Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SANAYE Sushant Vilas, PAWAR Ashwini Pandurang, RIVONKER Chandrasheker Umanath, SREEPADA Rayadurga Anantha, ANSARI Zakir Ali, RAM Anirudh
- Biochemical composition of the alligator pipefish, Syngnathoides biaculeatus (Bloch, 1785)
- Chinese Journal of Oceanology and Limnology, 35(6): 1501-1510
- http://dx.doi.org/10.1007/s00343-017-6070-0
Article History
- Received Mar. 15, 2016
- accepted in principle Aug. 19, 2016
- accepted for publication Aug. 26, 2016
2 Department of Marine Sciences, Goa University, Taleigao Plateau, Goa-403206, India;
3 CSIR-National Institute of Oceanography, Regional Centre, Mumbai, India
It is now well accepted that fish are a major and cheap source of protein and their importance in human nutrition is well recognized. They are also rich in polyunsaturated fatty acids (PUFAs) and highly unsaturated fatty acids (HUFA), which regulate prostaglandin synthesis and induce wound healing (Gibson, 1983; Chyun and Griminger, 1984; Zuraini et al., 2006), as well having beneficial effects on cardiovascular diseases and cancers (Conner, 1997). Many amino acids directly, or through their metabolites, act as antioxidants in human metabolism and play an important role in the immune system, acting as antiviral and antitumor agents, as well as inhibitors of inflammation (Wu, 2009).
Syngnathid fishes (mainly seahorses and pipefishes) constitute major ingredient in traditional Chinese medicine (TCM) (Kumaravel et al., 2012) for their curative properties in the treatment of various diseases including cancer and impotence. Published literature (Vincent, 1995, 1996; Moreau et al., 1998; Sreepada et al., 2002; Zhang et al., 2003; Alves and Rosa, 2006; Shi et al., 2006; Rosa et al., 2013) suggest the use of seahorses and pipefishes in TCM, as they have a significant role in increasing and balancing vital energy flows within the body, a curative role for impotence, infertility, asthma, high cholesterol, goitre, kidney disorders and skin afflictions such as severe acne and persistent nodules. Apart from medicinal use, syngnathid fishes also form a source of nutrition and serve as a powerful general health tonic in the form of soups, wine, pills and capsules (Vincent, 1995, 1996; Lv et al., 2002).
The biochemical composition and antioxidant properties of both wild and cultured seahorse species have been well documented (Hung et al., 2008; Lin et al., 2008, 2009; Qian et al., 2008, 2012; Rethna Priya et al., 2013; Sanaye et al., 2014). However, except for very few studies (Wijesekara et al., 2011; Sanaye et al., 2015), the bioactive properties of pipefish species are largely unknown.
The alligator or double-ended pipefish S. biaculeatus (Bloch, 1785) is widely distributed throughout the tropical Indo-Pacific with records from seagrass habitats extending from the northern Red Sea and the eastern coast of Africa, eastward to Japan, Samoa, the Tonga Islands and Australia (Dawson, 1985). Although S. biaculeatus is considered to be the most heavily exploited pipefish in TCM, there are few estimates of trade volume to corroborate this (Barrows et al., 2009). Vincent (1996) reported trade volumes (dried pipefishes) of 1 600– 16 500 kg/a into Taiwan Island over the period 1983– 1993, while Martin-Smith et al. (2003) put a figure in the range of 7 500–21 300 kg/a into Hong Kong during 1998–2002 with the further trade likely occurring from tropical countries, particularly from India, Malaysia, the Philippines and Thailand, including a mixture of species (Martin-Smith et al., 2003; Martin-Smith and Vincent, 2006). The recognized lack of information for S. biaculeatus is reflected in its 'Data Deficient' listing in the IUCN Red List (Bartnik et al., 2008).
Although the use of S. biaculeatus, known as 'Hailong' in TCM has a history of over 600 years (Shi et al., 1993; Pogonoski et al., 2002), absolutely no information, however, exists on its biochemical composition and nutritive value. Considering its economic importance in TCM, lack of baseline information and as a potential candidate species for syngnathid aquaculture, the present study has evaluated the biochemical constituents in the alligator pipefish, S. biaculeatus, collected from its natural environment. Furthermore, the fatty acid and amino acid profiles, trace element content and C:N ratio have also been elucidated. To our knowledge, this is the first comprehensive report on the biochemical composition of S. biaculeatus. The results of this study will help in developing appropriate diets for the culture of this economically and ecologically important pipefish species.
