Institute of Oceanology, Chinese Academy of Sciences
Article Information
- González GUTIÉRREZ Sergio(González GUTIÉRREZSergio), SARMA S.S.S.(SARMAS.S.S.), NANDINI S.(NANDINIS.)
- Seasonal variations of rotifers from a high altitude urban shallow water body, La Cantera Oriente (Mexico City, Mexico)
- Chinese Journal of Oceanology and Limnology, 35(6): 1387-1397
- http://dx.doi.org/10.1007/s00343-017-6101-x
Article History
- Received Apr. 8, 2016
- accepted in principle May. 16, 2016
- accepted for publication Sep. 1, 2016
2 Laboratory of Aquatic Zoology, Division of Research & Postgraduate Studies, National Autonomous University of Mexico, Campus Iztacala, Av. de los Barrios No. 1, AP 314, Los Reyes, Tlalnepantla, State of Mexico 54090, Mexico
Limnological research in Mexico has been increasing over the last two decades because of the realization that many freshwater bodies remain unexplored (Alcocer and Bernal-Brooks, 2010). Mexico has approximately 14 000 reservoirs, of which over 80% are less than ten hectares area (De la Lanza and García-Calderón, 2002). Studies on large, deep Mexican lakes, which are fewer than 50 in number, has contributed to the bulk of the national limnological data (Alcocer and Bernal-Brooks, 2010). Studies on the zooplankton of shallow lakes have been few and usually sporadic; long-term studies on shallow lakes such as Lake Xochimilco are not common (Nandini et al., 2005; García et al., 2009). Shallow lakes often have a greater variety of habitats and thus permit a greater diversity of zooplankton, which is composed mainly of protozoans, rotifers and crustaceans such as copepods and cladocerans (Moss, 2010). They often have a large variety of aquatic vegetation which in turn offers protection to zooplankton against fish predation (Gulati et al., 1990). Typically, Mexican shallow lakes are dominated by rotifers since crustaceans are vulnerable to predation around the year by fish (Zambrano et al., 1998; Contreras et al., 2009). Rotifers from shallow lakes get benefit not only from reduced competition from crustaceans but also feed on the detrital resources which are generated by the decomposing macrophytes (Wetzel, 2001). Therefore, it is not surprising that more than 100 rotifer species have been reported from point collections in just four Mexican shallow water bodies (Sarma and ElíasGutiérrez et al., 1999).
Rapid population growth and industrialization in major cities around the world take a toll on freshwater bodies with changes in water quality or begin to shrink considerably (Brown, 2008; Li et al., 2014). In spite of being a megacity, Mexico City still contains a few shallow lakes such as Lakes Xochimilco, Texcoco and La Cantera Oriente. La Cantera Oriente (altitude 2 270 m above sea level) is located within the Ecological Reserve of San Angel Pedregal zone of the Mexico City. It is an important heritage site with great biological diversity (administratively known as REPSA) and is presently protected and managed by the administration of the National Autonomous University of Mexico (Hortelano-Moncada et al., 2009). In order to ensure the conservation of biological heritage present in the REPSA, an inventory of species is available which contains algae, ciliates, insects, crustaceans, fish, amphibians, reptiles and birds (Lot, 2007). However, there are no records of rotifer species found in these ponds. Rotifers are good indicators of water quality (Sládeček, 1983) and hence it is useful to have an inventory of species and study their diversity over time. This information is needed to define management plans and appropriate conservation actions for the protection of this area.
For some high altitude water bodies exhaustive rotifer faunal listings are available but seasonal studies are few. For example, taxonomic information on rotifers is documented for many water bodies in Central Mexico, including descriptions of new records (Sarma and Elías-Gutierrez, 2000) but the seasonal variations of plankton is documented only in a few lakes and reservoirs such as Lake Xochimilco (Nandini et al., 2005) and the Valle de Bravo reservoir (Ramírez et al., 2002). In temporary water bodies, zooplankton sampling is done only during the period when water is present (see Kuczyńska-Kippen et al., 2013). However in permanent water bodies seasonal studies are also needed because they indicate how zooplankton species in nature vary both in terms of taxonomic diversity and species abundance, depending on the physico-chemical factors through the seasons. For example, Sarma et al. (1996) sampled two high-altitude (4 690 m above sea level) water bodies (La Luna and El Sol) in Central Mexico and reported a total of 35 rotifer species, but no taxon was common in both lakes. However, monthly collections for one year from the same water bodies revealed as many as 14 species in common for both lakes (DimasFlores et al., 2008). Therefore seasonal studies that describe patterns in rotifer community composition are better than sporadic collections.
The aim of this work was therefore to quantify the seasonal changes in the richness and density of rotifers in relation to selected physico-chemical variables during an annual cycle (2013–2014).
