates, largely of Gymnodinium, Ceratium, Gyrodinium, Procentrum and Noctiluca, and two diatom taxa Skeletonema costatum and Chaetoceros spp., have been reported to form harmful algal blooms in the Zhujiang River estuary (Tang et al., 2003).
This study evaluated decadal variations in diatom abundance, diatom species composition, dissolution level and percent planktonic abundance in a sediment core retrieved from the inner shelf of the SCS. In addition, the possible anthropogenic impacts and their correlation to diatom abundance and species composition were assessed.
2 STUDY AREAThe SCS, which is one of the largest continental marginal seas in the Pacific Ocean, is located in the western North Pacific and surrounded by land and islands that provide ample terrigenous materials (Fig. 1). The surface circulation is governed by the East Asia monsoon systems, which drive the cyclonic gyre during winter and anticyclonic gyre in summer. Consequently, there is strong seasonality in primary productivity and the quantity of organic material reaching the sea floor. The seasonality of Chl-a concentration, phytoplankton species composition and bloom formation have been reported in the Gulf of Beibu of the SCS (Tang et al., 2003). Hu et al. (2003) reported high concentrations of Chl-a throughout all seasons in the frontal water of Hainan Island based on satellite data. Zhao and Tang (2007) further explained the existence of a seasonal maximum of Chl-a concentration in the western SCS during summer, which is attributed to coastal upwelling, anticyclonic circulations and the Ekman Pumping effects. Huang et al. (2004) reported that Skeletonema costatum was the dominant species during rainy periods, whereas the dry season is dominated by Eucampia zoodiacus, which contributed 45% and 43.5% of the total phytoplankton abundance, respectively. The distribution of these species is mainly associated with variations in salinity, nutrient concentration and zooplankton abundance.

|
| Figure 1 Location of the sampling site in the South China Sea (red) |
The study site is located on the Leizhou (Zhanjiang) Peninsula coast at 20°85.765′N and 111°18.168 33′E along the northwestern SCS at a water depth of 38 m (Fig. 1). The Leizhou Peninsula is surrounded by Hainan Island to the south next to Qiongzhou Strait, the coast of China to the north and the SCS to the south. The Leizhou Peninsula is drained by two large rivers, mainly the Red River to the southwest and the Zhujiang River to the northeast. Qiongzhou Strait is a narrow ocean passage connecting the Gulf of Beibu (Gulf of Tonkin) on the west and the northeastern SCS basin on the east. The Leizhou Peninsula coast is an active site where different water masses exchange, including strong freshwater discharges from the Zhujiang River influencing shelf water masses and resulting in a lesser haline upper water mass, which may be transported to the coastal area of southeast China through Qiongzhou Strait (Bauer et al., 2013). Although this area has been regarded as one of the less-developed coastal areas of China until the last decade or so, recent data have shown environmental contamination and degradation along the coast (Li et al., 2014). Particularly, radical expansion of land and marine aquaculture, increased agricultural crop production via excessive use of artificial fertilizer, industrialization and untreated sewage disposal are among the main causes of the problems (Wang et al., 2013).
3 MATERIAL AND METHOD 3.1 Sediment processing and datingThe sediment core was retrieved by a gravity corer onboard the R/V Dongfanghong Ⅱ during the 2012 summer cruises to the NSCS organized by the CHOICE-C project (Carbon Cycling in the China Seas-budget, Controls and Ocean Acidification). After collection, the sediment core was sliced at 2 cm intervals and approximately 0.5 cm of the outer rim of each sediment slab was trimmed off to minimize contamination between layers, then placed in a freezer at -20℃ until laboratory analysis. Sediment dating was conducted by the α-spectrometry 210Pb (via 210Po) method (Huh and Su, 1999; Su and Huh, 2002). 210Pb dates were calculated using the Appleby and Oldfield (1978) method. To derive 210Pb-based rates, semi-log plots were used. Sediment accumulation rates (SPb210) were calculated from the slope (m) of the 210Pbex decrease (decay) from surface to bottom of the core, assuming a constant initial concentration of the radionuclide (despite variable sedimentation rates), as follows:
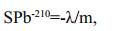
where, λ is the decay constant of 210Pb. In addition to 210Pb concentration, 137Cs concentration was also measured to validate the chronology based on 210Pb sediment dating.
3.2 Diatom abundanceThe sediment samples contained a large amount of clastic material and very low concentrations of diatom valves; therefore, the modified Renberg (1990) procedure was used. Approximately 1 g aliquots of samples were collected after drying at 65℃ for 24 h and then treated with H2O2 and HCl to remove organic matter and carbonates, respectively. Subsequently, samples were repeatedly (4–5 times) rinsed with distilled water to remove chemical residues (Renberg, 1990). Rinsed diatom suspensions were transferred into glass tubes and filled to 25 mL with distilled water, after which they were allowed to stand for 5 min to separate large-sized clastic materials and diatoms from smaller clastic materials and diatoms. The upper part, which consisted of the supernatant that settles slowly and was relatively full of smallsized clastic material and diatoms, was transferred to another tube and filled to 25 mL with distilled water. The lower, settled part, which contained relatively large sized clastic material and diatoms, was also filled to 25 mL. To identify and count diatom valves, well-mixed diatom suspensions (50 μL) were siphoned from each suspension tube and mounted on microscope slides using the mountant Naphrax®. Three slides were prepared from each suspension tube, indicating that six slides were prepared per sample. Prepared slides were observed under a light microscope at 400× or 1 000×. Moreover, subsamples from the top, middle and bottom sediment cores were observed under a JSM6390 scanning electron microscope. The number of valves per slide varied from 1 to 50. Broken valves were counted according to the protocol of Schrader and Gersonde (Zachariasse et al., 1978). Each specimen was identified to the species level, except for spores of Chaetoceros, which were identified at the genus level. In addition to the diatom abundance, diatom influx was calculated by multiplying the diatom abundance data by the dry bulk density (g/cm3) and the sedimentation rate (cm/a) for each level. Diatom flux was expressed as number of valves/cm2/a. Identification of taxa to the species and sub-species level was made using appropriate identification keys (Guo and Qian, 1984; Jin et al., 1985; Round et al., 1990; Cheng et al., 2013).