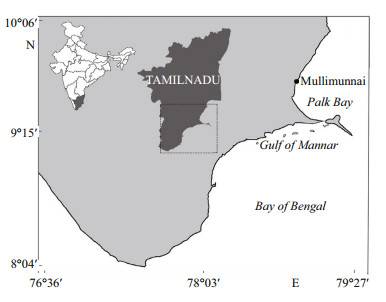
2 MATERIAL AND METHOD 2.1 Alligator pipefish collection and sample preparationA total of 40 dead specimens of alligator pipefish, S. biaculeatus (Bloch, 1785) landed as by-catch in wind-driven trawl or 'Vallams' (mainly operated for crab/shrimp fishing) at Mullimunnai village, Palk Bay, Tamil Nadu, India (9°39′24.48″N, 78°58′14.05″E; Fig. 1) served as material for the present study. Mean wet weight and total length (from the tip of the snout to the tip of the caudal end) of the fishes recorded were (5.45±0.57) g and (185±35) mm, respectively. Immediately after collection, the fishes were washed with ice-cold distilled water and then immediately frozen in liquid nitrogen and stored in zip-lock plastic bags at -80℃ until further analyses. The lyophilized and powdered sample was used for analyzing biochemical constituents, fatty acid and amino acid profiles.
|
| Figure 1 Collection site of alligator pipefish, S. biaculeatus shown with (●) along Palk Bay, Tamil Nadu, India |
Proximate principals such as crude protein, crude lipid, moisture and ash content were determined in duplicate following the standard methods as described in AOAC (2005). Moisture (% wet weight) was determined by drying the wet samples at 105℃ for 24 h to a constant weight in a hot air oven (Biotechnics India, Mumbai, India). Ash content was estimated by incinerating samples in a muffle furnace (Biotechnics India, Mumbai, India) at 600℃ for 6 h. Crude protein (N×6.25) was estimated following the micro-Kjeldahl after acid digestion. Crude lipid was estimated by using Soxhlet extraction apparatus using petroleum ether as solvent. The nitrogen-free extracts (carbohydrates, vitamins and other non-nitrogen soluble compounds) were computed by the remainder method (Woods and Aurand, 1977).
2.3 Fatty acid profileTotal lipids (TL) were extracted by homogenizing the lyophilized powdered samples in five columns of chloroform/methanol (2:1, v/v) and run according to the method of Folch et al. (1957). The lipids were converted into fatty acid methyl esters (FAMEs) then identified by gas chromatography after re-dissolution in hexane. The FAMEs were analyzed using a Shimadzu GC-Mass Spectrometer, QP-2010 Ultra EI & PCI (Shimadzu, Japan). Helium gas was used as the carrier gas. Identification of individual fatty acids in fish samples was performed by comparing with the chromatograms of fatty acid standards (C4–C24 fatty acids) from Sigma-Aldrich, India. Peaks in the chromatograms were identified by comparison with retention times and peaks of standard FAMEs. The contribution of each fatty acid was calculated from the total identified fatty acids and values are presented as % mean±standard deviation.
2.4 Amino acid profileThe composition of amino acids in S. biaculeatus was analyzed with Waters AccQ•TagTM amino acid analysis method. Lyophilized and powdered samples (50 mg) were subjected to acid hydrolysis in 6 N hydrochloric acid (HCl) for 24 h at 110℃, dried hydrolysate was again re-suspended in 100 mL of ultrapure H2O. 10 μL of the above solution was added to 90 μL of reaction buffer (AccQ• Fluor Borate Buffer, Waters, Milford, USA) to make a 100-μL solution. Then 10 μL solution was injected into a Waters AccQ• TagTM amino acid analysis column. Separation of the different amino acids was carried out using HPLC (Waters Corporation Amino Acid Analyzer, Milford, USA). Amino acids such as cysteine and tryptophan were undetectable after acid hydrolysis. Asparagine and glutamine were determined as aspartic acid and glutamic acid, respectively. Individual amino acids were identified by comparing their retention times with those of standards, after being carried out under identical conditions, and expressed as the percentage of total amino acid content.
2.5 Trace element analysisLyophilized and powdered samples (5 g) were used for determining the concentration of trace elements in duplicate. A microwave accelerated digestion system (CEM-MARS 5) was used to digest a wide variety of trace metals in the laboratory. This system condenses materials of different matrices, allowing for the analysis of volatile metals, such as Hg. During the digestion portion of the Hg analysis, 1 mL of HNO3 and 3 mL of HCl were added to 5 g of sample, and the volume was increased to 10 mL using Milli-Q water. Teflon vessels containing the samples were kept in the double walled, outer liner of the digestion bomb, capped with a sensor head and pressure rupture disc. Sealed vessels were then placed in the microwave carousels in the same manner as for digestion. Each set of samples was accompanied by a blank, spike and certified reference material. Trace metals were analyzed using Graphite Furnace Atomic Absorption Spectrometry (GF-AAS, PerkinElmer, Analyst 600) and an Inductively Coupled Plasma Optical Emission Spectrophotometer (ICP-OES, Optima 7300 DV, Perkin Elmer, Inc., Shelton, USA).The precision and accuracy of analysis were verified by replicate measurements (n=5) of target metals in a standard reference material of marine biota sample TORT-3 Lobster hepatopancreas reference material for trace metals (National Research Council, Canada). The analysed values obtained for the reference materials were found to be in good agreement with the certified values.
2.6 Carbon:nitrogen (C:N) ratioLyophilized and powdered samples were used for the measurement of total carbon and nitrogen. About 300 mg of alligator pipefish dried sample was taken into a tin container and kept in the auto sampler of NC organic elemental analyzer (FLASH 2000, Thermo Scientific, India). Samples were run in duplicate and measured levels of C and N in S. biaculeatus were noted.