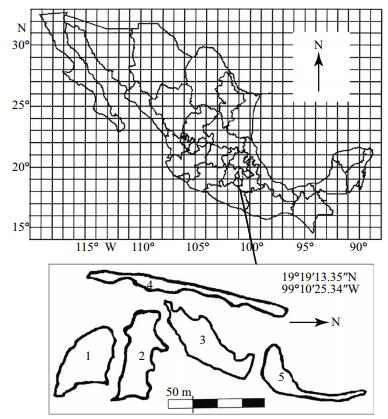
2 MATERIAL AND METHOD 2.1 Study siteThe rocks in the area of La Cantera Oriente (Mexico City, Mexico) (19°19′13.35″N and 99°10′25.34″W) were formed from the lava of Xitle volcano and are of basaltic type (Cervantes and Wallace, 2003). The volcanic rocks from this region were used for the construction of our University City and other surrounding urban development towards end of the first half of the twentieth century. These activities resulted in the excavation of rocks and the subsequent formation of ponds due to an underground spring and filtered water run-off. All five interconnected ponds are of different depths, but usually up to 6 m in maximum depth. These water bodies occupy an area of 11 906 m2 which represents about 14% of the total Ecological Reserve of REPSA (Lot, 2007). These are shallow ponds and are locally known as the lake. These ponds are located at an altitude of 2 270 m above sea level. The ponds have fish species belonging to the families Goodeidae and Cyprinidae (Pérez, 2009).
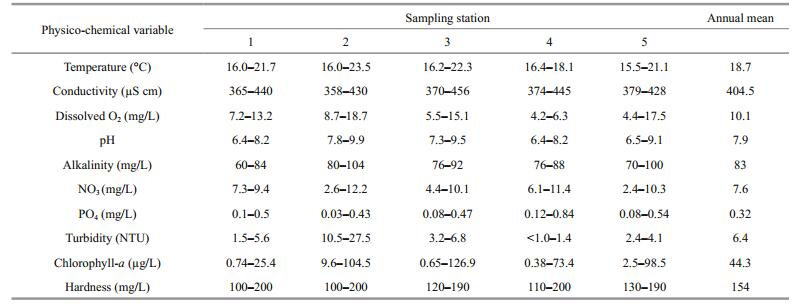
2.2 Rotifer collection and measurement of physicochemical parametersZooplankton collections were carried out monthly for one year (September 2013–August 2014) from each of the five ponds (Fig. 1). Zooplankton samples were obtained by filtering 80 L of surface water from the ponds through a plankton net of 50-μm mesh size, concentrated to 180 mL volume and immediately fixed in 4% formalin. Simultaneously, selected physico-chemical variables (dissolved oxygen and oxygen saturation percentage, temperature, pH and conductivity, alkalinity, nitrates and phosphates, chlorophyll-a and turbidity) were measured (American Public Health Association et al., 2012).
|
| Figure 1 Map of sampling stations from La Cantera Oriente (Mexico City, Mexico) Numbers 1 to 5 indicate the sampling stations of the five ponds. From each pond the zooplankton samples were collected from different points and then pooled them into a single sample. |
The rotifers were identified, as far as possible to the species level, using standard taxonomic keys (Koste, 1978; Segers, 1995; Wallace et al., 2016). The species specific densities of the rotifers were quantified in 3 aliquots samples of 1 ml of each using a Sedgewick-Rafter chamber and an inverted microscope (Wetzel and Likens, 2000). The data on the physico-chemical variables and the speciesspecific densities of rotifers were subjected to canonical correspondence analysis (CCA) using CANOCO program ver. 4.5 to determine the influence of different variables on the rotifer densities. ShannonWiener species diversity index was derived using the following formula (Krebs, 1989):
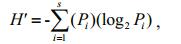
where H'=species diversity index (bits/ind.); Pi=proportion of numerical density of each species within the total density of all species present in the sample.
In order to determine the trophic status of the water bodies we used the index of Brachionus to Trichocerca ratio or QB/T (Sládeček, 1983). We also used other recent works involving the total rotifer density as an index of trophic status of freshwater bodies (EjsmontKarabin, 2012).
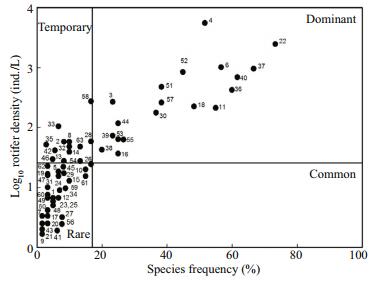
3 RESULT 3.1 Physico-chemical characteristics and rotifer species diversityData on the selected physico-chemical variables pooled from different sites of the La Cantera Oriente are presented in Table 1. Qualitative analysis of the zooplankton samples collected during the study period from different sites yielded 68 rotifer species which represented 24 genera spreading over 15 families (Table 2). The most frequently encountered species were Brachionus calyciflorus, B. quadridentatus, Polyarthra vulgaris, Lecane closterocerca and Keratella cochlearis. On the other hand, species that appeared rarely in these ponds were: Sinantherina semibullata, Pleurotrocha petromyzon, Lecane pyriformis, L. unguitata, Lophocharis oxysternon, L. salpina and Cephalodella remanei. Data on the frequency of rotifer species in relation to their log density, i.e., Preston plot, revealed that as many as 30% of the rotifer species became dominant throughout the year and among them were L. closterocerca, C. uncinata, C. obtusa, L. patella and K. cochlearis. In addition, more than 50% of the rotifers were infrequently present throughout the year (Fig. 2).
