Institute of Oceanology, Chinese Academy of Sciences
Article Information
- XIE Shijun(谢世筠), LI Fuhua(李富花), ZHANG Xiaojun(张晓军), ZHANG Jiquan(张继泉), XIANG Jianhai(相建海)
- Peritrophin-like protein from Litopenaeus vannamei (LvPT) involved in white spot syndrome virus (WSSV) infection in digestive tract challenged with reverse gavage
- Chinese Journal of Oceanology and Limnology, 35(6): 1524-1530
- http://dx.doi.org/10.1007/s00343-017-6109-2
Article History
- Received Apr. 6, 2016
- accepted in principle May. 11, 2016
- accepted for publication Aug. 24, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
The peritrophic membrane (PM) is an acellular structure lining the guts of many arthropods that separates the ingested food from the gut epithelium (Eiseman and Binnington, 1994). In Litopenaeus vannamei, the PM is an acellular membranous sac, enclosing ingested materials and passing them through the shrimp gut as fecal pellets (Wang et al., 2012). As well as digestion-related proteins and proteins related to the PM structure, immune-related and antioxidant proteins have been identified in the PM with liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/ MS) (Wang et al., 2012). This suggests that the PM also plays an important role in the gut defense system. White spot syndrome virus (WSSV) is a major pathogen in the shrimp culture industry, causing high mortality and enormous economic losses. Many studies have reported that WSSV can infect shrimp through the oral route (Chou et al., 1998; Di Leonardo et al., 2005). The digestive epithelial cells in the midgut of Marsupenaeus japonicus are considered the principal sites of WSSV replication (Di Leonardo et al., 2005). A study of Sicyonia ingentis reported that only particles smaller than 20 nm can physically pass through the PM (Martin et al., 2006). However, the size of WSSV (70–150 nm at its broadest point and 250–380 nm in length) greatly exceeds the permissible limit for penetrating the PM in S. ingentis (Wang et al., 1995; Escobedo-Bonilla et al., 2008).
In previous research, we found that a peritrophinlike protein from L. vannamei (LvPT) interacts with several envelope proteins of WSSV. LvPT is mainly expressed in the stomach and may participate in the formation of the PM (Xie et al., 2015). Therefore, it is reasonable to infer that LvPT plays a role in WSSV infection of the digestive tract. Several experiments were performed in this study to explore the functions of LvPT in WSSV infection of the digestive tract. We produced an LvPT polyclonal antibody and synthesized LvPT double-stranded RNA (dsRNA). After the expression of LvPT in shrimp was successfully interrupted with RNA interference (RNAi) at both the transcript and protein levels, the experimental animals were challenged with WSSV with reverse gavage. The amounts of WSSV in the pleopods, stomachs, and guts at different times after infection were measured.
2 MATERIAL AND METHOD 2.1 Experimental shrimp and virus preparationHealthy Pacific white shrimp (L. vannamei) with a body length of (5.5±0.5) cm and a weight of (3.5± 0.5) g, were cultured in the laboratory and used in this study. The WSSV used for the challenge experiment was purified from infected Exopalaemon carinicauda and quantified according to a previous study performed in our laboratory (Sun et al., 2013b).
2.2 Production of LvPT polyclonal antibody and binding specificity testThe full nucleotide sequence of LvPT was amplified from a yeast two-hybrid library of L. vannamei (Xie et al., 2015) with specific primers (Table 1). The deduced amino acid sequence of LvPT was analyzed with the GenScript OptimumAntigenTM design tool. The epitopic peptide CKQEGRYPDLLNQQ (amino acids 202–215) was synthesized, conjugated to keyhole limpet hemocyanin, and injected into rabbits to produce anti-LvPT antibodies. Both the preimmune serum and the polyclonal antibody were collected in this study, and the binding specificity of the antibody was tested against the total protein from the L. vannamei stomach.
Molecules of LvPT dsRNA and enhanced green fluorescent protein (EGFP) dsRNA were prepared according to Wen et al. (2014). Briefly, full-length LvPT with the T7 promoter sequence (858 bp) was amplified with specific primers (Table 1). The PCR product was purified with the Gel Extraction Kit (Omega, USA) and used as the template for dsRNA synthesis with the TranscriptAid T7 High Yield Transcription Kit (Thermo Fisher Scientific, USA). A fragment containing the T7 promoter sequence (289 bp) was amplified from pEGFP-N1 with specific primers (Table 1) and used as the template to synthesize EGFP dsRNA. EGFP dsRNA was synthesized with the same kit. The purity of the synthesized dsRNAs was examined with electrophoresis on 1% agarose gel and the concentrations of the dsRNAs were measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). After their quality and concentrations were determined, the synthesized dsRNAs were stored at -80℃ for later use.