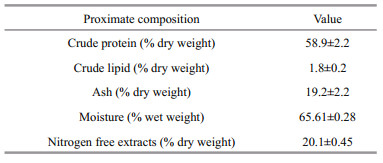
3 RESULT 3.1 Proximate compositionThe results of proximate analysis of S. biaculeatus are presented in Table 1. Mean percent moisture (wet weight) and ash (dry weight) in S. biaculeatus were (65.61±0.28)% and (19.2±2.2)%, respectively. Mean measured concentrations (% dry weight) of crude protein, crude lipid and nitrogen-free extracts were (58.9±2.2)%, (1.8±0.2)%, (20.1±0.45)%, respectively.
|
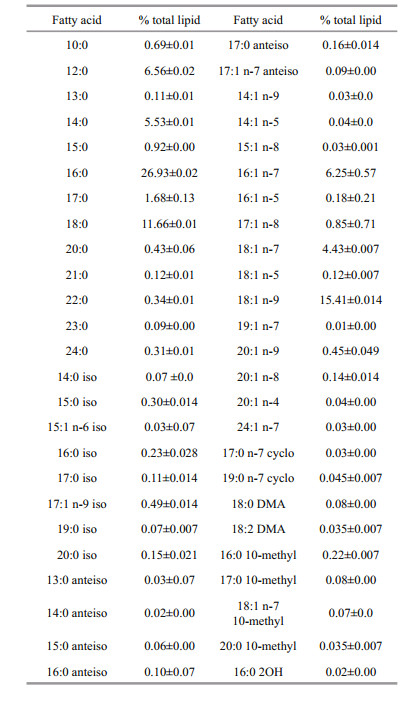
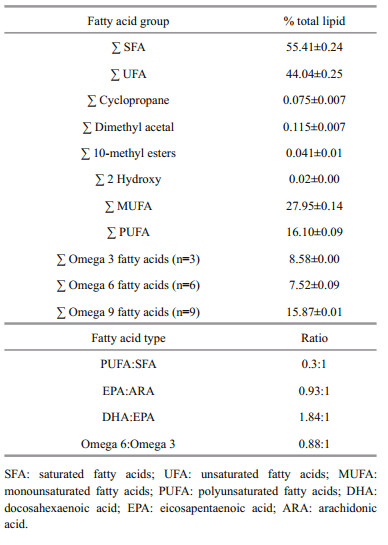
The fatty acid profile of S. biaculeatus revealed the presence of 27 saturated fatty acids (SFAs) with 13 straight chained and 14 branched chained, 28 unsaturated fatty acids (UFAs) with 14 monounsaturated fatty acids (MUFAs) and 14 polyunsaturated fatty acids (PUFAs) and nine other minor fatty acids (Table 2). The contributions of SFAs and UFAs (as a percentage of total lipids) in S. biaculeatus were found to be (55.41±0.24)% and (44.05±0.25)%, respectively (Table 3). The fatty acid composition revealed that, palmitic acid (16:0) was the major fatty acid ((26.93±0.02)%) followed by oleic acid (18:2) ((15.41±0.014)%), stearic acid (18:1) ((11.66±0.01)%), lauric acid ((6.56±0.02)%), palmitoleic acid ((6.25±0.57)%), docosahexaenoic acid (DHA) ((4.55±0.007)%) and vaccenic acid ((4.43±0.007)%). Amounts MUFA and PUFA in S. biaculeatus (as a percentage of total lipids) were (27.95±0.30)% and (16.10±0.09)%, respectively (Table 3).
|
|
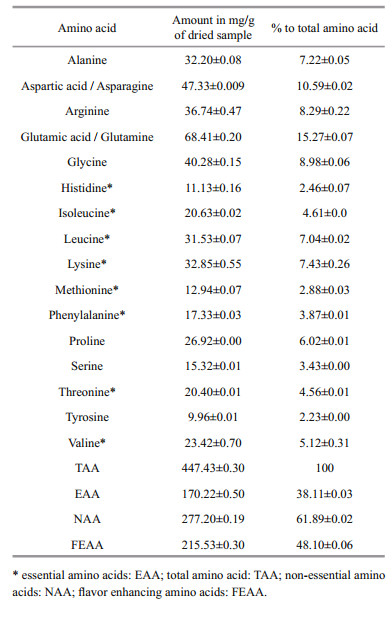
The amino acid composition (%) and essential amino acids (EAA) of the alligator pipefish, S. biaculeatus is presented in Table 4. Among all amino acids ((447.43±0.30) mg/g of dry weight), the major ones were glutamic acid/glutamine (15.27±0.07)%), aspartic acid/asparagine ((10.59±0.02)%), glycine ((8.98±0.06)%), arginine ((8.29±0.22)%), lysine ((7.43±0.26)%), alanine ((7.22±0.05)%), leucine ((7.04±0.02)%) and proline ((6.02±0.01)%). The contributions of EAA (total eight) and NAA (total eight) to the total amino acids were 38.11% and 61.89% respectively. Amongst EAA, Leucine and Lysine contributed (7.04±0.02)% and (7.43±0.26)%, respectively, while among NAA, glutamic acid/ glutamine ((15.27±0.07)%) and aspartic acid/ asparagine ((10.59±0.02)%) contributed significantly.