3.3 Formula and statistical analysisTo explain the causal correlation between diatom production and sediment preserved diatom valves and the extent of diatom valve dissolution, the fractional dissolution index (Fi) was calculated as the proportion of non-dissolved (or "pristine") to all classifiable valves within an assemblage (Ryves et al., 2009, 2011) by enumerating corroded and non-corroded valves. The values varied from 0 to 1, with Fi=1 indicating all valves were perfectly preserved and Fi =0 indicating all valves were dissolved. When the Fi value is approaching 1, the preserved assemblage contains almost pristine fragile species, some of which are fragmented, but without signs of significant dissolution. Linear regression and significance tests were implemented to examine causal relationships between the diatom abundance and diatom dissolution rate using Microsoft Excel. The Shannon-diversity index was calculated as a measure of diatom species diversity using the Multivariate Statistical Package (MVSP 3.2). To categorize diatoms into their closest groups (periods, in this case), TWINSPAN 2.3 (twoway indicator for species analysis (Hill et al., 1975; Hill and Šmilauer, 2005)) was used. Biostratographic analysis was conducted using C2 data analysis version 1.7.5. The percentage planktonic diatom abundance was calculated by dividing planktonic diatom abundance into total diatom abundance. To show the variations in diatom assemblages, the ratios of the dominant diatom species were analyzed (species that contributed more than 3% and occurred more than three times were considered dominant). Finally, longterm variations in diatom abundance and species composition were analyzed.
4 RESULT 4.1 Sediment datingThe excess 210Pb was determined by subtracting the 226Ra activity from the total 210Pb activity illustrated on Fig. 2. The 210Pb dating of the sediment core revealed an average sedimentation rate of 0.53 cm/a. Based on this sedimentation rate, each centimeter represents about two years of sediment accumulation. Measurement of the 137Cs concentration gave a sedimentation rate of about 0.59 cm/a. The 210Pb Constant Initial Concentration (CIC) model gave average accumulation rates that agree well with those derived from 137Cs (Chen Weifang, unpublished data). The recent comparison of the 210Pb age model based on independent dating methods Boer et al. (2006) also suggested the CIC model can give reliable results in environments with variable rates.

|
| Figure 2 Activity-depth profiles of 210Pbex in the sediment core |
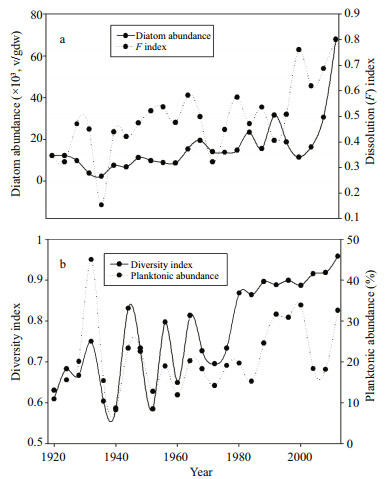
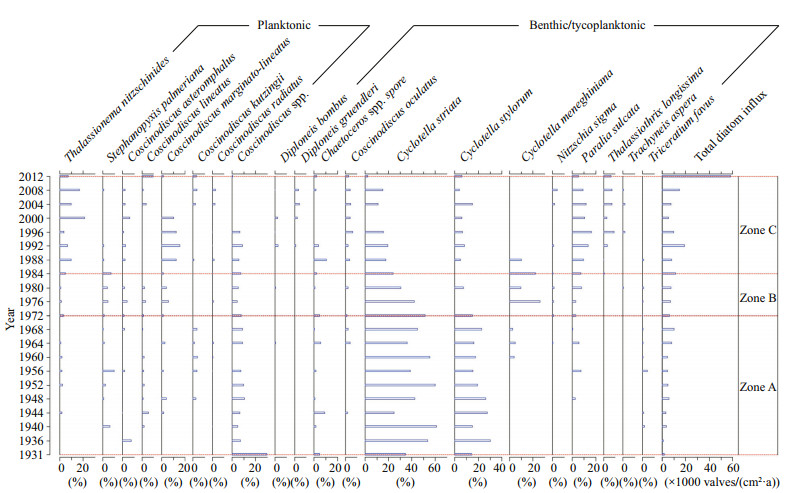
A total of 87 diatom taxa belonging to 25 genera were identified, with 25% of the species belonging to the centric genus Coscinodiscus. In addition to Coscinodiscus, the pennate genera Pleurosigma, Nitzschia and Trachyneis contributed a large number of species to the assemblage. Generally, the diatom abundance was very low. The absolute diatom abundance showed variations within two orders of magnitude (Fig. 3), ranging from 2 300 to 68 000 v/gdw with an average of 16 000 v/gdw. The average diatom flux was 10 000 valves/(cm2·a), with a range of 1 260 to 58 650 valves/(cm2·a) (Fig. 4). Shannon's species diversity index ranged from 0.59 to 0.96, with an average of 0.77, while the species richness varied from 7 to 52 species. At the inter-decadal time scale, species diversity increased erratically throughout the 20th century, before increasing consistently after the 1980s (Fig. 3). The majority of dominant species are benthic or tychoplanktonic diatoms such as Cyclotella striata, Cyclotella stylorum, Paralia sulcata, Planktoneilla blanda, Coscinodiscus oculatus, Actinoptychus undulatus, Thalassiothrix longissima and Thalassionema nitzschioides, which are frequently encountered in coastal marine waters. Conversely, the dominant planktonic taxa were Coscinodiscus marginato-lineatus, Coscinodiscus kutzingii and Coscinodiscus lineatus. The sum of the relative abundance of dominant species varied from 51% to 100%, with an average of 89.6% (Fig. 4).
|
| Figure 3 Decadal variations of a) absolute diatom abundance and diatom fractional dissolution index (Fi) and b) diatom diversity index and percent planktonic abundance |
|
| Figure 4 Decadal variations in relative abundance of major diatom species and total diatom influx in distinct zones |
The fractional diatom dissolution index showed high variations, with Fi values ranging from 0.15 to 0.8 and averaging 0.49. In general, diatom preservation was moderate, with an Fi value of 0.5. The diatom absolute abundance and fractional dissolution index showed strong and positive correlations (R=0.56; P < 0.05) with a similar pattern (Fig. 3). The percent planktonic abundance varied from 12% to 45% and averaged 21%, with most values being below 30%. The percent planktonic abundance increased after the mid-1980s, coinciding with increased total diatom production and an increase in the abundance of large-sized diatoms with robust frustules (Fig. 3).
