Institute of Oceanology, Chinese Academy of Sciences
Article Information
- XIE Jinlin(谢金林), YU Gongliang(虞功亮), XU Xudong(徐旭东), LI Shouchun(李守淳), LI Renhui(李仁辉)
- The morphological and molecular detection for the presence of toxic Cylindrospermopsis (Nostocales, Cyanobacteria) in Beijing city, China
- Chinese Journal of Oceanology and Limnology, 36(2): 263-272
- http://dx.doi.org/10.1007/s00343-018-6283-x
Article History
- Received Oct. 29, 2016
- accepted in principle Dec. 27, 2016
- accepted for publication Feb. 10, 2017
2 Key Laboratory of Algal Biology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
Cyanobacterial blooms have been reported to occur in freshwater bodies worldwide, and the occurrence of these blooms is becoming more frequent due to anthropogenic eutrophication and global warming (Paerl and Husiman, 2009; Bonilla et al., 2012; Sinha et al., 2012; Paerl and Otten, 2013). It was estimated that over 40 cyanobacterial genera were responsible for forming blooms, and subsequently likely to produce a wide range of harmful secondary metabolites, mainly including cyanotoxins and tasteodor substances (Paerl et al., 2015). Among these bloom-forming cyanobacteria, Cylindrospermopsis raciborskii (Woloszyńska) Seenayya et Subba Raju, the type species of Cylindrospermopsis genus, is a solitary heterocystous cyanobacterium that was initially found from the lakes of Java (Indonesia) in 1912 (Komárek and Kling, 1991). This species has been gradually recognized to poses a significant threat to ecological and human health because of its formation of blooms and production of toxic secondary metabolites such as hepatotoxic cylindrospemopsin and deoxy-cylindrospermopsin, and neurotoxic saxitoxins (Lagos et al., 1999; Saker and Eaglesham, 1999). Its occurrence was initially regarded to be limited in tropical environments, but over the past several decades its prevalence further into temperate regions, including northern Europe, New Zealand and the northern states of the USA, has rapidly increased (Hong et al., 2006; Burger et al., 2007; Kouzminov and Wood, 2007; Wiedner et al., 2007; Haande et al., 2008). The strategies adopted by this species to overgrow and out-compete other species has been summarized as its ecophysiological plasticity including its tolerance of a wide range of temperatures and light intensities, high phosphate affinity and storage capacity, nitrogen fixation or using dissolved inorganic nitrogen as nitrogen availability sources fluctuate (Burford et al., 2016), and these winning strategies may greatly contribute to its global expansion.
The planktonic genus Raphidiopsis, with R. curvata as the type species, is characterized by presence of akinetes (spores) but absence of heterocysts throughout the life cycle (Fritsch et al., 1929). Raphidiopsis is known to be morphologically similar to Cylindrospermopsis, especially in the cases where heterocytes and akinetes are not formed in Cylindrospermopsis species (Li et al., 2001; Moustaka-Gouni et al., 2009; Alster et al., 2010). It has been also found that both Cylindrospermopsis and Raphidiopsis genera shared the similar ecological niches, because they both often co-occur in the same water (Moustaka-Gouni et al., 2009). Further, the very highly genetic homologies were found between Cylindrospermopsis and Raphidiopsis genera, with more than 99% in 16S rRNA gene sequence and even more than 90% homology at genomic level (Stucken et al., 2009). Thus, the term Cylindrospermopsis/Raphidiopsis group has been used to represent their close association in many aspects.
Reports about occurrence of the blooms formed by Cylindrospermopsis/Raphidiopsis species and cylindrospermopsins in China have been recently increased, but mainly restricted in the south (Jiang et al., 2014; Lei et al., 2014). To obtain more information about distribution and expansion of Cylindrospermopsis in Chinese regions, surveys on waters along subtropical into temperate regions in China were performed during past years. In this study, we presented the findings about existence of Cylindrospermopsis/Raphidiopsis in Beijing city, and results based on morphological and molecular approaches clearly demonstrated that Cylindrospermopsis has quickly invaded into the north of China and the potential production of Cylindrospermopsin by this invasive species implicated water safety problem in this important huge city.