|

|
|
| Figure 2 Data on the frequency of rotifer species in relation to their log density 1. Brachionus angularis; 2. B. budapestinensis; 3. B. calyciflorus; 4. B. quadridentatus; 5. Keratella americana; 6. K. cochlearis; 7. K. tropica; 8. Platyias quadricornis; 9. Collotheca sp; 10. Cephalodella catellina; 11. C. gibba; 12. C. remanei; 13. C. ventripes; 14. Eosphora thoides; 15. Pleurotrocha petromyzon; 16. Dicranophorus grandis; 17. Euchlanis calpidia; 18. E. dilatata; 19. Filinia longiseta; 20. Lecane aculeata; 21. L. bifurca; 22. L. closterocerca; 23. L. curvicornis; 24. L. decipiens; 25. L. flexilis; 26. L. furcata; 27. L. hamata; 28. L. inermis; 29. L. luna; 30. L. lunaris; 31. L. nana; 32. L. pyriformis; 33. L. quadridentata; 34. L. stichaea; 35. L. unguitata; 36. Colurella obtusa; 37. C. uncinata; 38. Lepadella acuminata; 39. L. ovalis; 40. L. patella; 41. L. rhomboides; 42. L. triba; 43. L. triptera; 44. Squatinella mutica; 45. Limnias ceratophylli; 46. L. melicerta; 47. Sinantherina semibullata; 48. Lophocharis oxysternon; 49. L. salpina; 50. Mytilina mucronata; 51. M. ventralis; 52. Polyarthra vulgaris; 53. Proales decipiens; 54. Testudinella mucronata; 55. T. patina; 56. Trichocerca elongata; 57. T. porcellus; 58. T. pusilla; 59. T. ruttneri; 60. T. similis; 61. T. weberi; 62. Trichotria pocillum; 63. T. tetractis. Five other species not included due to extremely rare occurrence (one specimen each). |
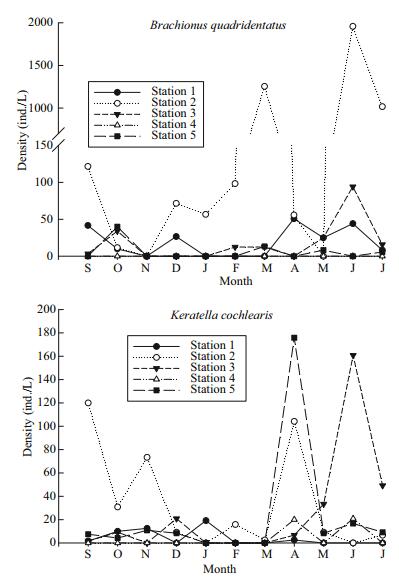
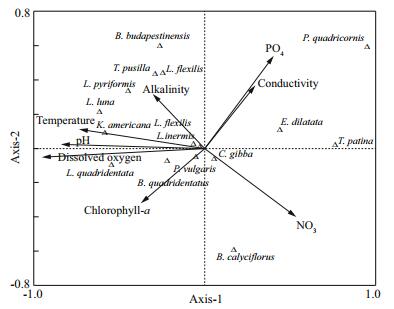
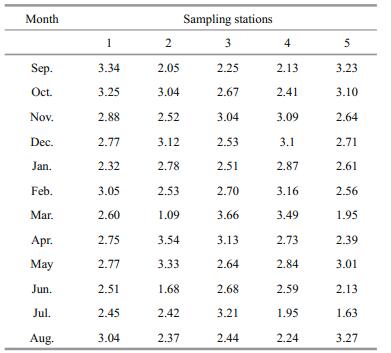
Changes in the densities of the four common rotifer species (Brachionus quadridentatus, Keratella cochlearis, Lecane closterocerca and Lepadella patella) are presented in Figs. 3 and 4. In general, all the four species showed higher densities at site 2. The peak densities for B. quadridentatus (ca. 2 000 ind./L), L. closterocerca (ca. 1 000 ind./L), K. cochlearis (ca. 180 ind./L), and for L. patella (ca. 90 ind./L) were observed during the summer months (May–July). Regardless of the sampling period, all the rotifers generally had the lowest densities at site 5. Canonical correspondence analysis of the relationship between some physico-chemical variables and the rotifer species showed that Platyias quadricornis was closely related to the quantity of phosphates available in the environment. On the other hand Lecane pyriformis, L. flexilis, Trichocerca pusilla and Brachionus budapestinensis were strongly influenced by alkalinity. Brachionus quadridentatus was positively correlated with chlorophyll-a, while Lecane quadridentata preferred areas with high levels of dissolved oxygen (Fig. 5). Shannon-Wiener species diversity index ranged from 1.1 to 3.7 bits/ind., depending on the sampling station and the period of sampling. In general, during the months of April and May, the average species diversity index of all the sampling stations was about 2.9 bits/ind. (Table 3).