4.3 Diatom assemblage zonesZone A encompasses the period before 1972, during this time the total diatom abundance was relatively low. Centric, tychoplanktonic and relatively small-sized diatoms such as C. striata, C. stylorum and small sized unidentified Coscinodiscus spp. were among dominant taxa. The relative abundance of Cyclotella in this zone was high, ranging from 50% to 85%. Zone B encompases the period beteween 1972– 1984, which was characterized by a rapid decline in the abundance of Cylotella species. In zone B, the relative abundance of Cyclottela ranged from 47% to 67% and the species diversity becomes more stable.
Zone C corresponds with the period after 1984, which coincided with 1) increased total diatom abundance, 2) increased relative abundance of centric species such as P. sulcata, C. marginato-lineatus and C. occulatus and 3) increased relative abundance of the penate planktonic and tychoplanktonic species T. nitzschioides and T. longissima (Fig. 4). During this period, the percent planktonic abundance, Fi and total diatom abundance increased considerably. Generally, there were five major distinct phenomena observed on the inner shelf of the SCS for the past 30 years after 1984: 1) replacement of the dominant species; the SCS inner shelf assemblages were largely dominated by Cyclotella species (mean=70%; range, 50% to 85%) before 1984. However, after 1984, the contribution of Cyclotella species decreased significantly (mean=21; range, 7% to 34%), which also coincided with the increased contribution of other larger diatoms. 2) Species diversity and species richness increased considerably after 1984 (from 0.68 to 0.82). 3) The percentage of planktonic abundance increased distinctly. 4) The mean total diatom abundance increased distinctly. 5) The fractional diatom dissolution index increased considerably.
4.4 The most dominant diatom speciesThe relative abundance of C. striata ranged from 2.8% to 61.5%. The relative abundance of C. striata decreased from 1972, coinciding with the increase in absolute abundance (Fig. 4). C. striata had a strong negative correlation with total diatom abundance. The relative abundance of C. stylorum varied from 4.5% to 30.8%. C. stylorum was the second most abundant species. Similar to C. striata, the relative abundance of C. stylorum was higher before 1972 than after 1972. C. stylorum was negatively correlated with total diatom abundance.
The relative abundance of P. sulcata varied from 0 to 16.5%. The percentage contribution of P. sulcata increased after 1972. Specifically, P. sulcata started to appear after 1950, and its relative abundance distinctly increased from the 1980s and peaked in the 1990s. The relative abundance of T. nitzschioides varied from 0 to 21% and was positively correlated with the total abundance (R=0.4, P < 0.005). The percentage contribution of T. nitzschioides increased after 1972 and its abundance was positively correlated with that of P. sulcata (R=0.62, P < 0.05). T. nitzschioides started to appear after 1950 and its relative abundance increased distinctly from the 1960s. The relative abundance of C. marginatolineatus ranged from 0–15%, while that of Chaetoceros spp. varied from 0–10%, that of Actinoptychus undulatus varied from 0–6.5%, that of C. oculatus varied from 0–6.9% and that of Thalassiothrix longissima varied from 0–9.6% (Fig. 4).
5 DISCUSSION 5.1 Diatom production and frustule dissolutionAccording to the calculated sedimentation rate, the 43 cm long sediment core spans about 81 years from AD 1931 to AD 2012. The rapid decline of 210Pb activity down core indicates the absence of substantial sediment mixing and reworking. The maximum diatom concentration that occurred in the surface sediment of the SCS is below the lower range of diatom concentrations reported from the Southwestern Atlantic (Romero and Hensen, 2002), as well as that reported from surface sediment of the SCS (Wu et al., 2013), from the Canary Island region (Nave et al., 2001), from the Yangtze River estuary of the East China Sea (Cheng et al., 2014) and from the surface sediment of the northeastern Kerguelen Plateau (Armand et al., 2008). However, it is higher than the diatom abundance reported from the surface sediment of the Málaga coast (Bárcena and Abrantes, 1998). On the inter-decadal time scale, the diatom abundance, diatom species diversity, percent planktonic abundance, diatom dissolution, and relative abundance of large-sized diatoms have increased throughout the last century. Generally, the period before and after 1972 can be considered as pre-warm and warm periods, respectively (Xie et al., 2010). The 1970s climate shift left a distinct mark on the SCS recorded by increasing the abundance of upwelling and productivity indicator cosmopolitan species (Zong, 1997; Kuwae et al., 2006) such as T. nitzschioides and P. sulcata, and decreasing eutrophication indicative marine-brackish diatom species such as C. striata and C. stylorum or causing variations in the preservation level or species composition. The diatom species composition and abundance are distinctly different for the periods before and after 1972.
The positive correlation of the relative abundance of P. sulcata and T. nitzschioides with the total absolute diatom abundance clearly shows that P. sulcata and T. nitzschioides contributed significantly to total diatom production (Fig. 4). There was a negative correlation between the relative abundance of C. striata and total diatom abundance, particularly after 1972. This inverse relationship most likely resulted from the appearance of larger and more cosmopolitan diatom species in the late 20th Century.