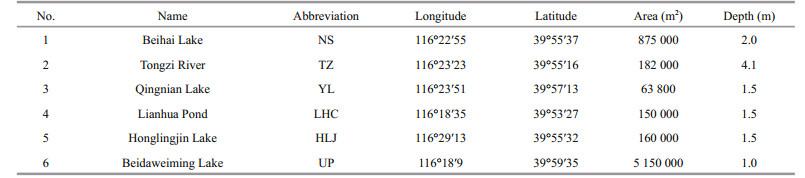
2 MATERIAL AND METHOD 2.1 Sampling sites and sample collectionBeijing is located at longitude 115.7°–117.4°E, latitude 39.4°–41.6°N, and its climate is the typical north temperate with semi-humid continental monsoon. A sampling was performed on September 18, 2015. As shown in Fig. 1, six sampling sites from different lakes/ponds were selected, and each sites were characterized as listed in Table 1. 500 mL of surface water samples were collected from the shoreline of each lakes, and all samples were kept in a cooler and brought back to the lab for further investigation.
|
| Figure 1 Location of six sampling sites The dark points represent the in situ stations during the sampling on September 18, 2015 and each name of the sampling sites is marked on each map. |
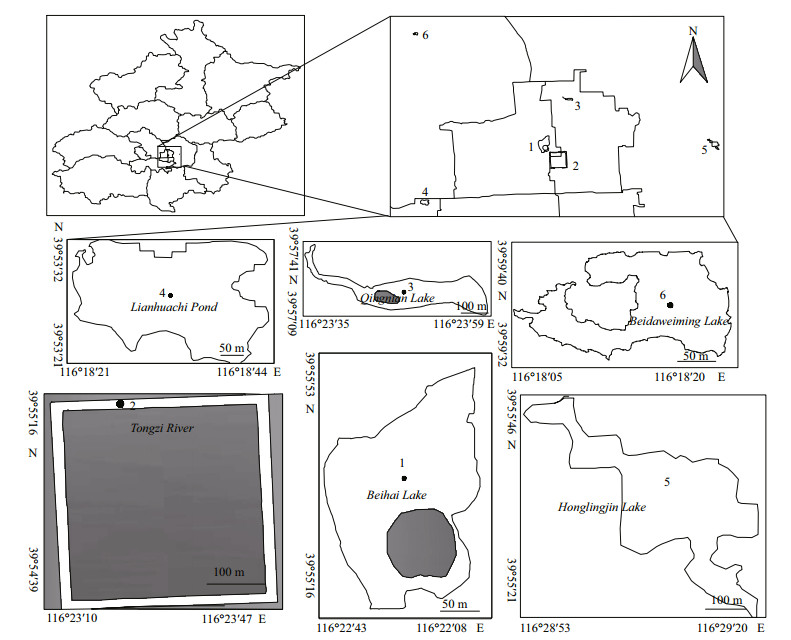
Chl-a concentrations were determined following the method of Jeffrey and Humphrey (1975). Water samples filtered through Whatman GF/C fiberglass membranes (NJ, USA), and add 90% ethanol into the filtered GF/C to extract the pigments. The extracts were then sonicated and kept at 4℃ for 24 h to ensure all pigments to fully extracted and eluted into the ethanol. Then the extracts were centrifuged at 5 000×g (Eppendorf, Germany) for 20 min and absorptances were measured at 750, 663, 645 and 630 nm. Chl-ɑ concentration was calculated according to the formula as:
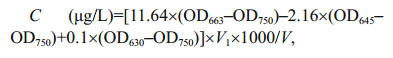
where C is Chl-a concentration (μg/L), V1 is the volume of ethanol (mL), V is the volume of water samples for filtration (L).
Total phosphorus (TP) was determined by the spectrophotometric determination of ammonium molybdate, and determination of total nitrogen (TN) by Ultraviolet Spectrophotometry with alkaline potassium persulfate digestion (Wu et al., 2006).
2.3 Morphological examinationThe morphological features of water samples were observed under a light microscope with Olympus BX-4l (Olympus, Japan), and the micrographs were captured by the microscope with DS-Ri1 digital camera (Nikon, Japan).
2.4 PCR, cloning and sequencingWater samples (1.5 mL) in Eppendorf tube were centrifuged (10 000×g, 5 min) and the supernatant removed by the sterile pipette, and 1 μL of the pellet was directly used for the PCR. The primer pair cyl-16sf56/cyl-16sr955 with specificity targeting C. raciborskii/Raphidiopsis was used to amplify the 16S rRNA gene fragment. Primers specific for current known CYN-producing species were cyrJf/cyrJr (Table 2). PCR was performed in a PTC-100 thermal cycler (MJ Research, USA). PCR mix was prepared in 50 μL volume containing 5 μL of 10×PCR buffer (TaKaRa, Japan), 10 nmol of each deoxynucleotide triphosphate, 10 pmol of each primer, 1 U of LA Taq (TaKaRa), and 1 μL intact cells as DNA templates. The cycling conditions were as follows: 95℃ for 5 min, followed by 35 cycles of 95℃ 30 s, 58℃ 30 s, 72℃ 30 s, and a final extension of 72℃ 5 min; and a 4℃ hold. PCR amplicons were visualized with 1% agarose gel electrophoresis with ethidium bromide staining under UV illumination.