|
| Figure 3 Seasonal changes in the densities (ind./L) of Brachionus quadridentatus and Keratella cochlearis from La Cantera Oriente during 2013–2014 |

|
| Figure 4 Seasonal changes in the densities (ind./L) of Lecane closterocerca and Lepadella patella from La Cantera Oriente during 2013–2014 |
|
| Figure 5 Canonical Correspondence Analysis for the relationship between selected physicochemical variables and rotifer species from the ponds of La Cantera Oriente, Mexico City (Mexico) during 2013–2014 |
|
A large set of data on the basic limnology of many different kinds of water bodies, rivers, lakes, ponds and temporary water bodies in Mexico is available (Alcocer and Bernal-Brooks, 2010). However, many of them are just based on one-time-sampling carried out during contrasting periods (e.g., winter vs summer collections). In addition, many studies do not identify and quantify rotifers in zooplankton collections because the interest of most limnological research is generally on functional groups rather than from a taxonomic point of view. The present work is one of the few aimed at understanding the seasonal changes in the rotifer diversity from a high altitude shallow water body. In spite of the ecological importance of shallow lakes, such as higher biodiversity and nutrient dynamics (Moss, 2010), researches on these water bodies from Mexico are scarce since these are thought to be poor sources for commercial activities such as harvesting fish or aquaculture. La Cantera Oriente is a man-made shallow water body largely neglected by limnologists as it is of little importance to fishery biologists since it harbors few fish species and almost no commercial (e.g., fishing) or recreational activity (e.g., boating) are permitted (Lot, 2007). Because of the uneven floor, the maximum depth of this water body varies from 0.5 to 6.0 m depending on the zone. The lake is fed by rainwater, wastewater and a few underground springs. Due to this, the water body has high nutrient levels during certain months of the year.
The data on the seasonal variations of the selected physico-chemical variables in this study agree in general with those reported in this region (De la Lanza-Espino and García-Calderón, 2002). For example, Nandini et al. (2005) have mentioned that the Lake Xochimilco has temperature, dissolved oxygen, pH, nitrates and phosphates in the range of 14–22℃, 1–15 mg/L, 7–9, 3–8 mg/L, and 2–4 mg/L, respectively.
Earlier ecological works from this water body dealt with sporadic collections of crustaceans and other arthropods but no rotifers were mentioned (Lot, 2007). This is the first comprehensive study on the diversity rotifers from this shallow water body. We were able to observe more than 60 rotifer species during one-year sampling. In the Ethiopian lakes located at 2 800 m above sea level, Degefu et al. (2014) recorded only 11 rotifer species, while in the present high-altitude water body we found much higher rotifer diversity. We also recorded some rare rotifer species in Mexican waters such as Eosphora thoides and Lecane bifurca (Sarma and ElíasGutiérrez, 1999, 2000). Most Mexican high altitude freshwater bodies have a rotifer species richness of up to 30 species and in rare cases higher than 70 (Sarma and Manuel, 1998). For example, in the Valle de Bravo reservoir, a high altitude drinking water reservoirs (about 1 830 m above sea level), the rotifer diversity was about 30 species (Nandini et al., 2008). For the shallow water bodies of comparable altitude, the rotifer diversity appears to be higher. For example, about 60 rotifer species have been reported from the shallow water body the lake Xochimilco (altitude 2 200 m above sea level) (Flores-Burgos et al., 2003; Nandini et al. 2005).
Among the rotifer genera recorded from La Cantera Oriente, Lecane was the most diverse. Lecane is the second most diverse genus in Rotifera (Segers, 1995). In Mexican waters too this genus is more diverse than many other genera of rotifers (Sarma et al., 2009; Vázquez-Sánchez et al., 2014). The distribution of the genus Brachionus in the high altitude water bodies of Mexico is intriguing. Brachionus is a warm-water genus (Xi and Huang, 2004) and hence its presence at low temperatures ( < 15℃) in high-altitude water bodies is surprising (Koste, 1978; Wallace et al., 2006). For example, in Valle de Bravo, Brachionus is poorly represented (only two species: JiménezContreras et al., 2009). On the other hand, Lake Xochimilco, with comparable altitude has as many as 13 species of Brachionus (Flores-Burgos et al., 2003). In the present study from La Cantera Oriente we encountered only four species. Water depth is an important factor determining the occurrence of Brachionus in high altitude ponds (Claps et al., 2011). The Valle de Bravo reservoir has a depth of 38 m (Merino-Ibarra et al., 2008) while lake Xochimilco and La Cantera Oriente are < 6 m deep (Lot, 2007). Yet, in another high-altitude shallow (mean depth: < 5 m) water body, Chimaliapan (a Ramsar site, altitude 2 560 m above sea level, Zepeda-Gómez et al., 2012), Brachionus was represented by 6 species (García-García et al., 2012). Therefore, the distribution of Brachionus in high altitude water bodies in Mexico requires further study. In addition, the conventional classification of rotifers into planktonic and semiplanktonic (Pontin, 1978; Koste, 1978) does not strictly apply for the shallow lakes (Nandini et al., 2005; Pociecha et al., 2015) because plankton collections made from the open waters of La Cantera Oriente contained several non-planktonic species including the dominant genus Lecane. In addition, non-planktonic rotifer genera such as Lecane and Lepadella are also occasionally found in planktonic collections even in deep lakes. For example, in Laguna Petén-Itza, a deep Guatemalan lake (40 m, max. depth), García-Morales and Elías-Gutiérrez (2007) reported as many as 17 species of Lecane and 3 species of Lepadella. Rotifer species of volcanic crater lakes at or near sea level may have genera such as Anuraeopsis, Brachionus, Polyarthra and Trichocerca. For example, Sichrowsky et al. (2014) have shown that the volcanic crater lakes of Uvea Island have 17 rotifer taxa including 3 species of Trichocerca (T. chattoni, T. pusilla and T. tenuior) and one species each from Anuraeopsis, Brachionus and Polyarthra. Ejsmont-Karabin and KuczyńskaKippen (2001) have noted that most urban waters in Poland contain Brachionus angularis, Keratella cochlearis, Colurella uncinata, Lecane closterocerca and Lepadella patella. In this present work too, we observed that all these rotifer species were common, suggesting that they possibly tolerate a wide range of physico-chemical factors in nature.