The strong and positive correlation between diatom abundance and dissolution index explicitly implies that diatom frustule dissolution plays a major role in determining diatom abundance in the SCS. The positive correlation between the abundance of T. nitzschioides and Fi may be due to the valve shape of this taxon (long and narrow) as it is more prone to fragmentation than centric diatoms. It seems that diatom preservation increased distinctly after 1972; however, the increase in total Fi after 1972 might not only indicate improved diatom preservation, but may also reflect species-specific differences in the resistance to dissolution in the sediment. Both diatom preservation and diatom abundance increased after 1972, which coincided with relatively increased abundance of larger pennate and centric cosmopolitan diatoms and a decreased relative contribution of Cyclotella species. The dominance of benthic/ tychoplanktonic and/or large diatom species such as T. nitzschioides, T. longissima, P. sulcata and C. marginato-lineatus suggests that a change in sea conditions such as a higher concentration of dissolved silica and/or lower salinity is associated with the increased rainfall in the region and river inputs that have occurred since the 1970s. This has favored the increased production of those species in the SCS.
The preservation of diatom valves in the coastal SCS has increased erratically for the last century, with a dramatic increase after 2000. The change in the ENSO/PDO or local climate pattern accompanied by increased rainfall and river input most likely had a prominent impact on diatom species composition and abundance. Specifically, increased rainfall and river input 1) enhance the loading of dissolved silica, which favors increased production of diatoms with heavy cell walls, 2) dilute sea water, which reduces frustule dissolution, and 3) increase sedimentation rate and the rapid burial of diatom frustules. Jiang et al. (2004) also found a strong correlation between diatom species assemblages and the hydrographic conditions that are intimately connected with monsoonal variations. The results of the present study show that planktonic diatom abundance increased recently, which could be attributed to the distinct decline of Cyclotella (tychoplanktonic) abundance. The main possible reason for the decline of Cyclotella in the coastal SCS after 1972 is likely the decline of salinity in the coastal SCS because of increased rainfall associated with climate warming in the region. C. striata and C. stylorum are marine-brackish species that are dominant in the western Pacific marginal seas as well (Huang et al., 2009). Furthermore, Huang et al. (2009) reported the association of a higher abundance of C. striata and C. stylorum with high salinity in the coastal SCS.
Inter-decadal variations of the calculated dissolution index also conform to the same pattern, showing a congruence with the increased diatom abundance and percent planktonic abundance, which indicates the increased level of preservation of total diatoms after 1972. This, in turn, implies that environmental conditions become more suitable for frustule preservation and/or that the dissolution resistant species become dominant in the assemblage. The co-occurrence of decreased relative abundance of the most dominant species, C. striata, with increased total diatom abundance after 1972 and the negative correlation between the relative abundance of C. striata and absolute abundance suggests that C. striata has different environmental preferences than the majority of the other species preserved in the assemblage. Moreover, Cyclotella generally has a smaller size than the other dominant species in sediment samples, which further strengthens the assertion that smaller diatoms were selectively removed from the sediment. Taken together, these findings suggest that diatom dissolution plays a significant role in controlling diatom valve abundance. Shemesh et al. (1989) also suggested dissolution can account for the temporal and spatial variations in sedimentary diatom assemblages observed in Southern Ocean sediments.
Owing to the high influence of frustule dissolution on diatom valve composition and concentration, it is possible that Fi is a very important factor that determines the quantity (abundance) and quality (type of species) of preserved diatoms in the sediment of the coastal SCS. Liu et al. (2002) observed that the contribution of smaller diatoms to total phytoplankton was almost equal to larger diatoms in the surface waters of the SCS. However, at lower depths (125 m), the contribution of larger diatoms was higher. Therefore, these authors suggested that the larger phytoplankton/diatoms play a bigger role in the primary production and export flux of particulate organic carbon to the bottom. Almost all species that increased in relative abundance after 1972 are largesized diatoms. In contrast, the species showing decreased abundance are those with relatively weakly silicified frustules and smaller size. Therefore, it is possible to argue that the diatom abundance/ production variation observed before and after 1972 could also have resulted from alteration of the diatom assemblage; however, total organic carbon content (Abate et al., unpublished data) showed increased TOC content after 1972. Taken together, these findings support the hypothesis of a general increase in production after 1972.
Despite the location of the sampling station in the coastal upwelling area, the observed low to moderate diatom valve concentration might be related to the selective dissolution of a few dominant weakly silicified and poorly preserved diatom species. Moreover, the total absence of species such as Skeletonema costatum from the sediment core indicates the occurrence of intense species-specific dissolution of diatom frustules, implying that selective dissolution is one of the major factors that lead to reduced diatom abundance in the sediment core. For example, frequently occurring and bloom-forming fragile and weakly silicified species such as Rhizosolenia spp. and Skeletonema costatum are reported from the Zhujiang River estuary and adjacent coastal SCS water (Yin et al., 2001; Qiu et al., 2010); however, none of these species were observed in the sediment samples. Similarly, Sangiorgi and Donders (2004) discussed the total absence of the remains of diatoms with very fragile frustules (e.g., Chaetoceros spp. and Skeletonema spp.) in the sediment, while they were abundant in surface waters of the North Adriatic Sea, and concluded that this was an indication of opal dissolution. Fragmentation and dissolution of diatom valves are to be expected given that the study site is located in a shallow area experiencing upwelling with frequent periods of mixing and turbulence (Bauer, 2012).
Poor preservation in the sediment is a frequent characteristic of coastal regions in which there is strong upwelling (Nave et al., 2001). Accordingly, Shuman (1978) noted that the counts of intact diatom cells in sediment traps in Dabob Bay along the coast of Washington were lower than diatom crops in the overlaying water column. However, in addition to selective dissolution, heterotrophic decomposition, grazing and transport by current could play a significant role in the disappearance of diatom valves as well (Swann and Mackay, 2006; Ryu et al., 2008). Extremely low abundance of diatom valves (71 valves/g) has previously been reported from the continental shelf of the SCS (Wu et al., 2013). These authors also reported that diatom valve concentration varied significantly from place to place throughout the SCS.