|
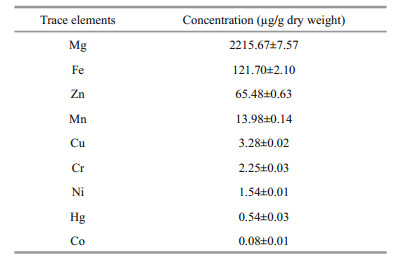
Measured concentrations (mean±SD) of nine different trace elements in S. biaculeatus collected from its natural environment are presented in Table 5. Trace element concentrations in S. biaculeatus were generally low and their distribution followed the order, Mg>Fe>Zn>Mn>Cu>Cr>Ni>Hg>Co (Table 5). Concentrations of magnesium, iron and zinc were found to be relatively higher than other trace elements and contributed (2 215.67±7.57), (121.70±2.10) and (65.48±0.63) μg/g dry weight of alligator pipefish, respectively. Other trace elements included cobalt, chromium, copper, manganese, nickel and mercury.
|
Measured levels of carbon and nitrogen in S. biaculeatus were (50.55±0.04)% and (11.57±0.01)%, respectively with a C:N ratio of (4.37±0.04)%.
4 DISCUSSIONThe alligator pipefish, S. biaculeatus forms the second most important ingredient in TCM and is the only pipefish species that has been extensively traded for TCM purposes. In spite of economic value in TCM, unfortunately, there are no systematic and scientific published reports on biochemical profile of S. biaculeatus. Here, an attempt has been made to provide the biochemical composition of S. biaculeatus collected from Palk Bay, Tamil Nadu, east coast of India.
4.1 Proximate compositionAmongst proximate principals, crude protein ((58.9±2.2)% dry wt.) formed the major component in S. biaculeatus. This level is marginally lower than the reported values of six different species of wild seahorses, yellow seahorse Hippocampus kuda ((70.70±2.12)%); three spotted seahorse H. trimaculatus ((77.59±1.06)%); Kellogg's seahorse H. kelloggi ((78.31±1.74)%); Hedgehog seahorse H. spinosissimus; Thorny seahorse H. histrix ((68.07±1.96)%) and tiger tail seahorse H. comes ((76.59±3.25)%), collected from the Chinese coast by Lin et al. (2008). On the other hand, Lin et al. (2009) reported crude protein levels of (72.2±2.55)% and (68.9±3.4)%, respectively in wild and cultured H. kuda and marginally higher levels in wild ((78.5±4.2)%) and cultured ((75.6±2.8)%) H. trimaculatus. Measured levels of other proximate principals (moisture, crude lipids and ash) in S. biaculeatus are in accordance with Lin et al.(2008, 2009). In the case of snake pipefish, Entelurus aequoreus collected from Bay of Biscay, Spain, proximate levels of crude protein, lipids and ash are 14.7%, 1.9% and 6.8%, respectively (Spitz et al., 2010). The biochemical composition and the nutritional content of wild fishes are often variable and mostly depend upon their feed, geographical conditions, sex and growth stage (Payne and Rippingale, 2000; Lin et al., 2009). The difference in biochemical composition of alligator pipefish and seahorses species may be influence by such conditions.
It is well known that the nutritional composition of fish species is strongly affected by their food rather than other physical parameters (Henderson and Tocher, 1987; Orban et al., 2007; Lin et al., 2008). The natural food of syngnathid fishes consists mostly of small crustaceans such as copepods, amphipods and mysids (Tipton and Bell, 1988; Garcia et al., 2005; personal observation). As cited in Lin et al. (2008), the natural foods of the seahorses may be rich in protein (>75% of dry weight) and poor in lipid (~3% of dry weight) which is reflected in their biochemical composition. Proximate composition of major food organisms of seahorses from Palk Bay, the east coast of India has been studied by Murugan et al. (2009). Levels of crude protein, crude lipids and carbohydrates were found in the range of 52% and 45%, 11% and 13%, 6% and 8%, respectively in amphipods and sergestid shrimps. In a similar study, Perumal et al. (2009) reported proximate and amino acid composition of two copepod species, Acartia spinicauda and Oithona similis from Palk Bay, India. These authors report the levels of crude protein, lipids and carbohydrates to be the range, 67.33%–75.45%, 12.42%–17.81% and 4.01%–7.98%, respectively in A. spinicauda and 59.53%–69.61%, 9.89%–15.44% and 3.43%–6.59%, respectively in O. similis. A total 16 amino acids were also reported from these two copepod species. The diet of alligator pipefishes collected from Palk Bay also revealed the dominance of copepods, amphipods, decapods, isopods and other peracarids (personal observation). In view of the above published reports and the observations made in the present study, it is not surprising that the nutritional composition of the alligator pipefish collected from its natural habitat is largely influenced by its diet and feeding habits.