The trophic status of the water bodies can be assessed using different methods: chemical constituents (e.g., phosphate levels) and occurrence (absence or presence) of certain biota (e.g., bioindicators) or their densities (e.g., Secchi transparency) or their pigments (e.g., chlorophyll a) (Wetzel, 2001; Moss, 2010). The Redfield ratio for this shallow water body could not be derived as we did not measure total nitrogen and phosphorus levels. However, the chlorophyll-a levels for most part of the year represent a eutrophic condition (Wetzel, 2001). Sládeček (1983) developed an index of Brachionus to Trichocerca ratio or QB/T, for determining the trophic status of freshwater bodies. When the ratio is < 1, it indicates oligotrophic condition, 1–2 mesotrophic and > 2 the eutrophic level. In this study, the QB/T ratio (0.8) for this water body indicates an oligotrophic level. It is also not reliable in the Valle de Bravo reservoir which is meso-to eutrophic but has a higher number of Trichocerca than Brachionus species (Ramírez et al., 2002) and further studies are needed to ascertain the feasibility of applying the QB/T ratio in high altitude tropical waters. Because of the difficulty in identification, the use of rotifers as indicators of trophic status in tropical and subtropical regions of the world is still limited (Snell and Joaquim-Justo, 2007). There are methods using the total rotifer density as a measure of trophic status of a given water body. It is known that in eutrophic waters, rotifer density is high but the diversity is low. On the other hand, in most oligotrophic lakes and ponds, the rotifer density is low (Wallace et al., 2006). Therefore, independent of species diversity, if the total density of rotifers from a given water body is < 500 ind./L, then it reflects an oligotrophic condition, while 500– 1 000 ind./L is mesotrophic, 1 000–2 500 ind./L is eutrophic and 3 000–4 000 ind./L is hypertrophic (Ejsmont-Karabin, 1995; May and O'Hare, 2005; Ejsmont-Karabin, 2012). In this work, the mean total density of rotifers from all measured sites for most months was < 400 ind./L. However, in the month of July, it exceeded this value but was below < 1 000 ind./L. This also suggests, though the water body has oligotrophic conditions for most months and in summer, it tends to be mesotrophic. EjsmontKarabin (2012) noted that in Polish water bodies, Keratella cochlearis f. tecta become more abundant in summer months. In this study, we did not identify rotifers to infra-specific level. However, in another on the morphometric variations of loricate rotifers from the same water body, Sarma et al. (2015) have indeed noted the predominance of Keratella cochlearis f. tecta (=unspined form) especially during summer months. Ejsmont-Karabin (2012) also developed a rotifer trophic-state index (TSIROT). If the values of TSIROT < 45 vary between 45–55, 44–55, 55–65 or > 65, then the water body is, respectively, mesotrophic, meso-eutrophic, eutrophic or hypertrophic. For deriving this TSIROT for the present water body, we applied the rotifer numbers in the equation developed by Ejsmont-Karabin (2012) (TSIROT=5.38ln(N)+ 19.28). We obtained a mean annual value of < 40 TSI Rot. This too suggests that the water body is mesotrophic.
On average, sampling station 1 had the highest species diversity index of rotifers (2.81 bits/ind.) since the presence of macrophytes in this pond favored the high diversity of Rotifera (Bakker et al., 2013). A low mean annual species diversity index was observed at station 2 (2.54 bits/ind.). In this pond we observed a high density of Brachionus which exploited the food resources and kept other species at low density (Pejler and Bērzinš, 1989; Nandini et al., 2005). The mean annual species diversity index of the five water bodies in La Cantera Oriente was about 2.7, which indicates the ecosystem is relatively little contaminated (Wetzel, 2001). The zooplankton species diversity index values in other high altitude water bodies in Mexico vary considerably. For example, Nandini et al. (2016) have recorded a mean species diversity of about 2 for the lake Xochimilco, for the high altitude drinking water reservoir, while Figueroa-Sánchez et al. (2014) recorded a wide range (0.57 to 2.47 bits/ind.). Kuczyńska-Kippen et al. (2013) found relatively much lower rotifer species diversity values (0.17 to 1.94) for four crater ponds in Poland. They attributed the low rotifer diversity to the temporary nature of the water bodies, that is, the water bodies periodically dry.
We did not estimate the density of algae in our study. However, it is known that La Cantera Oriente a diverse community ( > 135 taxa) of microalgae of which most species belong to the Chlorophyta and the Bacillariophyceae (Novelo et al., 2009). Most members of Chlorophyta can provide food for rotifers of the Brachionidae, the Lecanidae, the Lepadellidae and the Trichocercidae (Wallace et al., 2006). The range of tolerance of freshwater rotifers to certain physico-chemical variables including temperature, dissolved oxygen, hardness and conductivity varies depending on the species. The results of the CCA showed that L. pyriformis, L. flexilis, T. pusilla and B. budapestinensis were positively affected by alkalinity. Bērzinš and Pejler (1989a, b) have documented the occurrence of various rotifers including Lecane, Trichocerca and Brachionus with respect to different abiotic factors including pH, temperature, conductivity and dissolved oxygen. The occurrence of different rotifer species in La Cantera Oriente agrees with the data presented in Bērzinš and Pejler (op.cit.). Some of the most common rotifers such as Testudinella patina, Euchlanis dilatata and Cephalodella gibba showed no clear relation in relation to the chosen physicochemical variables. It is known that some of these species have a wide range of tolerance to temperature, dissolved oxygen and chlorophyll a levels (Koste, 1978). Species of Cephalodella and Euchlanis are benthic-littoral, living where the physico-chemical variables vary considerably (Moss, 2010) and therefore it is not surprising that these rotifers show little relation to the measured abiotic factors.