5.2 Emergence of cosmopolitan speciesThe emergence of upwelling of indicative cosmopolitan species, P. sulcata and T. nitzschioides, dramatically increased after 1972, coinciding with increased SST and anthropogenic pressures such as land agriculture, aquaculture and dam construction. The presence of T. nitzschioides indicates increased nutrient supply in response to the coastal upwelling process in combination with some freshwater fluvial inputs (Sancetta, 1982; Van Iperen et al., 1987; Romero and Hebbeln, 2003; Kuwae et al., 2006). T. nitzschioides was also previously reported as a dominant species in the surface sediment (Jiang et al., 2004) and sediment core (Huang et al., 2009) of the SCS, as well as in surface sediment of the northeastern Kerguelen Plateau (Armand et al., 2008). A high abundance of T. nitzschioides was also reported from the Bering and Okhotsk Seas (Sancetta, 1982).
Thus, it is possible to suggest that the dominance of relatively large cosmopolitan and high nutrient indicator diatom species such as P. sulcata and T. nitzschioides (Van Iperen et al., 1987; Gebühr et al., 2009) could be attributed to increased nutrient availability, which is in turn related to climate change and anthropogenic effects. Similarly, previous studies of the Changjiang River estuary of the East China Sea revealed that changes in the nutrient concentrations caused increased P. sulcata and T. nitzschioides abundance, while the abundance of Cyclotella species declined after the 1980s (Cheng et al., 2014).
5.3 The relative abundance of planktonic and benthic diatomsBecause the sampling site is located in the shallow coastal part of the SCS, the dominance of benthic diatom taxa would be expected. Moreover, the observed negative correlation between diatom absolute abundance and percent planktonic abundance in conjunction with very low values of the percent planktonic abundance indicates that the diatom production is significantly influenced by the contribution of benthic diatoms. One reason for the increased percent planktonic abundance since the 1980s is the decline of Cyclotella abundance. Nevertheless, the conjunction of increased percent planktonic abundance with increased absolute diatom abundance since the mid-1980s is probably related to increased rainfall in eastern and southern China and increased human intervention in the coastal areas of the northwestern SCS. These factors further influence the system in different ways, such as: 1) increased rainfall is associated with increased river input and runoff, which in turn enhances input of sediment particulate material that reduces light availability for the algae, especially for the benthos, and 2) nutrient input is enhanced by increasing runoff and river discharge, favoring planktonic diatoms by increasing nutrient concentrations in the open water. These conditions ultimately resulted in reduced benthic diatom production or enhanced planktonic diatoms production. Furthermore, increased input of nutrients from farms and aquaculture contribute to the enhancement of planktonic diatom production.
6 CONCLUSIONThe results of the present study show that the SCS has clearly responded to the late 20th century climate change and anthropogenic impacts. These changes are reflected in the observed inter-decadal changes in total diatom abundance, diatom species composition, diatom dissolution index and percent planktonic abundance. In the SCS, the quantity and quality of diatom valves preserved in the sediment are significantly controlled by environmental variables that dictate the composition of the assemblage and the intensity of dissolution. The occurrence and dominance of upwelling indicator species such as T. nitzschioides and P. sulcata after 1972 clearly indicates that the changes in physico-chemical environmental conditions in the western Pacific marginal seas have induced changes in diatom species composition. Frustule dissolution is one of the major factors that determines diatom species composition and abundance; thus, future studies using diatombased environmental reconstruction should consider diatom dissolution as an important factor. Furthermore, future research describing sediment cores from inshore, mid-shelf and offshore sites could help show the specific effects of diatom dissolution on total diatom abundace and species composition. Since the preservation of diatom valves in the sediment was found to be intimately connected to diatom dissolution, further research should be conducted to integrate the analysis of diatom abundance and species composition in samples collected from the water column, by sediment traps and from the surface sediment to provide better estimates of dissolution and preservation processes.
| Abrantes F, 1988. Diatom assemblages as upwelling indicators in surface sediments off Portugal. Marine Geology, 85(1): 15–39. Doi: 10.1016/0025-3227(88)90082-5 |
| Appleby P G, Oldfield F, 1978. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena, 5(1): 1–8. Doi: 10.1016/S0341-8162(78)80002-2 |
| Armand L K, Crosta X, Quéguiner B, Mosseri J, Garcia N, 2008. Diatoms preserved in surface sediments of the northeastern Kerguelen Plateau. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 55(5-7): 677–692. Doi: 10.1016/j.dsr2.2007.12.032 |