The PCR amplicon was ligated into pMD18-T vector (TaKaRa, Japan) that was used to transform Escherichia coil DH5ɑ competent cells. The plasmid containing the inserted DNA fragment was purified using a PCR purification kit (Generay, China) according to the manufacturer's instructions. Two positive bacterial clones were randomly picked per clone library, and then sequencing was performed using an ABI 3730 automated sequencer (Applied Biosystems) in both directions. All available nucleotide sequences have been deposited in the GenBank database under accession numbers KX955225-KX955233 and KY234284-KY234286.
2.5 PhylogeneticanalysisThe obtained sequences of 16S rRNA and cyrJ genes in this study were used to perform the phylogenetic analyses along with the previously published sequences in the GenBank. Multiple sequence alignments were undertaken using MEGA 6 (Tamura et al., 2013), manually checked for the correction, and assessed for the best substitution model for DNA sequence evolution through the calculation by akaike information criterion (AIC) using ModelTest 3.06 (Posada and Crandall, 1998). For 16S rRNA sequences, the multiple-aligned data using the neighbor-joining (NJ) algorithmic in MEGA6 program package (Tamura and Dudley, 2013), was performed with 1 000 bootstrap analysis under Kimura's two-parameter. The maximum-likelihood (ML) trees were constructed in PAUP 4.0v10b (Swofford, 2003), and performed with 100 bootstrap analysis based on the parameters [Nreps=100 Keepall=yes Brlens=yes], [PAUP command lines for each criterion Lset Base=(0.269 7 0.208 9 0.325 6) Nst=6 Rmat=(0.658 3 2.412 4 0.591 2 0.468 0 5.081 3) Rates=gamma Shape=0.661 4 Pinvar=0.565 0] under a best-fit model (GTR+I+G). Bayesian analyses were performed in MrBayes version 3.1.2 (Huelsenbeck and Ronquist, 2001), and parameters in MrBayes were set to five million generations and 50 000 trees, sampled every 100th generation, using the GTR+I+G model of DNA substitution, Nst=6, rates=invgamma, Burnin=10 000 and the default random tree option was set to begin the analysis. For cyrJgenes sequences, the multiple-aligned data using the neighbor-joining (NJ) algorithmic Kimura's two-parameter was implemented within MEGA6 program package (Tamura et al., 2013). The final phylogenetic tree was drawn with Adobe Photoshop CS6.
3 RESULT 3.1 Water quality parametersThe TN, TP and Chl-a concentrations of the examined water samples were shown in Table 3. TN concentrations of all samples were above 1.2 mg/L, varied among the lakes, with the highest concentration (4.4 mg/L) measured in Qingnian Lake. TP concentrations at all sites ranged from 0.07 to 0.24 mg/L. TN/TP ratios were in the range from 7.4 to 61, and Chl-a concentrations varied from 11.3 to 68.8 μg/L.

|
Straight and spiral morphotypes of C. raciborskii were identified, but the coiled one was not found. Typical Raphidiopisis species were not observed (Fig. 2). The morphotype with straight trichomes was found in four samples and the spiral one was found in Qingnian Lake. Trichomes were free-floating and solitary, and apical cells were conically narrowed and rounded. Terminal heterocysts were located at one or both of ends of the trichome with drop-like and rounded-pointed ends. Akinetes were cylindrical to oval, developing near the ends of trichomes, adjacent to the terminal heterocysts or slightly distant (separated by one to several vegetative cells) from heterocysts, usually solitary, less frequently up to 3 in a row.

|
| Figure 2 The morphological characteristic of Cylindrospermopsis raciborskii |
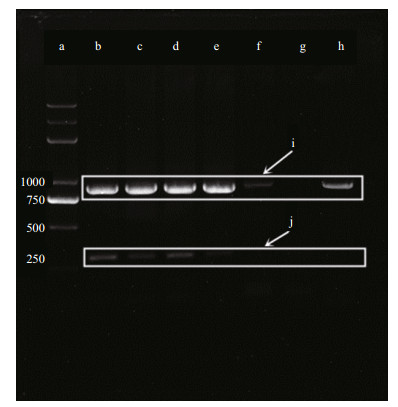
Amplification by primers for C. raciborskii/Raphidiopsis -specific 16S rRNA gene and CYN synthetase gene cyrJ from these samples were shown in Fig. 3. Using the culture of C. raciborskii CHAB3438 as the reference strain, the result shows that five samples may contain C. raciborskii/Raphidiopsis cells and three of them with positive amplification of the cyrJ gene.
|
| Figure 3 PCR products of 16S rRNA and cyrJ genes displayed on a 1% agarose gel a. DNA marker; b. control group; c. LHC; d. NS; e. HLJ; f. TZ; g. YL; h. UP; i. 16S rRNA band; j. cylindrospermopsin synthetase CyrJ(cyrJ) gene band. |
We obtained nine sequences of 16S rRNA gene from this study. Sequence alignment produced a matrix including these nine sequences and those from cyanobacterial strains deposited in GenBank including 7 Raphidiopsis strains, 19 Cylindrospermopsis strains and 7 other different cyanobacterial strains.