5 CONCLUSIONThis study reveals that the shallow lakes of La Cantera Oriente have more than 60 rotifer species including the taxa rare for Mexico such as Eosphora thoides and Lecane bifurca. The physico-chemical data agree with those available for similar water bodies in Central Mexico. The trophic status of this shallow lake, based on chlorophyll a is eutrophic while the QB/T ratio and the rotifer densities suggest an oligotrophic condition for most part of the year. When the trophic state index (TSIROT) is applied the water body it indicates a mesotrophic condition. This warrants further studies, especially with reference to the nutrient dynamics in this shallow lake.
6 ACKNOWLEDGEMENTWe thank E. Ortega Mayagoitia, M. Silva Briano, A. Lugo Vazquez, M. Merino Ibarra and F. S. Castillo Sandoval for help during the preparation of this manuscript. Francisco Martínez Pérez helped us during the field collections. The first author thanks CONACyT-Mexico for a scholarship (No. 563067).
| Alcocer J, Bernal-Brooks F W, 2010. Limnology in Mexico. Hydrobiologia, 644(1): 1–54. Doi: 10.1007/s10750-010-0108-z |
| American Public Health Association, American Water Works Association, Water Environment Federation. 2012. Standard Methods for the Examination of Water and Wastewater. 22nd edn. Amer Public Health Assn, Washington DC. 1 496p. |
| Bakker E S, Sarneel J M, Gulati R D, Liu Z W, van Donk E, 2013. Restoring macrophyte diversity in shallow temperate lakes:biotic versus abiotic constraints. Hydrobiologia, 710(1): 23–37. Doi: 10.1007/s10750-012-1142-9 |
| Bērziņš B, Pejler B, 1989a. Rotifer occurrence in relation to temperature. Hydrobiologia, 175(3): 223–231. Doi: 10.1007/BF00006092 |
| Bērziņš B, Pejler B, 1989b. Rotifer occurrence and trophic degree. Hydrobiologia, 182(2): 171–180. Doi: 10.1007/BF00006043 |
| Brown L R. 2008. Plan B 3. 0: Mobilizing to Save Civilization. 3rd edn. Earth Policy Institute/W. W. Norton & Company, New York. 398p. |
| Cervantes P, Wallace P, 2003. Magma degassing and basaltic eruption styles:a case study of~2000 year BP Xitle volcano in central Mexico. Journal of Volcanology and Geothermal Research, 120(3-4): 249–270. Doi: 10.1016/S0377-0273(02)00401-8 |
| Claps M C, Gabellone N A, Benítez H H, 2011. Seasonal changes in the vertical distribution of rotifers in a eutrophic shallow lake with contrasting states of clear and turbid water. Zoological Studies, 50(4): 454–465. |
| Contreras J J, Sarma S S S, Merino-Ibarra M, Nandini S, 2009. Seasonal changes in the rotifer (Rotifera) diversity from a tropical high altitude reservoir (Valle de Bravo, Mexico). Journal of Environmental Biology, 30(2): 191–195. |
| De la Lanza E G, García-Calderón J L. 2002. Lagos y Presas de México. 2nd edn. AGT Publishers, Mexico City, Mexico. 680p. |
| Degefu F, Herzig A, Jirsa F, Schagerl M, 2014. First limnological records of highly threatened tropical highmountain crater lakes in Ethiopia. Tropical Conservation Science, 7(3): 365–381. Doi: 10.1177/194008291400700302 |
| Dimas-Flores N, Alcocer J, Ciros-Pérez J, 2008. The structure of the zooplankton assemblages from two neighboring tropical high mountain lakes. Journal of Freshwater Ecology, 23(1): 21–31. Doi: 10.1080/02705060.2008.9664554 |
| Ejsmont-Karabin J, Kuczyńska-Kippen N, 2001. Urban rotifers:structure and densities of rotifer communities in water bodies of the Poznań agglomeration area (western Poland). Hydrobiologia, 446(1): 165–171. |
| Ejsmont-Karabin J, 1995. Rotifer occurrence in relation to age, depth and trophic state of quarry lakes. Hydrobiologia, 313(1): 21–28. |
| Ejsmont-Karabin J, 2012. The usefulness of zooplankton as lake ecosystem indicators:rotifer trophic state index. Polish Journal of Ecology, 60(2): 339–350. |
| Figueroa-Sanchez M A, Nandini S, Sarma S S S, 2014. Zooplankton community structure in the presence of low levels of cyanotoxins:a case study in a high altitude tropical reservoir (Valle de Bravo, Mexico). Journal of Limnology, 73(1): 157–166. |