| Bárcena M A, Abrantes F, 1998. Evidence of a highproductivity area off the coast of Málaga from studies of diatoms in surface sediments. Marine Micropaleontology, 35(1-2): 91–103. Doi: 10.1016/S0377-8398(98)00012-7 |
| Bauer A, Radziejewska T, Liang K, Kowalski N, Dellwig O, Bosselmann K, Stark A, Xia Z, Harff J, Böttcher M E, Schulz-Bull D E, Waniek J J, 2013. Regional differences of hydrographical and sedimentological properties in the Beibu Gulf, South China Sea. Journal of Coastal Research(S66): 49–71. |
| Bauer A. 2012. Hydrographical and Biogeochemical Characterization of the Beibu Gulf, South China Sea. der Mathematisch-Naturwissenschaftlichen Fakultät, der Universität Rostock, Rostock. 146p. |
| Belkin I M, 2009. Rapid warming of large marine ecosystems. Progress in Oceanography, 81(1-4): 207–213. Doi: 10.1016/j.pocean.2009.04.011 |
| Bianchi T S, Engelhaupt E, McKee B A, Miles S, Elmgren R, Hajdu S, Savage C, Baskaran M, 2002. Do sediments from coastal sites accurately reflect time trends in water column phytoplankton? A test from Himmerfjärden Bay(Baltic Sea proper). Limnology and Oceanography, 47(5): 1537–1544. Doi: 10.4319/lo.2002.47.5.1537 |
| Boer W, Van den Bergh G, De Haas H, De Stigter H, Gieles R, Van Weering T C, 2006. Validation of accumulation rates in Teluk Banten (Indonesia) from commonly applied 210 Pb models, using the 1883 Krakatau tephra as time marker. Marine Geology, 227(3): 263–277. |
| Brush G S, 2009. Historical land use, nitrogen, and coastal eutrophication:a paleoecological perspective. Estuaries and Coasts, 32(1): 18–28. Doi: 10.1007/s12237-008-9106-z |
| Cai W J, Dai M H, Wang Y C, Zhai W D, Huang T, Chen S T, Zhang F, Chen Z Z, Wang Z H, 2004. The biogeochemistry of inorganic carbon and nutrients in the Pearl River estuary and the adjacent Northern South China Sea. Continental Shelf Research, 24(12): 1301–1319. Doi: 10.1016/j.csr.2004.04.005 |
| Cheng F J, Yu Z M, Song X X, 2014. Long-term changes in sedimentary diatom assemblages and their environmental implications in the Changjiang (Yangtze) River estuary, China. Chinese Journal of Oceanology and Limnology, 32(1): 155–161. Doi: 10.1007/s00343-014-3133-3 |
| Cheng Z D, Gao Y H, Liu S C, Wang D Z, Chen C P, Liang J R, Qi Y Z. 2013. Flora Algarum Marinarum Sinicarum: Tomus V. Bacillariophyta, No. Ⅱ. Pennatae Ⅰ, Diatomales, Achnanthales, Phaeodactylales, Eunotiales. Science Press, Beijing. 137p. (in Chinese) |
| Finkelstein S A, Gajewski K, 2007. A palaeolimnological record of diatom-community dynamics and late-Holocene climatic changes from Prescott Island, Nunavut, central Canadian Arctic. The Holocene, 17(6): 803–812. Doi: 10.1177/0959683607080521 |
| Gebühr C, Wiltshire K H, Aberle N, van Beusekom J E, Gerdts G, 2009. Influence of nutrients, temperature, light and salinity on the occurrence of Paralia sulcata at Helgoland Roads, North Sea. Aquatic Biology, 7(3): 185–197. |
| Giani M, Djakovac T, Degobbis D, Cozzi S, Solidoro C, Umani S F, 2012. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuarine, Coastal and Shelf Science, 115: 1–13. Doi: 10.1016/j.ecss.2012.08.023 |
| Guo Y, Qian S, 1984. Flora Algarum Marinarum Sinicarum(Tomus Ⅴ) Bacillariophyta (No. Ⅰ) Centricea. Science Press, Beijing, China. |
| Harrison P J, Yin K, Lee J H W, Gan J P, Liu H B, 2008. Physical-biological coupling in the Pearl River Estuary. Continental Shelf Research, 28(12): 1405–1415. Doi: 10.1016/j.csr.2007.02.011 |
| Hill M O, Bunce R G H, Shaw M W, 1975. Indicator Species Analysis, a divisive polythetic method of classification, and its application to a survey of native pinewoods in scotland. The Journal of Ecology, 63(2): 597–613. Doi: 10.2307/2258738 |
| Hill M O, Šmilauer P. 2005. TWINSPAN for Windows Version 2. 3. Software and user Guide. Centre for Ecology and Hydrology, University of South Bohemia, Huntingdon, Ceske Budejovice. |
| Hinder S L, Hays G C, Edwards M, Roberts E C, Walne A W, Gravenor M B, 2012. Changes in marine dinoflagellate and diatom abundance under climate change. Nature Climate Change, 2(4): 271–275. Doi: 10.1038/nclimate1388 |
| Hu J F, Sun X S, Peng P A, Zhang G, Chivas A R, 2009. Spatial and temporal variation of organic carbon in the northern South China Sea revealed by sedimentary records. Quaternary International, 206(1-2): 46–51. Doi: 10.1016/j.quaint.2009.02.016 |
| Hu J Y, Kawamura H, Tang D L, 2003. Tidal front around the Hainan Island, northwest of the South China Sea. Journal of Geophysical Research:Oceans, 108(C11): 3342. Doi: 10.1029/2003JC001883 |
| Huang L M, Jian W J, Song X Y, Huang X P, Liu S, Qian P Y, Yin K D, Wu M, 2004. Species diversity and distribution for phytoplankton of the Pearl River estuary during rainy and dry seasons. Marine Pollution Bulletin, 49(7-8): 588–596. Doi: 10.1016/j.marpolbul.2004.03.015 |
| Huang Y, Jiang H, Svante B, Li T G, Lv H Y, Ran L H, 2009. Surface sediment diatoms from the western Pacific marginal seas and their correlation to environmental variables. Chinese Journal of Oceanology and Limnology, 27(3): 674–682. Doi: 10.1007/s00343-009-9211-2 |