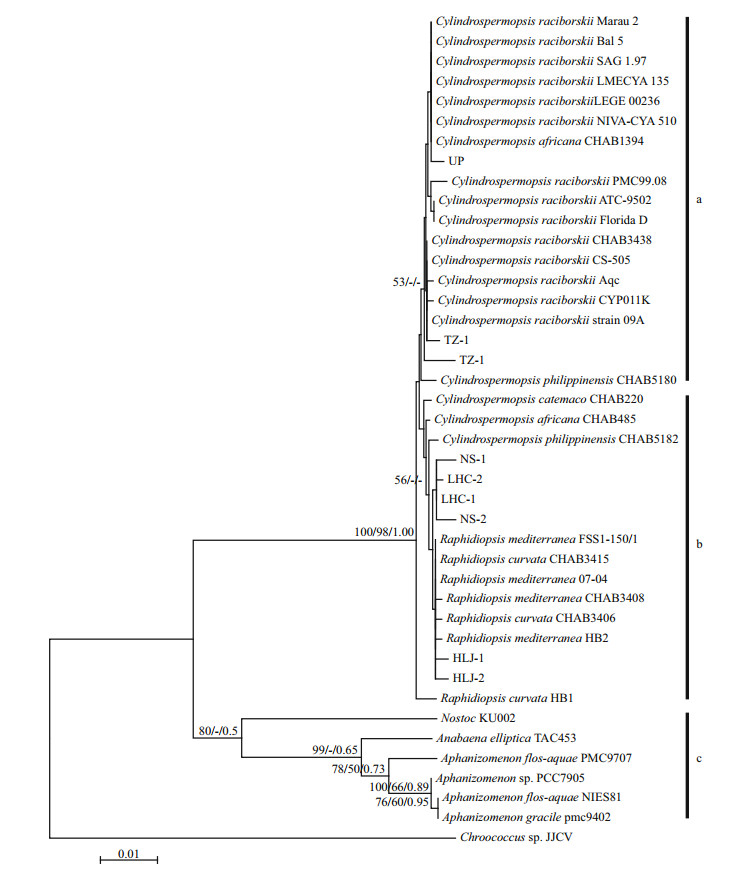
The phylogenetic trees based on the above 16S rRNA gene sequences using the three method as the Neighbor-joining (NJ), Maximum likelihood (ML) and Bayesian inference (BI) led to the similar topological structures. As shown in Fig. 4, there are three clades: a, b and c. The nine sequences obtained in this study were distributed in clades a and b. The all sequences in a clade belonged to Cylindrospermopsis strains and those in b as a mixture of Raphidiopsis and Cylindrospermopsis spp. strains, indicating high similarity of 16S rRNA gene sequences between Cylindrospermopsis and Raphdiopsis species with addition of Chinese strains.
|
| Figure 4 Neighbor-joining (NJ) phylogenetic tree based on the 16S rRNA gene sequences containing 7 strains Raphidiopsis, 19 strains Cylindrospermopsis, and 7 strains from different cyanobacterial species (obtained from NCBI) and 9 cyanobacteria clones (this study) Bootstrap values greater than 50% are given in front of the corresponding nodes for NJ and ML analyses followed by Bayesian posterior probabilities (over 0.5 are illustrated). |
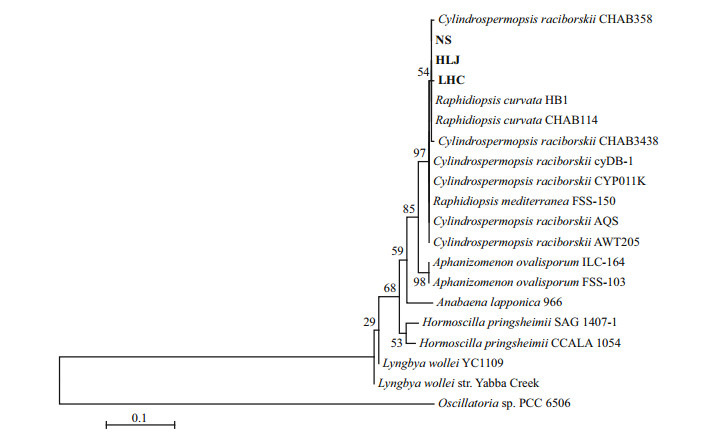
Three cyrJ gene sequences were obtained from this study. A phylogenetic tree was constructed based on cyrJ sequences from these three sequences and cyrJ sequences deposited in GenBank from 6 Cylindrospermopsis strains, 3 Raphidiopsis strains and 9 other cyanobacterial ones. The tree, as shown in Fig. 5, was built using the neighbor-joining (NJ) algorithmic Kimura's two-parameter as implemented within MEGA6 program package (Tamura et al., 2013). The tree showed that all cyrJ sequences obtained in this study were belonging to the Cylindrospermopsis/Raphidiopsis group with high bootstrap supports, suggesting that Cylindrospermopsis or Raphidiopsis species in these samples have potential ability to produce hepatotoxic cylindrospermopsins.