| Flores-Burgos J, Sarma S S S, Nandini S. 2003. Estudio preliminar sobre la fauna de rotíferos de Xochimilco(México). El agua de cuenca de México. Sus problemas históricos y perspectivas de solución. In: Proceedings of the Second International Conference on Xochimilco. Ecological Park of Xochimilco, UAM Xochimilco, Mexico City, Mexico, 1: 163-171. |
| García C E, Nandini S, Sarma S S S, 2009. Seasonal dynamics of zooplankton in Lake Huetzalin, Xochimilco (Mexico City, Mexico). Limnologica-Ecology and Management of Inland Waters, 39(4): 283–291. Doi: 10.1016/j.limno.2009.06.010 |
| García-García G, Nandini S, Sarma S S S, Martínez-Jerónimo F, Jiménez-Contreras J, 2012. Impact of chromium and aluminium pollution on the diversity of zooplankton:a case study in the Chimaliapan wetland (RAMSAR Site)(Lerma basin, Mexico). Journal of Environmental Science and Health A, 47(4): 534–547. Doi: 10.1080/10934529.2012.650554 |
| García-Morales A, Elías-Gutiérrez M, 2007. The rotifer fauna of Guatemala and Belize:survey and biogeographical affinities. Revista de Biologia Tropical, 55(2): 569–584. |
| Gulati R D, Lammens E H R R, Meijer M L, van Donk E, 1990. Biomanipulation, tool for water management. Hydrobiologia, 200-201: 619–627. Doi: 10.1007/BF02530378 |
| Hortelano-Moncada Y, Cervantes F A, Trejo-Ortiz A, 2009. amíferos silvestres de la Reserva Ecológica del Pedregal de San Ángel en Ciudad Universitaria, Universidad Nacional Autónoma de México, México, D. F. Revista Mexicana de Biodiversidad, 80: 507–520. |
| Koste W. 1978. Rotatoria. Die Rädertiere Mitteleuropas. Ein Bestimmungswerk begründet von Max Voigt. Bornträger, Stuttgart. 673p. |
| Krebs J R, 1989. Ecological Methodology. Harper Collins Publishers, New York.Kuczyńska-Kippen N, Basińska A M, Świdnicki K. 2013.Specificity of zooplankton distribution in meteorite crater ponds (Morasko, Poland). Knowledge and Management of Aquatic Ecosystems, 409: 08. |
| Li X Y, Yu H X, Ma C X, 2014. Zooplankton community structure in relation to environmental factors and ecological assessment of water quality in the Harbin Section of the Songhua River. Chinese Journal of Oceanology and Limnology, 32(6): 1344–1351. Doi: 10.1007/s00343-014-3303-3 |
| Lot A. 2007. Guía Ilustrada de la Cantera Oriente. Caracterización Ambiental e Inventario Biológico. Secretaria Ejecutiva de la Reserva Ecológica del Pedregal de San Ángel. Coordinación de la Investigación Científica, UNAM, Mexico City. 246p. |
| May L, O'Hare M, 2005. Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK. Hydrobiologia, 546(1): 397–404. Doi: 10.1007/s10750-005-4282-3 |
| Merino-Ibarra M, Monroy-Ríos E, Vilaclara G, Castillo F S, Gallegos M E, Ramírez-Zierold J, 2008. Physical and chemical limnology of a wind-swept tropical highland reservoir. Aquatic Ecology, 42(3): 335–345. Doi: 10.1007/s10452-007-9111-5 |
| Moss B R. 2010. Ecology of Fresh Waters: A View for the twenty-First Century. 4th edn. Wiley-Blackwell, London. 480p. http://www.cabdirect.org/abstracts/20113029218.html |
| Nandini S, Merino-Ibarra M, Sarma S S S, 2008. Seasonal changes in the zooplankton abundances of the reservoir Valle de Bravo.(State of Mexico, Mexico). Lake and Reservoir Management, 24(4): 321–330. Doi: 10.1080/07438140809354842 |
| Nandini S, Ramírez G P, Sarma S S S, 2016. Water quality indicators in Lake Xochimilco, Mexico:zooplankton and Vibrio cholerae. Journal of Limnology, 75(1): 91–100. |
| Nandini S, Ramírez-García P, Sarma S S S, 2005. Seasonal variations in the species diversity of planktonic rotifers in Lake Xochimilco, Mexico. Journal of Freshwater Ecology, 20(2): 287–294. Doi: 10.1080/02705060.2005.9664968 |
| Novelo E, Ponce M E, Ramírez R. 2009. Las microalgas de la Cantera Oriente. In: Biodiversidad del Ecosistema del Pedregal: Reserva Ecológica del Pedregal de San Ángel. Secretaria Ejecutiva de la Reserva Ecológica del Pedregal de San Ángel, UNAM, Mexico City. p. 71-80. |
| Pejler B, Bērziņš B, 1989. On choice of substrate and habitat in brachionid rotifers. Hydrobiologia, 186-187(1): 137–144. Doi: 10.1007/BF00048905 |
| Pérez H E. 2009. Los peces y sus hábitats. Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. p. 357-362. |
| Pociecha A, Wilk-Woźniak E, Mróz W, Bielańska-Grajner I, Gadzinowska J, Walusiak E, 2015. Biodiversity of rotifers in urban water reservoirs of Southern Poland. Oceanological & Hydrobiological Studies, 44(3): 335–342. |