| Huh C A, Su C C, 1999. Sedimentation dynamics in the East China Sea elucidated from 210Pb, 137Cs and 239, 240Pu. Marine Geology, 160(1-2): 183–196. Doi: 10.1016/S0025-3227(99)00020-1 |
| Itoh S, Yasuda I, Nishikawa H, Sasaki H, Sasai Y, 2009. Transport and environmental temperature variability of eggs and larvae of the Japanese anchovy (Engraulis japonicus) and Japanese sardine (Sardinops melanostictus) in the western North Pacific estimated via numerical particle-tracking experiments. Fisheries Oceanography, 18(2): 118–133. Doi: 10.1111/fog.2009.18.issue-2 |
| Jiang H, Zheng Y L, Ran L H, Seidenkrantz M S, 2004. Diatoms from the surface sediments of the South China Sea and their relationships to modern hydrography. Marine Micropaleontology, 53(3-4): 279–292. Doi: 10.1016/j.marmicro.2004.06.005 |
| Jin D X, Cheng Z D, Lin J M, Liu S C. 1985. The marine benthic diatoms in China. China Ocean Press, SpringerVerlag, Berlin Heidelberg, Beijng. 313p. |
| Klais R, Tamminen T, Kremp A, Spilling K, Olli K, 2011. Decadal-scale changes of dinoflagellates and diatoms in the anomalous Baltic Sea spring bloom. PLoS One, 6(6): e21567. Doi: 10.1371/journal.pone.0021567 |
| Kuwae M, Yamashita A, Hayami Y, Kaneda A, Sugimoto T, Inouchi Y, Amano A, Takeoka H, 2006. Sedimentary records of multidecadal-scale variability of diatom productivity in the Bungo Channel, Japan, associated with the Pacific Decadal Oscillation. Journal of Oceanography, 62(5): 657–666. Doi: 10.1007/s10872-006-0084-0 |
| Lee H S, Yamashita T, Mishima T, 2012. Multi-decadal variations of ENSO, the Pacific Decadal Oscillation and tropical cyclones in the western North Pacific. Progress in Oceanography, 105: 67–80. Doi: 10.1016/j.pocean.2012.04.009 |
| Lee V, Olsen S, 1985. Eutrophication and management initiatives for the control of nutrient inputs to Rhode Island coastal lagoons. Estuaries, 8(2): 191–202. Doi: 10.2307/1352200 |
| Li F, Lin J Q, Liang Y Y, Gan H Y, Zeng X Y, Duan Z P, Liang K, Liu X, Huo Z H, Wu C H, 2014. Coastal surface sediment quality assessment in Leizhou Peninsula (South China Sea) based on SEM-AVS analysis. Marine Pollution Bulletin, 84(1-2): 424–436. Doi: 10.1016/j.marpolbul.2014.04.030 |
| Liu K K, Chao S Y, Shaw P T, Gong G C, Chen C C, Tang T Y, 2002. Monsoon-forced chlorophyll distribution and primary production in the South China Sea:observations and a numerical study. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 49(8): 1387–1412. Doi: 10.1016/S0967-0637(02)00035-3 |
| Liu Y, Peng Z C, Chen T G, Wei G J, Sun W D, Sun R Y, He J F, Liu G J, Chou C L, Zartman R E, 2008. The decline of winter monsoon velocity in the South China Sea through the 20th century:evidence from the Sr/Ca records in corals. Global and Planetary Change, 63(1): 79–85. Doi: 10.1016/j.gloplacha.2008.05.003 |
| Menge B A, Chan F, Nielsen K J, Lorenzo E D, Lubchenco J, 2009. Climatic variation alters supply-side ecology:impact of climate patterns on phytoplankton and mussel recruitment. Ecological Monographs, 79(3): 379–395. Doi: 10.1890/08-2086.1 |
| Miller K R, Chapman M R, Andrews J E, Koç N, 2011. Diatom phytoplankton response to Holocene climate change in the Subpolar North Atlantic. Global and Planetary Change, 79(3-4): 214–225. Doi: 10.1016/j.gloplacha.2011.03.001 |
| Montes-Hugo M, Doney S C, Ducklow H W, Fraser W, Martinson D, Stammerjohn S E, Schofield O, 2009. Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic peninsula. Science, 323(5920): 1470–1473. Doi: 10.1126/science.1164533 |
| Nave S, Freitas P, Abrantes F, 2001. Coastal upwelling in the Canary Island region:spatial variability reflected by the surface sediment diatom record. Marine Micropaleontology, 42(1-2): 1–23. Doi: 10.1016/S0377-8398(01)00008-1 |
| Oey L Y, Chang M C, Chang Y L, Lin Y C, Xu F H, 2013. Decadal warming of coastal China Seas and coupling with winter monsoon and currents. Geophysical Research Letters, 40(23): 6288–6292. Doi: 10.1002/2013GL058202 |
| Perren B B, Douglas M S V, Anderson N J, 2009. Diatoms reveal complex spatial and temporal patterns of recent limnological change in West Greenland. Journal of Paleolimnology, 42(2): 233–247. Doi: 10.1007/s10933-008-9273-8 |
| Qiu D J, Huang L M, Zhang J L, Lin S J, 2010. Phytoplankton dynamics in and near the highly eutrophic Pearl River Estuary, South China Sea. Continental Shelf Research, 30(2): 177–186. Doi: 10.1016/j.csr.2009.10.015 |
| Renberg I, 1990. A procedure for preparing large sets of diatom slides from sediment cores. Journal of Paleolimnology, 4(1): 87–90. |
| Romero O, Hebbeln D, 2003. Biogenic silica and diatom thanatocoenosis in surface sediments below the PeruChile Current:controlling mechanisms and relationship with productivity of surface waters. Marine Micropaleontology, 48(1-2): 71–90. Doi: 10.1016/S0377-8398(02)00161-5 |
| Romero O, Hensen C, 2002. Oceanographic control of biogenic opal and diatoms in surface sediments of the Southwestern Atlantic. Marine Geology, 186(3-4): 263–280. Doi: 10.1016/S0025-3227(02)00210-4 |
| Rosenberg R, Elmgren R, Fleischer S, Jonsson P, Persson G, Dahlin H, 1990. Marine eutrophication case studies in sweden. Ambio, 19(3): 102–108. |