4.2 Fatty acid profileIn the present study, an analysis of the fatty acid composition of S. biaculeatus revealed 64 different types of fatty acids represented by 27 saturated fatty acids, SFA ((55.41±0.24)%), 28 unsaturated fatty acids, UFA ((44.05±0.25)%) and nine other minor fatty acids ((0.62±0.08)%). The number of SFAs quantified during the present study is relatively higher in comparison with the values reported earlier by Lin et al.(2008, 2009) in seahorse species. In contrast, the total amounts of UFAs in alligator pipefishes are comparatively lower than seahorse species. Generally, high levels of saturated fats are not recommended in foods by the Department of Health, United Kingdom and the ideal ratio of polyunsaturated fatty acids (PUFA) to SFA for food consumption should not be less than 0.1 (Wood et al., 2004). The calculated ratio of PUFA:SFA in S. biaculeatus was determined to be 0.3, which is comparatively lower than the ratio reported in wild seahorse (0.40–0.93) collected along the Chinese coast (Lin et al., 2008). In another study conducted by Lin et al. (2009), this ratio was found to be 0.44–0.91. The proportion of monounsaturated fatty acids (MUFA) in alligator pipefish ((27.95±0.30)%) are comparatively higher than corresponding values reported in seahorses (Lin et al., 2008, 2009), while proportions of PUFA ((16.10±0.09)%) are comparatively less. According to Mazereeuw et al. (2012) and Larsson (2013) Omega 3 fatty acids are helpful for humans to reduce risk of cardiovascular diseases, inflammation, developmental disorders and in mental health. The content of omega 3 (n-3), omega 6 (n-6) and omega 9 (n-9) fatty acids determined in alligator pipefish were (8.58±0.0)%, (7.52±0.09)% and (15.87±0.01)%, respectively. It has been recommended that the ratio of omega 6 to omega 3 fatty acids should be 1:1 for better human health (Simopoulos, 2006). This ratio in alligator pipefish was determined to be 0.88:1 which is superior to many vegetable oils such as Canola oil (2:1), Soybean oil (7:1), Olive oil (3-13:1) and Corn oil (41:1) (Hibbeln et al., 2006). Among omega 3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are most important for normal human health and these are abundant in marine fishes (Morris et al., 1995; Osman et al., 2001). The sum of DHA and EPA levels in S. biaculeatus is 7.02% of TL and the calculated ratio of DHA:EPA is 1.84:1. It is not surprising that alligator pipefish are the second most important ingredient in TCM after seahorses, perhaps owing to their relevantly high content of PUFA (DHA and EPA).
4.3 Amino acid profileA total 16 amino acids were retrieved from alligator pipefish, S. biaculeatus, with an equal number of essential (EAA) and non-essential (NAA) ones. EAA contributed 38.11% and NAA contributed 61.89% of the total amino acids (TAA) content. The content of EAA in S. biaculeatus is quite high compared to reported values ((17.62±2.32)% to (20.45±2.19)%) in wild seahorses from the Chinese coast (Lin et al., 2008) and two cultured seahorse species ((19.08±3.56)% to (25.04±2.37)%) (Lin et al., 2009). EAAs are beneficial to enhance the immune system and recovery processes in human health (Chyun and Griminger, 1984; Mat Jais et al., 1994; Witte et al., 2002; Wu, 2009). It has been demonstrated that amino acids such as alanine, arginine, isoleucine and proline possess the ability to bind together with glycine and also to form polypeptides, thereby triggering tissue re-growth and recovery in humans (Heimann, 1980; Witte et al., 2002). According to the studies of Lin et al.(2008, 2009), seahorses contain high levels of flavor enhancing amino acids (FAA) such as aspartic acid, glutamic acid, glycine and alanine. In addition to these, phenylalanine and tyrosine are also known to possess flavor enhancing properties. The content of FAA in alligator pipefish ((48.20±0.27)%) is somewhat higher than those recorded in seahorses by Lin et al.(2008, 2009).
4.4 Trace elementsTrace elements are generally dietary elements that are needed in minute quantities for proper growth, development and physiology in an organism (Bowen, 1966). A total of nine trace elements have been reported in alligator pipefish during the present study. Trace elements from six seahorse species along the Chinese coast as well as from cultured and wild seahorses are reported by Lin et al.(2008, 2009). Concentration of Mg and Zn are quite high in alligator pipefish compared to seahorse species while Mn is marginally lower in alligator pipefish. Other trace elements are more or less similar in concentration in both seahorses and alligator pipefish. As cited in Lin et al.(2008, 2009), Zn and Mn, in general have roles to play in sperm development and also strengthen functioning of the kidneys (Xu et al., 2003; Meng et al., 2005). This supports the known efficacy of seahorses and pipefishes in TCM. Optimum concentrations of Fe are helpful in maintaining blood circulation through improved hemoglobin-oxygen carrying capacity of the blood in body as described in TCM (Zhang et al., 1998; Alves and Rosa, 2006; Rosa et al., 2013). Reported Fe concentration in S. biaculaetus ((121.70±2.10) μg/g) was on a par with concentrations observed in seahorses (160 μg/g) by Lin et al. (2008). Among toxic heavy metals, the concentrations of Hg were far below the toxic level.
4.5 C:N ratioDetermination of the C:N ratio has been considered an accurate indicator of the condition of fish (Fagan et al., 2011; Martinez-Cardenas et al., 2013). It has been hypothesized that the fish in good condition is expected to have a C:N ratio of 3 (Harris et al., 1986). In the present study, the C:N ratio was determined to be 4.37±0.04, suggesting that the populations of S. biaculeatus inhabiting the study area are not nutritionally stressed. These results are in agreement with the rearing experiment study of o possum pipefish, Microphis brachyurus by Martinez-Cardenas et al. (2013).