2.4 Determination of optimized injection dose for dsRNA interference (dsRNAi)To achieve the optimal silencing efficiency with LvPT dsRNA, three groups (n=3) of shrimp were injected with different doses of dsRNA (1, 2, or 4 μg/shrimp). LvPT or EGFP dsRNA was injected into the last abdominal segment of the shrimp and each treatment was performed in triplicate. At 72 h postinjection (hpi), the three shrimp from each group were collected, and their stomachs were isolated and frozen in liquid nitrogen for total RNA and protein extraction.
Total RNA was extracted with RNAiso Plus (TaKaRa, Japan), according the user manual, and quantified with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). The total RNA was reverse transcribed to cDNA with the PrimeScriptTM RT Reagent Kit with gDNA Eraser (TaKaRa, Dalian). The relative expression of LvPT was analyzed with realtime quantitative PCR (qPCR), using 18S rRNA as the internal control, according to previous studies (Du et al., 2013; Lan et al., 2013; Wen et al., 2014; Yang et al., 2015). The primers used in qPCR are shown in Table 1. The qPCR analysis of 18S rRNA was performed with the following cycling parameters: 40 cycles of 95℃ for 15 s, 57℃ for 15 s, and 72℃ for 20 s. The qPCR for LvPT was performed with the following cycling parameters: 40 cycles of 95℃ for 15 s, 59℃ for 15 s, and 72℃ for 20 s. The relative expression of LvPT was calculated with the comparative Ct method using the formula 2-ΔΔCt (Livak and Schmittgen, 2001). An unpaired two-tailed t test and Tukey's multiple comparison test were used for the statistical analysis, with the GraphPad Prism software (version 5.0). A P value less than 0.01 was considered statistically significant.
To confirm the efficiency of LvPT dsRNA silencing, the total protein in samples taken from the group with the lowest relative LvPT expression was extracted with the Total Protein Extraction Kit (BestBio, China), according to the user manual. After the proteins were separated with 10% SDS-PAGE, they were transferred to a polyvinylidene difluoride (PVDF) membrane, which was incubated with blocking buffer (TBS; 50 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 2% nonfat milk) at room temperature (25℃) for 2 h and then with blocking buffer containing the rabbit anti-LvPT polyclonal antibody or a mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (CWBIO, China) for 1 h at room temperature. The membrane was then washed three times with 1% Tween 20 in TBS. A horseradish peroxidase (HRP)-conjugated goat antirabbit IgG antibody or HRP-conjugated goat antimouse IgG antibody was used as the secondary antibody. Both secondary antibodies were detected with a commercial HRP-DAB Chromogenic Substrate Kit (Tiangen, China).
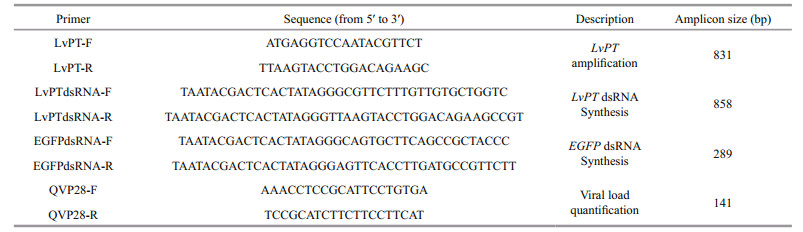
2.5 WSSV challenge and tissue samplingThe dose of dsRNA used for injection was optimized, and 4 μg of EGFP dsRNA or LvPT dsRNA was used in the WSSV challenge experiment. Ninety shrimp were divided into two groups. In the first group, each shrimp was injected with 4 μg of EGFP dsRNA, and in the second group, the shrimp were injected with 4 μg of LvPT dsRNA. At 72 h after injection, nine shrimp from each group (three individuals per replicate) were collected as the baseline (0 h) samples. The other shrimp in both groups were challenged with WSSV using reverse gavage. The WSSV solution was injected with a pipette with a 10-μL tip and the tip was inserted 2–3 mm into the anus during injection (Fig. 1a). Each shrimp was injected with 1×105 copies of WSSV in 10 μL of solution. Phenol red (which had no adverse effect on WSSV infectivity) was added to the solution at a final concentration at 0.1% (w/v) as an internal marker to evaluate the success of injection (Fig. 1b,c). The time at WSSV injection was deemed to be 0 h, and samples were collected separately at 24, 48, and 72 hpi. At each sampling time, the pleopods, stomachs, and guts of nine shrimp in each group (3 individuals per replicate) were collected and frozen separately in liquid nitrogen for DNA extraction.