|
| Figure 5 Neighbor-joining (NJ) phylogenetic tree based on the cyrJ gene sequences from 3 strains Raphidiopsis, 6 strains Cylindrospermopsis, and 9 strains from different cyanobacterial species (obtained from NCBI) and 3 cyanobacteria clones (this study) Bootstrap values greater than 50% are given in front of the corresponding nodes for NJ analyses. |
Recently, more and more studies on C. raciborskii have been worldwide performed since C. raciborskii is able to form water blooms and produce cylindrospermopsin and neurotoxins. These studies mainly targeted on detection of C. raciborskii blooms, the geographical distribution of C. raciborskii, types of cyanotoxins produced by this species and toxicity of cylindrospermopsins on aquatic animals and mammals (St.Amand, 2002; Griffiths and Saker, 2003; Haande et al., 2008). In contrast to other global regions/countries, only a few investigations on the occurrence, toxin detection and molecular characterization of Cylindrospermopsis/Raphidiopsis group have been done from Chinese waters. Li et al. (2001) reported the production of deoxy-cylindrospermopsin and cylindrospermopsin from a strain of Raphidiopsis curvata HB1 isolated from a fish pond in Wuhan, China, representing the first study on cylindrospermopsin of Cylindrospermopsis/Raphidiopsis group in China. Wu et al. (2011) used the 21 strains, from species of C. raciborskii, R. curvata and R. mediteriann isolated from subtropical southern provinces of Hubei, Yunnan, Fujian and Guangdong, to perform phylogenetic analyses based on multi-gene sequences for molecular differentiation between Cylindrospermopsis and Raphidiopsis genera. Further, Lei et al. (2014) documented the occurrence and dominance of C. raciborskii and production of cylindrospermopsin in 25 urban reservoirs used for drinking water supply in Dongguan city, Guangdong Province, and they even detected dissolved cylindrospermopsin in more than half of these reservoirs with up to 8.25 μg/L concentrations. Apparently, C. raciborskii has been found in tropical to subtropical regions of China, even in the transitional zone between subtropical to temperate climate, such as in Jiangsu and Shandong provinces (data not shown). It is challenging to determine if the detection of C. raciborskii in new regions has occurred due to invasions or as a result of an increase in its abundance caused by changes in environmental conditions (e.g., Wood et al., 2014). Previous studies have suggested that phylo-biogeographic patterns can be used to reflect global origin and spreading routes of C. raciborskii (Dyble et al., 2002; Neilan et al., 2003; Gugger et al., 2005; Moreira et al., 2015). However, recent works have shown a more nuanced story when a greater number of strains from more regions are included (Cirés et al., 2014; Antunes et al., 2015). Therefore, the confirmation for the spreading of C. raciborskii in China may not be able to be determined by phylo-biogeographic analysis. Based on the absence of this species in previous phycological studies in this region we suggestion the most likely scenario is that it is a new incursion. The occurrence in the transitional zone in China is of particular concern with very characteristic signs of invasion and expansion of C. raciborskii. Therefore, more investigations on the occurrence of C. raciborskii and its impact in further northern regions in China are much needed to understand the invasive feature of C. raciborskii in China and physio-ecological mechanisms for its expansion.
In this study, we examined and confirmed the occurrence of cylindropsermopsin-producing Cylindrospermopsis/Raphdiopsisin urban lakes of Beijing city, China, and this result represented the most northern line of Cylindrospermopsis distribution so far reported in China, and therefore contributes to the knowledge and methods in morphological identification of Cylindrospermopsis, and further in molecular detection of Cylindrospermopsis/Raphdiopsis and their potential production of cylindrospermopsin. The future studies will mainly include the isolation of Cylindrospermopsis/Raphdiopsis strains, detection of their cylindrospermopsin production and monitoring dynamics of Cylindrospermopsis/Raphdiopsis and cylindrospermopsin in Beijing waters.