5 CONCLUSIONIn conclusion, the results of the present study highlighted that the proximate composition, fatty acid profile, amino acid profile, trace elements as well as C:N ratio of the alligator pipefishes are at least as beneficial as seahorses, and can be more efficaciously used for TCM as well as for human nutrition. As alligator pipefish are a potential candidate species in syngnathid aquaculture, these results are of significance in the selection and development of optimum diets and feeding protocols during culture.
6 ACKNOWLEDGEMENTThe authors are grateful to the Director, CSIRNational Institute of Oceanography, Goa (India) for encouragement and facilities. Permission granted by Chief Wildlife Warden, Forest department, Govt. of Tamil Nadu (India) for the collection of alligator pipefishes from Mullimunnai village, Palk Bay is gratefully acknowledged. This represents contribution No. 5939 of the CSIR-National Institute of Oceanography, Goa (India).
| Alves R R N, Rosa I L, 2006. From cnidarians to mammals:the use of animals as remedies in fishing communities in NE Brazil. Journal of Ethnopharmacology, 107(2): 259–276. Doi: 10.1016/j.jep.2006.03.007 |
| AOAC, 2005. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC) International. Gaithersburg, Maryland, USA.. |
| Barrows A P W, Martin-Smith K M, Baine M S P, 2009. Population variables and life-history characteristics of the alligator pipefish Syngnathoides biaculeatus, in Papua New Guinea. Journal of Fish Biology, 74(4): 806–819. Doi: 10.1111/jfb.2009.74.issue-4 |
| Bartnik S, Morgan, Pogonoski J, Pollard D, Paxton J. 2008. Syngnathoides biaculeatus. The IUCN Red List of Threatened Species 2008: e. T40715A10357159. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T40715A10357159.en. Assessed on 2015-04-16. |
| Bowen H J M, 1966. Trace Elements in Biochemistry. London, Academic Press241p. |
| Chyun J H, Griminger P, 1984. Improvement of nitrogen retention by arginine and glycine supplementation and its relation to collagen synthesis in traumatized mature and aged rats. Journal of Nutrition, 114(9): 1. |
| Conner W E, 1997. The beneficial effects of omega-3 fatty acids:cardiovascular disease and neuro development. Current Opinion in Lipidology, 8(1): 1–3. Doi: 10.1097/00041433-199702000-00001 |
| Dawson C E. 1985. Indo-Pacific Pipefishes: Red Sea to the Americas. Gulf Coast Research Laboratory, Ocean Springs, MS. |
| Fagan K A, Koops M A, Arts M T, Power M, 2011. Assessing the utility of C:N ratios for predicting lipid content in fishes. Canadian Journal of Fisheries and Aquatic Sciences, 68(2): 374–385. Doi: 10.1139/F10-119 |
| Folch J, Less M, Sloane Stanley G H, 1957. A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry, 226(1): 497–509. |
| Garcia A M, Geraldi R M, Vieira J P, 2005. Diet composition and feeding strategy of the southern pipefish, Syngnathus folletti in a Widgeon grass bed of the Patos Lagoon Estuary, RS, Brazil. Neotropical Ichthyology, 3(3): 427–432. Doi: 10.1590/S1679-62252005000300011 |
| Gibson R A, 1983. Australian fish-an excellent source of both arachidonic acid and ω-3 polyunsaturated fatty acids. Lipids, 18(11): 743–752. Doi: 10.1007/BF02534631 |
| Harris R K, Nishiyama T, Paul A J, 1986. Carbon, nitrogen and caloric content of eggs, larvae, and juveniles of the walleye pollock. Theragra chalcogramma.Journal of Fish Biology, 29(1): 87–98. Doi: 10.1111/jfb.1986.29.issue-1 |
| Heimann W. 1980. Fundamentals of Food Chemistry. AVI Publishing Company, Westport, Connecticut, USA. 344p. |
| Henderson R J, Tocher D R, 1987. The lipid composition and biochemistry of freshwater fish. Progress in Lipid Research, 26(4): 281–347. Doi: 10.1016/0163-7827(87)90002-6 |
| Hibbeln J R, Nieminen L R, Blasbalg T L, Riggs J A, Lands W E, 2006. Healthy intakes of n-3 and n-6 fatty acids:estimations considering worldwide diversity. The American Journal of Clinical Nutrition, 83(6): S1483–S1493. |
| Hung Y L, Hwang P A, Gau S Y, Wu C H, 2008. Antioxidative and Immune activities of Hippocampus kuda extract. Journal of Taiwan Fisheries Research, 16(2): 97–105. |
| Kumaravel K, Ravichandran S, Balasubramanian T, Sonneschein L, 2012. Seahorses-A source of traditional medicine. Natural Product Research, 26(24): 2330–2334. Doi: 10.1080/14786419.2012.662650 |
| Larsson S C, 2013. Dietary fats and other nutrients on stroke. Current Opinion in Lipidology, 24(1): 41–48. Doi: 10.1097/MOL.0b013e3283592eea |