|
| Figure 1 Shrimp (body length (5.5±0.5) cm) challenged with WSSV using reverse gavage a. shrimp injected with a pipette with a 10 μL tip; b. dorsal view; c. lateral view. |
DNA was extracted from each sample with a Plant Genomic DNA Kit (Tiangen), according to the user manual, and proteinase K was added at a final concentration of 0.5 μg/μL during sample digestion. The extracted DNA was subjected to electrophoresis on 1% agarose gel and quantified with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). The WSSV copy number was determined in all the DNA samples. The method used in this study has been reported previously (Sun et al., 2013a). Briefly, the WSSV VP28 gene was inserted into the pMD19-T Simple vector (TaKaRa, Dalian) and the plasmid was purified. After purification and quantification with a NanoDrop 2000 spectrophotometer, the copy number of the plasmid was calculated. Serially diluted solutions of the plasmid (1×103, 1×104, 1×105, 1×106, 1×107, and 1×108 copy/μL) were used as the samples to generate a standard curve for qPCR with primers QVP28-F and QVP28-R (Table 1). The copy number in each sample was converted to copies/ng DNA, based on the DNA concentration measured above. The statistical analysis is described in Section 2.4.
3 RESULT 3.1 Binding specificity of anti-LvPT polyclonal antibodyAccording to the immunoblotting results, the LvPT polyclonal antibody bound a band of ~27 kDa, whereas the preimmune serum bound no protein (Fig. 2). Excluding the signal peptide, the theoretical molecule weight of LvPT is 29 kDa. Therefore, the band indicated by the asterisk in Fig. 2 was considered to be LvPT. Immunoblotting also confirmed that the LvPT polyclonal antibody specifically bound LvPT, at ~27 kDa.

|
| Figure 2 Binding specificity of the anti-LvPT polyclonal antibody Total proteins from the stomach were used to test the binding specificity of the anti-LvPT polyclonal antibody. Preimmune serum was incubated with the PVDF membrane as the negative control. The band indicated by the asterisk at ~27 kDa is LvPT. |
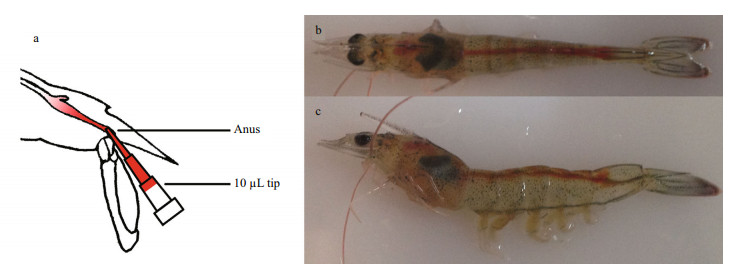
The quality of the synthesized dsRNA was evaluated with electrophoresis on 1% agarose gel. The expected sizes of the EGFP dsRNA and LvPT dsRNA were 289 bp and 858 bp, respectively. According to the result of electrophoresis (Fig. 3), both the quality and sizes of the dsRNAs were suitable for the interference experiment. The concentrations of synthesized EGFP dsRNA and LvPT dsRNA were 1.86 μg/μL and 1.44 μg/μL, respectively.
|
| Figure 3 Quality of dsRNAs synthesized in vitro Synthesized EGFP dsRNA and LvPT dsRNA were subjected to electrophoresis on 1% agarose gel. Lane M contains the DL 2000 DNA Marker (TaKaRa, Dalian). Lanes dsEGFP and dsLvPT contain the synthesized EGFP dsRNA and LvPT dsRNA, respectively. |
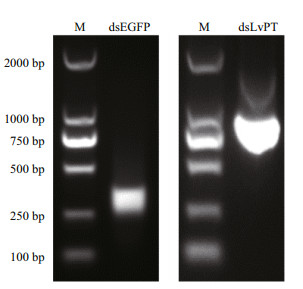
The relative transcriptional expression of LvPT 72 h after the injection of different doses of EGFP or LvPT dsRNA is shown in Fig. 4a. Shrimp injected with 4 μg of LvPT dsRNA showed the most efficient silencing, with about 0.4% LvPT expression compared with that in the control group (shrimp injected with 4 μg of EGFP dsRNA). The expression of LvPT protein in the group injected with 4 μg of dsRNA was also detected with immunoblotting (Fig. 4b). Therefore, 4 μg of dsRNA per shrimp was used for the subsequent interference experiments.