In addition to the description about the ecophysiological plasticity of C. raciborskii, Padisák (1997) summarized that the reasons for C. raciborskii succeeding in the world's lakes might be attributed to multiple factors such as good floating ability, superior shade tolerance, high affinity ammonia uptake and resistance to grazing. Therefore, climate change with global warming tendency and ecophysiological advantage of C. raciborskii have been regarded as possible mechanisms for the gradual expansion and dominance of C. raciborskii into northern regions in China. According to the data from Intergovernmental Panel on Climate Change (IPCC), the average temperatures in the northern and southern hemispheres during the 20th century have risen 0.52℃ and 0.7℃, respectively (IPCC, 2001). Further, the annual average temperature in Beijing was the rise of 0.5℃ per 10 years (Zhu et al., 2012), much faster than the average in global range. Result of this study may explain that this temperature rise in Beijing already provided the condition with a suitable temperature for C. raciborskii to grow and proliferate. In the present study, water temperatures in the six sites in mid-September, with average 25.6℃, were similar to those in waters of typical subtropical regions in China. In Chinese subtropical areas, our previous surveys showed that C. raciborskii samples were always found in autumn season, after the summer months with frequent occurrence of Microcystis dominated blooms at higher temperatures. Several investigations have indicated that the Microcystis blooms have been previously reported in urban lakes in Beijing during summer season (Tu et al., 2004; Du et al., 2005). Therefore, the pattern for C. raciborskii occurrence after summer in Beijing is also similar to the case in Chinese southern waters.
Water quality parameters, with major concern to nutrition such as TN and TP in this study, showed that the six water bodies were already eutrophicated (HLJ, TZ and LHC as super-eutrophicated) (Table 2), according to Classification References Standard of Lake Nutrition (Smith et al., 1999), and such eutrophic levels in the lakes create the proper nutrient conditions for C. raciborskii (Briand et al., 2004). Site YL did not have C. raciborskii but had the highest TN value as 4.398 mg/L, and this high value much exceeded the total nitrogen levels for C. raciborskii occurrence from previous reports at different parts of the world.
The morphological and molecular similarities between Cylindrospermopsis and Raphidiopsis genera led to the difficulty in distinguishing them. McGregor and Fabbro (2000) proposed that the Raphidiopsis-like trichomes were considered as environmental morphotypes of C. raciborskii. Moustaka-Gouni et al. (2009) suggested from the morphological and 16S rRNA phylogenetic analyses that R. mediterranea from Lake Kastoria was the non-heterocystous stage in a complex life cycle of Cylindrospermopsis. In this study, 16S rRNA gene sequences from the positive PCR amplification at the five respective sites were divided into the different sub-clusters in each of which both Cylindrospermopsis and Raphdiopsis were included, although the big Cylindrospermopsis/Raphdiopsis cluster was significantly shown. Molecular detection revealed that three of the five sites them were found to contain cylindrospermopsin synthesis gene cyrJ. These lakes/ponds are located at the center of Beijing city, and these waters (LHC, NS, HLJ) have the potential ability to produce cylindrospermopsin. Even they are not currently used as for drinking water, but it is likely for them to contaminate other water resources including drinking waters due to the expansive and transportable paths of Cylindrospermopsis/Raphdiopsis within watershed network in the whole Beijing city. Therefore, it is strongly suggested that the monitoring on C. raciborskii/Raphidiopsis species and their production of cylindrospemopsin should be emphasized in Beijing and even more northern parts of China.
5 CONCLUSIONThis study used morphological and molecular methods to detect toxic Cylindrospermopsis/Raphidiopsis group in Beijing city. The eutrophic waters and global warming provide ideal conditions for C. raciborskii to spread, and the electrophoretic results of PCR products and the phylogenetic tree based on 16S rRNA indicated that C. raciborskii/Raphidiopsis sp. has already invaded into Beijing and existthe possibility to produce cylindrospermopsin.