| Round F E, Crawford R M, Mann D G, 1990. The Diatoms:Biology & Morphology of the Genera. Cambridge University Press, Cambridge. |
| Rühland K, Paterson A M, Smol J P, 2008. Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Global Change Biology, 14(11): 2740–2754. |
| Ryu E, Lee S J, Yang D Y, Kim J Y, 2008. Paleoenvironmental studies of the Korean peninsula inferred from diatom assemblages. Quaternary International, 176-177: 36–45. Doi: 10.1016/j.quaint.2007.05.015 |
| Ryves D B, Battarbee R W, Fritz S C, 2009. The dilemma of disappearing diatoms:incorporating diatom dissolution data into palaeoenvironmental modelling and reconstruction. Quaternary Science Reviews, 28(1-2): 120–136. Doi: 10.1016/j.quascirev.2008.08.021 |
| Ryves D B, Juggins S, Fritz S C, Battarbee R W, 2001. Experimental diatom dissolution and the quantification of microfossil preservation in sediments. Palaeogeography, Palaeoclimatology, Palaeoecology, 172(1-2): 99–113. Doi: 10.1016/S0031-0182(01)00273-5 |
| Sancetta C, 1982. Distribution of diatom species in surface sediments of the Bering and Okhotsk seas. Micropaleontology, 28(3): 221–257. Doi: 10.2307/1485181 |
| Sangiorgi F, Donders T H, 2004. Reconstructing 150 years of eutrophication in the north-western Adriatic Sea (Italy) using dinoflagellate cysts, pollen and spores. Estuarine, Coastal and Shelf Science, 60(1): 69–79. Doi: 10.1016/j.ecss.2003.12.001 |
| Shemesh A, Burckle L H, Froelich P N, 1989. Dissolution and preservation of Antarctic diatoms and the effect on sediment thanatocoenoses. Quaternary Research, 31(2): 288–308. Doi: 10.1016/0033-5894(89)90010-0 |
| Shuman F R. 1978. The fate of phytoplankton chlorophyll in the euphotic zone: Washington coastal waters. University of Washington, Washington. 250p. |
| Su C C, Huh C A, 2002. 210Pb, 137Cs and 239, 240Pu in East China Sea sediments:sources, pathways and budgets of sediments and radionuclides. Marine Geology, 183(1-4): 163–178. Doi: 10.1016/S0025-3227(02)00165-2 |
| Swann G E A, Mackay A W, 2006. Potential limitations of biogenic silica as an indicator of abrupt climate change in Lake Baikal, Russia. Journal of Paleolimnology, 36(1): 81–89. Doi: 10.1007/s10933-006-0005-7 |
| Tang D L, Kawamura H, Lee M A, Van Dien T, 2003. Seasonal and spatial distribution of chlorophyll-a concentrations and water conditions in the Gulf of Tonkin, South China Sea. Remote Sensing of Environment, 85(4): 475–483. Doi: 10.1016/S0034-4257(03)00049-X |
| Trenberth K E, Hurrell J W, 1994. Decadal atmosphere-ocean variations in the Pacific. Climate Dynamics, 9(6): 303–319. Doi: 10.1007/BF00204745 |
| Treppke U F, Lange C B, Donner B, Fischer G, Ruhland G, Wefer G, 1996. Diatom and silicoflagellate fluxes at the Walvis Ridge:an environment influenced by coastal upwelling in the Benguela system. Journal of Marine Research, 54(5): 991–1016. Doi: 10.1357/0022240963213655 |
| Van Iperen J M, Van Weering T C E, Jansen J H F, Van Bennekom A J, 1987. Diatoms in surface sediments of the Zaire deep-sea fan (SE Atlantic Ocean) and their relation to overlying water masses. Netherlands Journal of Sea Research, 21(3): 203–217. Doi: 10.1016/0077-7579(87)90013-5 |
| Wang B, Huang F, Wu Z W, Yang J, Fu X H, Kikuchi K, 2009. Multi-scale climate variability of the South China Sea monsoon:a review. Dynamics of Atmospheres and Oceans, 47(1-3): 15–37. Doi: 10.1016/j.dynatmoce.2008.09.004 |
| Wang S L, Xu X R, Sun Y X, Liu J L, Li H B, 2013. Heavy metal pollution in coastal areas of South China:a review. Marine Pollution Bulletin, 76(1-2): 7–15. Doi: 10.1016/j.marpolbul.2013.08.025 |
| Weckström K, Korhola A, Weckström J, 2007. Impacts of eutrophication on diatom life forms and species richness in coastal waters of the Baltic Sea. Ambio, 36(2): 155–160. Doi: 10.1579/0044-7447(2007)36[155:IOEODL]2.0.CO;2 |
| Wu R, Gao Y H, Fang Q, Chen C P, Lan B B, Sun L, Lan D Z, 2013. Diatom assemblages in surface sediments from the South China Sea as environmental indicators. Chinese Journal of Oceanology and Limnology, 31(1): 31–45. Doi: 10.1007/s00343-013-1181-8 |
| Xie S P, Du Y, Huang G, Zheng X T, Tokinaga H, Hu K M, Liu Q Y, 2010. Decadal shift in El Niño influences on IndoWestern Pacific and East Asian climate in the 1970s. Journal of Climate, 23(12): 3352–3368. Doi: 10.1175/2010JCLI3429.1 |
| Yin K D, Qian P Y, Wu M C S, Chen J C, Huang L M, Song X Y, Jian W J, 2001. Shift from P to N limitation of phytoplankton growth across the Pearl River estuarine plume during summer. Marine Ecology Progress Series, 221: 17–28. Doi: 10.3354/meps221017 |
| Zachariasse W J, Riedel W R, Sanfilippo A, Schmidt R R, Brolsma M J, Schrader H J, Gersonde R, Drooger M M, Broekman J A. 1978. Micropaleontological counting methods and techniques: an exercise on an eight metres section of the lower Pliocene of Capo Rossello, Sicily. Utrecht Micropaleontological Bulletins, Vol. 17. Utrecht University, Utrecht. |
| Zhao H, Tang D L, 2007. Effect of 1998El Niño on the distribution of phytoplankton in the South China Sea. Journal of Geophysical Research, 112(C2): C02017. |
| Zong Y Q, 1997. Implications of Paralia sulcata abundance in Scottish isolation basins. Diatom Research, 12(1): 125–150. Doi: 10.1080/0269249X.1997.9705407 |
 2017, Vol. 35
2017, Vol. 35