|
| Figure 4 Silencing efficiency of LvPT dsRNA injection a. transcript expression of LvPT in the stomach 72 h after the injection of different doses of dsRNA. **P<0.01; b. expression of LvPT protein in the stomachs of shrimp injected with 4 μg of dsRNA. |
To study the function of LvPT during WSSV infection of the digestive tract, the WSSV copy numbers in the shrimp from the EGFP dsRNAi + WSSV group and the LvPT dsRNAi + WSSV group were determined at different times after infection (Fig. 5). At 0 h and 24 hpi, the WSSV copy numbers in the two groups did not differ in any of the three tissues tested. However, at 48 hpi and 72 hpi, the WSSV copy numbers in the EGFP dsRNAi + WSSV group were significant higher than those in the LvPT dsRNAi + WSSV group in all three tissues.
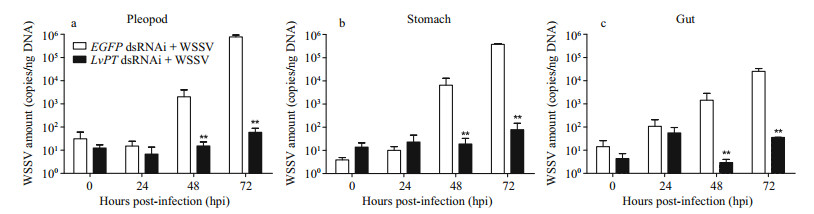
|
| Figure 5 Comparison of WSSV copy numbers in shrimp from the EGFP dsRNAi + WSSV group and LvPT dsRNAi + WSSV group a. WSSV copy numbers in pleopod; b. WSSV copy numbers in stomach; c. WSSV copy numbers in gut. **P<0.01. |
The interactions between LvPT and the envelope proteins of WSSV (VP32, VP37, VP38A, VP39B, and VP41A) have been reported (Xie et al., 2015). However, whether LvPT facilitates WSSV infection remained unknown. To explore the functions of LvPT in WSSV infection of the digestive tract, an RNAi experiment with dsRNA and a WSSV challenge experiment were performed. In Drosophila S2 cells, the natural uptake of dsRNAs is length dependent, with longer dsRNAs showing more efficient silencing (Saleh et al., 2006). Therefore, we used the full length of LvPT as the template for LvPT dsRNA synthesis. Silencing efficiency also differs when different doses of dsRNA are injected (Wen et al., 2014). Therefore, to knock down LvPT expression as far as possible, we optimized the injection dose for dsRNAi.
To evaluate the expression of LvPT at the protein level in the dsRNAi experiment, we produced an LvPT polyclonal antibody. In a previous study, a polyclonal antibody was prepared using a purified recombinant protein as the antigen (Wen et al., 2013; Yang et al., 2015). Here, we predicted the epitope peptide of LvPT with bioinformatic software and used the predicted peptide as the antigen to generate the LvPT polyclonal antibody. Immunoblotting showed that this antibody specifically bound to LvPT, with no nonspecific bands between 25 kDa and 35 kDa. Relative to the molecular-weight marker, the approximate molecular weight of LvPT was 27 kDa. In previous research, a variety of PM proteins, ranging in length from 30 kDa to 116 kDa, were identified with LC-ESI-MS/MS (Wang et al., 2012). However, the proteins around 27 kDa were not included in the sample used for the LC-ESI-MS/MS analysis, which explains why no peritrophin-like protein was identified (Wang et al., 2012).