| Alster A, Kaplan-Levy R N, Sukenik A, Zohary T, 2010. Morphology and phylogeny of a non-toxic invasive Cylindrospermopsis raciborskii from a Mediterranean Lake. Hydrobiologia, 639(1): 115–128. Doi: 10.1007/s10750-009-0044-y |
| Antunes J T, Leão P N, Vasconcelos V M, 2015. Cylindrospermopsis raciborskii:review of the distribution, phylogeography, and ecophysiology of a global invasive species. Frontiers in Microbiology, 6: 473. |
| Bonilla S, Aubriot L, Soares M C S, González-Piana M, Fabre A, Huszar V L M, Lürling M, Antoniades D, Padisák J, Kruk C, 2012. What drives the distribution of the bloomforming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii?. FEMS Microbiology Ecology, 79(3): 594–607. Doi: 10.1111/fem.2012.79.issue-3 |
| Briand J F, Leboulanger C, Humbert J F, Bernard C, Dufour P, 2004. Cylindrospermopsis raciborskii (cyanobacteria)invasion at mid-latitudes:selection, wide physiological tolerance, or global warming?. Journal of Phycology, 40(2): 231–238. Doi: 10.1111/j.1529-8817.2004.03118.x |
| Burford M A, Beardall J, Willis A, Orr P T, Magalhaes V F, Rangel L M, Azevedo S M F O E, Neilan B A, 2016. Understanding the winning strategies used by the bloomforming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae, 54: 44–53. Doi: 10.1016/j.hal.2015.10.012 |
| Burger D F, Hamilton D P, Hall J A, Ryan E F, 2007. Phytoplankton nutrient limitation in a polymictic eutrophic lake:community versus species-specific responses. Fundamental and Applied Limnology, 169(1): 57–68. Doi: 10.1127/1863-9135/2007/0169-0057 |
| Cirés S, Wörmer L, Ballot A, Agha R, Wiedner C, Velázquez D, Casero M C, Quesada A, 2014. Phylogeography of cylindrospermopsin and paralytic shellfish toxin-producing Nostocales cyanobacteria from Mediterranean Europe(Spain). Applied and Environmental Microbiology, 80(4): 1 359–1 370. Doi: 10.1128/AEM.03002-13 |
| Du G S, Wu Y M, Yang Z S, Wu D W, Liu J, 2005. Analysis of water quality on urban rivers and lakes in Beijing. Journal of Lake Sciences, 17(4): 373–377. Doi: 10.18307/2005.0416 |
| Dyble J, Paerl H W, Neilan B A, 2002. Genetic characterization of Cylindrospermopsis raciborskii (Cyanobacteria)isolates from diverse geographic origins based on nifH and cpcBA-IGS nucleotide sequence analysis. Applied and Environmental Microbiology, 68(5): 2 567–2 571. Doi: 10.1128/AEM.68.5.2567-2571.2002 |
| Fritsch F E, Rich E, Stephens M E L, 1929. Contributions to our knowledge of the freshwater algae of Africa:7. Freshwater algae (exclusive of diatoms) from Griqualand West. Transactions of the Royal Society of South Africa, 18: 1–92. Doi: 10.1080/00359192909518804 |
| Griffiths D J, Saker M L, 2003. The Palm Island mystery disease 20 years on:a review of research on the cyanotoxin cylindrospermopsin. Environmental Toxicology, 18(2): 78–93. Doi: 10.1002/(ISSN)1522-7278 |
| Gugger M, Molica R, Le Berre B, Dufour P, Bernard C, Humbert J F, 2005. Genetic diversity of Cylindrospermopsis strains (Cyanobacteria) isolated from four continents. Applied and Environmental Microbiology, 71(2): 1 097–1 100. Doi: 10.1128/AEM.71.2.1097-1100.2005 |
| Haande S, Rohrlack T, Ballot A, Røberg K, Skulberg R, Beck M, Wiedner C, 2008. Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, cyanobacteria) isolates from Africa and Europe. Harmful Algae, 7(5): 692–701. Doi: 10.1016/j.hal.2008.02.010 |
| Hong Y, Steinman A, Biddanda B, Rediske R, Fahnenstiel G, 2006. Occurrence of the toxin-producing cyanobacterium Cylindrospermopsis raciborskii in Mona and Muskegon Lakes, Michigan. Journal of Great Lakes Research, 32(3): 645–652. Doi: 10.3394/0380-1330(2006)32[645:OOTTCC]2.0.CO;2 |
| Huelsenbeck J P, Ronquist F, 2001. MRBAYES:Bayesian inference of phylogenetic trees. Bioinformatics, 17(8): 754–755. Doi: 10.1093/bioinformatics/17.8.754 |
| IPCC. 2001. Climate Change 2001-the Scientific Basis. Summary for Policymakers, A Report of Working Group I. Intergovernmental Panel on Climate Change, Geneva. |
| Jeffrey S W, Humphrey G F, 1975. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen, 167(2): 191–194. Doi: 10.1016/S0015-3796(17)30778-3 |
| Jiang Y G, Xiao P, Yu G L, Sano T, Pan Q Q, Li R H, 2012. Molecular basis and phylogenetic implications of deoxycylindrospermopsin biosynthesis in the cyanobacterium Raphidiopsis curvata. Applied and Environmental Microbiology, 78(7): 2 256–2 263. Doi: 10.1128/AEM.07321-11 |
| Jiang Y G, Xiao P, Yu G L, Shao J H, Liu D M, Azevedo S M F O, Li R H, 2014. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Applied and Environmental Microbiology, 80(17): 5 219–5 230. Doi: 10.1128/AEM.00551-14 |