| Pontin R M. 1978. A Key to the Freshwater Planktonic and Semi-Planktonic Rotifera of the British Isles. Freshwater Biological Association, Windermere. 192p. http://ci.nii.ac.jp/ncid/BA20492193 |
| Ramírez G P, Nandini S, Sarma S S S, Robles V E, Cuesta I, Hurtado M D, 2002. Seasonal variations of zooplankton abundance in the freshwater reservoir Valle de Bravo(Mexico). Hydrobiologia, 467(1-3): 99–108. |
| Sarma S S S, Elías-Gutiérrez M, Carmen S S, 1996. Rotifers from high altitude crater-lakes at Nevado de Toluca Volcano, México. Hidrobiologica, 6(1-2): 33–38. |
| Sarma S S S, Elías-Gutiérrez M, 1999. Rotifers (Rotifera) from four natural water bodies of central Mexico. LimnologicaEcology and Management of Inland Waters, 29(4): 475–483. Doi: 10.1016/S0075-9511(99)80054-1 |
| Sarma S S S, Elías-Gutiérrez M, 2000. Rotifers from Mexico:new records in high altitude ponds. The Southwestern Naturalist, 45(3): 366–373. Doi: 10.2307/3672847 |
| Sarma S S S, Jiménez-Nigó A, Nandini S. 2015. Estudios morfométricos de especies de rotíferos (Rotifera) en la Cantera Oriente (México, D. F. ). In: Alcocer J, Merino-Ibarra M, Escobar-Briones E eds. Tendencias de investigación en Limnología tropical: Perspectivas universitarias en Latinoamérica. Asociación Mexicana de Limnología, A. C., Instituto de Ciencias del Mary Limnología, UNAM, y Consejo Nacional de Ciencias y Tecnología. México. p. 315-327. |
| Sarma S S S, Manuel E G, 1998. Rotifer diversity in a central Mexican pond. Hydrobiologia, 387-388: 47–54. |
| Sarma S S S, Serranía-Soto C, Nandini S. 2009. Rotíferos. In: Ceballos G, List R, Garduño G, López-Cano R, Muñozcano-Quintanar M J, Collado E, San-Román J E eds. La Diversidad Biológica del Estado de México. Estudio de Estado. Biblioteca Mexiquense del Bicentenario. Colección Mayor, Gobierno del Estado de México. |
| Segers H. 1995. Rotifera 2. The lecanidae. In: Nogrady T, Dumont H J eds. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 6. SPB Academic Publishing BV, Hague. 226p. |
| Sichrowsky U, Schabetsberger R, Sonntag B, Stoyneva M, Maloney A E, Nelson D B, Richey J N, Sachs J P, 2014. Limnological characterization of volcanic crater lakes on uvea island (Wallis and Futuna, South Pacific). Pacific Science, 68(3): 333–343. Doi: 10.2984/68.3.3 |
| Sládeček V, 1983. Rotifers as indicators of water quality. Hydrobiologia, 100(1): 169–201. Doi: 10.1007/BF00027429 |
| Snell T W, Joaquim-Justo C, 2007. Workshop on Rotifers in Ecotoxicology. Hydrobiologia, 593(1): 227–232. Doi: 10.1007/s10750-007-9045-x |
| Vázquez-Sánchez A, Reyes-Vanegas G, Nandini S, Sarma S S S, 2014. Diversity and abundance of rotifers during an annual cycle in the reservoir Valerio Trujano(Tepecoacuilco, Guerrero, Mexico). Inland Waters, 4(3): 293–302. Doi: 10.5268/IW |
| Wallace R L, Snell T W, Ricci C, Nogrady T. 2006. Rotifera, Part 1: biology, ecology and systematics. In: Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. Kenobi Productions Gent, Belgium/Backhuys Publishers, The Hague. 299p. |
| Wallace R L, Snell T W, Walsh E J, Sarma S S S, Segers H. 2016. Phylum rotifera. In: Thorp J H, Rogers D C eds. Keys to Nearctic fauna. Thorp and Covich's Freshwater Invertebrates. 4thedn. Elsevier, Amsterdam, Vol. 2. p. 131-167. |
| Wetzel R G, Likens G E, 2000. Limnological Analyses. Springer, New York429p. |
| Wetzel R G, 2001. Limnology:Lake and River Ecosystems. Academic Press, London1006p. |
| Xi Y L, Huang X F, 2004. Temperature effect on the life history of three types of Brachionus calyciflorus females. Chinese Journal of Oceanology and Limnology, 22(2): 192–197. Doi: 10.1007/BF02842592 |
| Zambrano L, Perrow M R, Macías-García C, Aguirre-Hidalgo V, 1998. Impact of introduced carp (Cyprinus carpio) in subtropical shallow ponds in Central Mexico. Journal of Aquatic Ecosystem Stress and Recovery, 6(4): 281–288. Doi: 10.1023/A:1009958914016 |
| Zepeda-Gómez C, Lot-Helgueras A, Nemiga X A, MadrigalUribe D, 2012. Florística y diversidad de las ciénagas del río Lerma Estado de México, México. Acta Botánica Mexicana, 98: 23–49. |
 2017, Vol. 35
2017, Vol. 35