After the silencing efficiency of dsRNAi was confirmed at both the transcript and protein levels, we performed a WSSV challenge experiment with reverse gavage. WSSV challenge experiments are usually performed with immersion (Chou et al., 1995, 1998; Arts et al., 2007), abdominal segment injection (Sun et al., 2013a; Wen et al., 2014; Yang et al., 2015), or oral injection (Escobedo-Bonilla et al., 2005). Although the amount of WSSV in the solution used for immersion can be quantified, the amount of WSSV that enters each experimental shrimp remains unknown. Abdominal segment injection ensures that the experimental animals are challenged with a specific amount of WSSV. However, the viral particles directly enter the hemolymph, so abdominal segment injection does not simulate WSSV infection of the digestive tract. Oral injection entails a high risk of physical damage to the oral cavity, and even the stomach, during injection, which would directly expose the hemolymph to WSSV. To solve these problems, we used the modified approach of reverse gavage in the WSSV challenge experiment based on previous bacterial challenge experiments (Aranguren et al., 2010; Lee et al., 2015). Reverse gavage has obvious advantages over immersion, abdominal segment injection, and oral injection. First, it ensures that the experimental animals are infected with a specific amount of WSSV and guarantees that the digestive tract is the initially targeted tissue. Second, all the experimental individuals infected with 1×105 copies of WSSV die within 7 days (data not shown; this result was obtained during the optimization of the WSSV dose to be injected, performed before the WSSV challenge experiment). Third, injection via the anus is simple and causes less damage to the experimental animal than oral injection. To clearly determine whether each experimental individual was successfully injected with WSSV solution, phenol red was used as an internal marker.
According to qPCR, the amounts of WSSV were significantly lower in the three tissues of the LvPT dsRNAi + WSSV group than in those of the EGFP dsRNAi + WSSV group at 48 and 72 hpi. These results suggest that reducing LvPT reduces WSSV infection. Penetrating the physical barrier of the PM is essential for WSSV infection of the digestive tract, and LvPT is associated with the PM structure. Therefore, we believe that LvPT might act as an attachment factor during WSSV infection of the digestive tract. Previous research in E. carinicauda also demonstrated that silencing the expression of EcPT increased the survival rate after WSSV challenge (Wang et al., 2013). The results of both studies suggest that silencing the expression of the peritrophin-like protein protects shrimp from WSSV infection and that this protein plays an important role during WSSV infection of shrimp.
5 CONCLUSIONThe results of this study suggest that LvPT acts as an attachment factor during WSSV infection of the digestive tract. However, WSSV infection of the digestive tract is a complex process. The PM is an acellular structure and WSSV attachment to the PM is only the first step in overcoming the physical barrier it presents in the digestive tract. Many other factors, such as the receptors on the surface epithelial cells in the digestive tract, remain unknown and further studies are required to determine the complete mechanism of WSSV infection in the digestive tract.
6 ACKNOWLEDGMENTWe sincerely thank the Guangtai Company (Hainan, China) for providing the postlarval shrimp for this study.
| Aranguren L F, Tang K F J, Lightner D V, 2010. Quantification of the bacterial agent of necrotizing hepatopancreatitis(NHP-B) by real-time PCR and comparison of survival and NHP load of two shrimp populations. Aquaculture, 307(3-4): 187–192. Doi: 10.1016/j.aquaculture.2010.07.022 |
| Arts J A J, Taverne-Thiele A J, Savelkoul H F J, Rombout J H W M, 2007. Haemocyte reactions in WSSV immersion infected Penaeus monodon. Fish & Shellfish Immunology, 23(1): 164–170. |
| Chou H Y, Huang C Y, Lo C F, Kou G H, 1998. Studies on transmission of white spot syndrome associated baculovirus (WSBV) in Penaeus monodon and P.japonicus via waterborne contact and oral ingestion. Aquaculture, 164(1-4): 263–276. Doi: 10.1016/S0044-8486(98)00192-6 |
| Chou H Y, Huang C Y, Wang C H, Chiang H C, Lo C F, 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Diseases of Aquatic Organisms, 23(3): 165–173. |