| Lin Q, Lin J D, Lu J Y, Li B J, 2008. Biochemical composition of six seahorse species,Hippocampus sp. from the Chinese Coast. Journal of the World Aquaculture Society, 39(2): 225–234. Doi: 10.1111/j.1749-7345.2008.00159.x |
| Lin Q, Lin J D, Wang C, 2009. Biochemical composition of the wild and cultured seahorses, Hippocampus kuda Bleeker and Hippocampus trimaculatus Leach. Aquaculture Research, 40(6): 710–719. Doi: 10.1111/are.2009.40.issue-6 |
| Lv J Y, Li B J, Sun Y Y, Yang D W, Huang K, 2002. The ingestion, growth and ecological conversion efficiency of Hippocampus kuda under the intensive rearing conditions. Journal of Fisheries of China, 26(1): 61–66. |
| Martinez-Cardenas L, Sumaya-Martínez M T, ValdezHernández E F, González-Díaz A A, Soria-Barreto M, Castañeda-Chavez M R, Ruiz-Velazco J M, Peña-Messina E, 2013. Effect of Temperature on growth and survival in juvenile Opossum pipefish, Microphis brachyurus:first observations on the species in culture conditions. Journal of the World Aquaculture Society, 44(5): 735–742. Doi: 10.1111/jwas.2013.44.issue-5 |
| Martin-Smith K M, Lam T F, Lee S K, 2003. Trade in pipehorses, Solegnathus spp. for traditional medicine in Hong Kong. TRAFFIC Bulletin, 19(3): 139–148. |
| Martin-Smith K M, Vincent A C J, 2006. Exploitation and trade of Australian seahorses, pipehorses, sea dragons and pipefishes (family Syngnathidae). Oryx, 40(2): 141–151. Doi: 10.1017/S003060530600010X |
| Mat Jais A M, McCulloh R, Croft K, 1994. Fatty acid and amino acid composition in haruan as a potential role in wound healing. General Pharmacology, 25(5): 947–950. Doi: 10.1016/0306-3623(94)90101-5 |
| Mazereeuw G, Lanctôt K L, Chau S A, Swardfager W, Herrmann N, 2012. Effects of omega-3 fatty acids on cognitive performance:a meta-analysis. Neurobiology of Aging, 33(7): 1482. Doi: 10.1016/j.neurobiolaging.2011.12.014 |
| Meng X Q, Xu D H, Mei X T, Xu S B, Lv J Y, Li B J, 2005. Research on Hippocampus capsule therapy of experimental benign prostatic hyperplasia. Chinese Pharmaceutical Journal, 40(3): 190–193. |
| Moreau M A, Hall H J, Vincent A C J. 1998. Proceedings of the First International Workshop on The Management and Culture of Marine Species Used in Traditional Medicines. Project Seahorse, Montreal, Canada. |
| Morris C A, Haynes K C, Keeton J T, Gatlin D M, 1995. Fish oil dietary effects on fatty acid composition and flavor of channel catfish. Journal of Food Science, 60(6): 1. Doi: 10.1111/j.1365-2621.1995.tb04561.x |
| Murugan A, Dhanya S, Sreepada R A, Rajagopal S, Balasubramanian T, 2009. Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture, 290(1-2): 87–96. Doi: 10.1016/j.aquaculture.2009.01.033 |
| Orban E, Nevigato T, Masci M, Di Lena G, Casini I, Caproni R, Gambelli L, De Angelis P, Rampacci M, 2007. Nutritional quality and safety of European perch (Perca fluviatilis) from three lakes of central Italy. Food Chemistry, 100(2): 482–490. Doi: 10.1016/j.foodchem.2005.09.069 |
| Osman H, Suriah A R, Law E C, 2001. Fatty acid composition and cholesterol content of selected marine fish in Malaysian waters. Food Chemistry, 73(1): 55–60. Doi: 10.1016/S0308-8146(00)00277-6 |
| Payne M F, Rippingale R J, 2000. Rearing West Australian seahorse, Hippocampus subelongatus juveniles on copepod nauplii and enriched Artemia. Aquaculture, 188(3-4): 353–361. Doi: 10.1016/S0044-8486(00)00349-5 |
| Perumal P, Rajkumar M, Santhanam P, 2009. Biochemical composition of wild copepods, Acartia spinicauda and Oithona similis, from Parangipettai coastal waters in relation to environmental parameters. Journal of Environmental Biology, 30(6): 995–1. |
| Pogonoski J J, Pollard D A, Paxton J R, 2002. Conservation overview and action plan for Australian threatened and potentially threatened marine and estuarine fishes. Canberra, Australia, Environment Australia. |
| Qian Z J, Kang K H, Kim S K, 2012. Isolation and antioxidant activity evaluation of two new phthalate derivatives from seahorse, Hippocampus kuda Bleeker. Biotechnology and Bioprocess Engineering, 17(5): 1031–1040. Doi: 10.1007/s12257-012-0115-1 |
| Qian Z J, Ryu B, Kim M M, Kim S K, 2008. Free radical and reactive oxygen species scavenging activities of the extracts from seahorse, Hippocampus kuda Bleeker. Biotechnology and Bioprocess Engineering, 13(6): 705–715. Doi: 10.1007/s12257-008-0093-5 |