| Di Leonardo V A, Bonnichon V, Roch P, Parrinello N, Bonami J R, 2005. Comparative WSSV infection routes in the shrimp genera Marsupenaeus and Palaemon. Journal of Fish Diseases, 28(9): 565–569. Doi: 10.1111/jfd.2005.28.issue-9 |
| Du Z Q, Lan J F, Weng Y D, Zhao X F, Wang J X, 2013. BAX inhibitor-1 silencing suppresses white spot syndrome virus replication in red swamp crayfish, Procambarus clarkii. Fish & Shellfish Immunology, 35(1): 46–53. |
| Eiseman C H, Binnington K C, 1994. The peritrophic membrane:its formation, structure, chemical composition and permeability in relation to vaccination against ectoparasitic arthropods. International Journal for Parasitology, 24(1): 15–26. Doi: 10.1016/0020-7519(94)90055-8 |
| Escobedo-Bonilla C M, Alday-Sanz V, Wille M, Sorgeloos P, Pensaert M B, Nauwynck H J, 2008. A review on the morphology, molecular characterization, morphogenesis and pathogenesis of white spot syndrome virus. Journal of Fish Diseases, 31(1): 1–18. |
| Escobedo-Bonilla C M, Wille M, Sanz V A, Sorgeloos P, Pensaert M B, Nauwynck H J, 2005. In vivo titration of white spot syndrome virus (WSSV) in specific pathogenfree Litopenaeus vannamei by intramuscular and oral routes. Diseases of Aquatic Organisms, 66(2): 163–170. |
| Lan J F, Li X C, Sun J J, Gong J, Wang X W, Shi X Z, Shi L J, Weng Y D, Zhao X F, Wang J X, 2013. Prohibitin interacts with envelope proteins of white spot syndrome virus and prevents infection in the red swamp crayfish, Procambarus clarkii. Journal of Virology, 87(23): 12756–12765. Doi: 10.1128/JVI.02198-13 |
| Lee C T, Chen I T, Yang Y T, Ko T P, Huang Y T, Huang J Y, Huang M F, Lin S J, Chen C Y, Lin S S, Lightner D V, Wang H C, Wang A H J, Wang H C, Hor L I, Lo C F, 2015. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proceedings of the National Academy of Sciences of the United States of America, 112(34): 10798–10803. Doi: 10.1073/pnas.1503129112 |
| Livak K J, Schmittgen T D, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ct method. Methods, 25(4): 402–408. Doi: 10.1006/meth.2001.1262 |
| Martin G G, Simcox R, Nguyen A, Chilingaryan A, 2006. Peritrophic membrane of the penaeid shrimp Sicyonia ingentis:structure, formation, and permeability. Biological Bulletin, 211(3): 275–285. Doi: 10.2307/4134549 |
| Saleh M C, van Rij R P, Hekele A, Gillis A, Foley E, O'Farrell P H, Andino R, 2006. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nature Cell Biology, 8(8): 793–802. Doi: 10.1038/ncb1439 |
| Sun Y M, Li F H, Chi Y H, Xiang J H, 2013b. Enhanced resistance of marine shrimp Exopalamon carincauda Holthuis to WSSV by injecting live VP28-recombinant bacteria. Acta Oceanologica Sinica, 32(2): 52–58. Doi: 10.1007/s13131-013-0261-0 |
| Sun Y M, Li F H, Xiang J H, 2013a. Analysis on the dynamic changes of the amount of WSSV in Chinese shrimp Fenneropenaeus chinensis during infection. Aquaculture, 376(379): 124–132. |
| Wang C H, Lo C F, Leu J H, Chou C M, Yeh P Y, Chou H Y, Tung M C, Chang C F, Su M S, Kou G H, 1995. Purification and genomic analysis of baculovirus associated with white spot syndrome (WSBV) of Penaeus monodon. Diseases of Aquatic Organisms, 23(3): 239–242. |
| Wang L Y, Li F H, Wang B, Xiang J H, 2012. Structure and partial protein profiles of the peritrophic membrane (PM) from the gut of the shrimp Litopenaeus vannamei. Fish & Shellfish Immunology, 33(6): 1285–1291. |
| Wang L Y, Li F H, Wang B, Xiang J H, 2013. A new shrimp peritrophin-like gene from Exopalaemon carinicauda involved in white spot syndrome virus (WSSV) infection. Fish & Shellfish Immunology, 35(3): 840–846. |
| Wen R, Li F H, Li S H, Xiang J H, 2014. Function of shrimp STAT during WSSV infection. Fish & Shellfish Immunology, 38(2): 354–360. |
| Wen R, Li F H, Sun Z, Li S H, Xiang J H, 2013. Shrimp MyD88 responsive to bacteria and white spot syndrome virus. Fish & Shellfish Immunology, 34(2): 574–581. |
| Xie S J, Zhang X J, Zhang J Q, Li F H, Xiang J H, 2015. Envelope proteins of white spot syndrome virus (WSSV) interact with Litopenaeus vannamei peritrophin-like protein (LvPT). PLoS One, 10(12): e0144922. Doi: 10.1371/journal.pone.0144922 |
| Yang H, Li S H, Li F H, Wen R, Xiang J H, 2015. Analysis on the expression and function of syndecan in the Pacific white shrimp Litopenaeus vannamei. Developmental & Comparative Immunology, 51(2): 278–286. |
 2017, Vol. 35
2017, Vol. 35