| Rethna Priya E, Ravichandran S, Ezhilmathi R, 2013. Bioactive proteins from pipefishes. Journal of Coastal Life Medicine, 1(1): 1–5. Doi: 10.14511/jlm.2329.5430 |
| Rosa I L, Defavari G R, Alves R R N, Oliveira T P R. 2013. Seahorses in traditional medicines: a global review. In: Alves R R N, Rosa I L eds. Animals in Traditional Folk Medicine. Springer-Verlag, Berlin Heidelberg. p. 207-240, http://dx.doi.org/10.1007/978-3-642-29026-8-10. |
| Sanaye S V, Pise N M, Pawar A P, Parab P P, Sreepada R A, Pawar H B, Revankar A D, 2014. Evaluation of antioxidant activities in captive-bred cultured yellow seahorse, Hippocampus kuda (Bleeker, 1852). Aquaculture, 434: 100–107. Doi: 10.1016/j.aquaculture.2014.08.007 |
| Sanaye S V, Pise N M, Pawar A P, Parab P P, Sreepada R A, Pawar H B, Murugan A, 2015. Total phenolic content and in-vitro antioxidant activities from methanolic extract of alligator pipefish, Syngnathoides biaculeatus (Bloch, 1785). Indian Journal of Geo-Marine Sciences, 44(9): 1352–1357. |
| Shi R C, Yao L X, Bei X F, Zhu J P, Wang J, 2006. Chinese medicine rehabilitative therapy for periarthritis of shoulder. Chinese Journal of Clinical Rehabilitation, 10(47): 150–152. |
| Shi R, Zhang Y H, Wang Z G, 1993. Experimental studies on Hailong extracts from Syngnathoides biaculeatus. 1. The influences of Hailong extracts on human PBL proliferation and human tumor cell lines. Chinese Journal of Marine Drugs(2): 4–6. |
| Simopoulos A P, 2006. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation:nutritional implications for chronic diseases. Biomedicine & Pharmacotherapy, 60(9): 502–507. Doi: 10.1016/j.biopha.2006.07.080 |
| Spitz J, Mourocq E, Schoen V, Ridoux V, 2010. Proximate composition and energy content of forage species from the Bay of Biscay:high-or low-quality food?. ICES Journal of Marine Science, 67(5): 909–915. Doi: 10.1093/icesjms/fsq008 |
| Sreepada R A, Desai U M, Naik S, 2002. The plight of Indian seahorses:need for conservation and management. Current Science, 82(4): 377–378. |
| Tipton K, Bell S S, 1988. Foraging patterns of two syngnathid fishes:importance of harpacticoid copepods. Marine Ecology Progress Series, 47: 31–43. Doi: 10.3354/meps047031 |
| Vincent A C J, 1995. Exploitation of seahorses and pipefishes. NAGA, the ICLARM Quarterly, 18(1): 18–19. |
| Vincent A C J, 1996. The international trade in seahorse. TRAFFIC International Cambridge, UK: 172p. |
| Wijesekara I, Qian Z J, Ryu B, Ngo D H, Kim S K, 2011. Purification and identification of antihypertensive peptide from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Research International, 44(3): 703–707. Doi: 10.1016/j.foodres.2010.12.022 |
| Witte M B, Thornton F J, Tantry U, Barbul A, 2002. L-Arginine supplementation enhances diabetic wound healing:involvement of the nitric oxide synthase and arginase pathways. Metabolism, 51(10): 1269–1273. Doi: 10.1053/meta.2002.35185 |
| Wood J D, Richardson R I, Nute G R, Fisher A V, Campo M M, Kasapidou E, Sheard P R, Enser M, 2004. Effects of fatty acids on meat quality:a review. Meat Science, 66(1): 21–32. Doi: 10.1016/S0309-1740(03)00022-6 |
| Woods A E, Aurand L W, 1977. Laboratory Manual in Food Chemistry. The AVI Publishing Company, Inc.Westport, CT.: 77p. |
| Wu G Y, 2009. Amino acids:metabolism, functions, and nutrition. Amino Acids, 37(1): 1–17. Doi: 10.1007/s00726-009-0269-0 |
| Xu D H, Mei X T, Li B J, Lin Z L, Xu S B, 2003. The pharmacological effects of Hippocampus capsule on enhancing sexual functions of rats. Journal of Chinese Medicinal Materials, 26(11): 807–808. |
| Zhang C H, Ni Q G, Wu L Y, Wang Q, Xu L S, Xu G J, 1998. Studies on antitumor activity of Trachyrhamphus serratus. Chinese Journal of Marine Drugs(4): 10–12. |
| Zhang N, Xu B, Mou C Y, Yang W L, Wei J W, Lu L, Zhu J J, Du J C, Wu X K, Ye L T, Fu Z Y, Lu Y, Lin J H, Sun Z Z, Su J, Dong M L, Xu A L, 2003. Molecular profile of the unique species of traditional Chinese medicine, Chinese seahorse(Hippocampus kuda Bleeker). FEBS Letters, 550(1-3): 124–134. Doi: 10.1016/S0014-5793(03)00855-X |
| Zuraini A, Somchit M N, Solihah M H, Goh Y M, Arifah A K, Zakaria M S, Somchit N, Rajion M A, Zakaria Z A, Mat Jais A M, 2006. Fatty acid and amino acid composition of three local Malaysian Channa spp. fish. Food Chemistry, 97(4): 674–678. Doi: 10.1016/j.foodchem.2005.04.031 |
 2017, Vol. 35
2017, Vol. 35