| Komárek J, Kling H, 1991. Variation in six planktonic cyanophyte genera in Lake Victoria (East Africa). Algological Studies/Archive für Hydrobiologie, 61: 21–45. |
| Kouzminov A, Ruck J, Wood S A, 2007. New Zealand risk management approach for toxic cyanobacteria in drinking water. Australian and New Zealand Journal of Public Health, 31(3): 275–281. Doi: 10.1111/j.1467-842X.2007.00061.x |
| Lagos N, Onodera H, Zagatto P A, Andrinolo D, Azevedo S M F Q, Oshima Y, 1999. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon, 37(10): 1 359–1 373. Doi: 10.1016/S0041-0101(99)00080-X |
| Lei L M, Peng L, Huang X H, Han B P, 2014. Occurrence and dominance of Cylindrospermopsis raciborskii and dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China. Environmental Monitoring and Assessment, 186(5): 3 079–3 090. Doi: 10.1007/s10661-013-3602-8 |
| Li R H, Carmichael W W, Brittain S, Eaglesham G K, Shaw G R, Liu Y D, Watanabe M M, 2001. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis curvata(Cyanobacteria). Journal of Phycology, 37(6): 1 121–1 126. Doi: 10.1111/jpy.2001.37.issue-6 |
| McGregor G B, Fabbro L D, 2000. Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in Queensland tropical and subtropical reservoirs:implications for monitoring and management. Lakes & Reservoirs:Research and Management, 5(3): 195–205. |
| Moreira C, Fathalli A, Vasconcelos V, Antunes A, 2015. Phylogeny and biogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Archives of Microbiology, 197(1): 47–52. Doi: 10.1007/s00203-014-1052-5 |
| Moustaka-Gouni M, Kormas K A, Vardaka E, Katsiapi M, Gkelis S, 2009. Raphidiopsis mediterranea Skuja represents non-heterocytous life-cycle stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), its type locality:evidence by morphological and phylogenetic analysis. Harmful Algae, 8(6): 864–872. Doi: 10.1016/j.hal.2009.04.003 |
| Neilan B A, Saker M L, Fastner J, Törökné A, Burns B P, 2003. Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Molecular Ecology, 12(1): 133–140. |
| Padisák J, 1997. Cylindrospermopsis raciborskii (Woloszynska)Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium:worldwide distribution and review of its ecology. Archiv Für Hydrobiologie Supplementband Monographische Beitrage, 107(4): 563–593. |
| Paerl H W, Huisman J, 2009. Climate change:a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports, 1(1): 27–37. Doi: 10.1111/emi4.2009.1.issue-1 |
| Paerl H W, Otten T G, 2013. Harmful cyanobacterial blooms:causes, consequences, and controls. Microbial Ecology, 65(4): 995–1 010. Doi: 10.1007/s00248-012-0159-y |
| Paerl H W, Xu H, Hall N S, Rossignol K L, Joyner A R, Zhu G W, Qin B Q, 2015. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China:implications for controlling eutrophication and harmful algal blooms. Journal of Freshwater Ecology, 30(1): 5–24. Doi: 10.1080/02705060.2014.994047 |
| Posada D, Crandall K A, 1998. MODELTEST:testing the model of DNA substitution. Bioinformatics, 14(9): 817–818. Doi: 10.1093/bioinformatics/14.9.817 |
| Saker M L, Eaglesham G K, 1999. The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the redclaw crayfish Cherax quadricarinatus. Toxicon, 37(7): 1 065–1 077. Doi: 10.1016/S0041-0101(98)00240-2 |
| Sinha R, Pearson L A, Davis T W, Burford M A, Orr P T, Neilan B A, 2012. Increased incidence of Cylindrospermopsis raciborskii in temperate zones-is climate change responsible?. Water Research, 46(5): 1 408–1 419. Doi: 10.1016/j.watres.2011.12.019 |
| Smith V H, Tilman G D, Nekola J C, 1999. Eutrophication:impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution, 100(1-3): 179–196. Doi: 10.1016/S0269-7491(99)00091-3 |
| St.Amand A, 2002. Cylindrospermopsis:an invasive toxic algae. Lakeline, 22(1): 36–38. |
| Stucken K, Murillo A A, Soto-Liebe K, Fuentes-Valdés J J, Méndez M A, Vásquez M, 2009. Toxicity phenotype does not correlate with phylogeny of Cylindrospermopsis raciborskii strains. Systematic and Applied Microbiology, 32(1): 37–48. Doi: 10.1016/j.syapm.2008.10.002 |
| Swofford D L. 2003. PAUP*: phylogenetic analysis using parsimony, version 4. 0 b10. Sinauer Associates, Sunderland, UK. |
| Tamura K, Stecher G, Peterson D, Filipski A, Kumar S, 2013. MEGA6:molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2 725–2 729. Doi: 10.1093/molbev/mst197 |
| Tu Q Y, Zhang Y T, Yang X Z, 2004. Approaches to the ecological recovery engineering in Lake Shishahai, Beijing. Journal of Lake Sciences, 16(1): 61–67. |
| Wiedner C, Rücker J, Brüggemann R, Nixdorf B, 2007. Climate change affects timing and size of populations of an invasive cyanobacterium in temperate regions. Oecologia, 152(3): 473–484. Doi: 10.1007/s00442-007-0683-5 |
| Wood S A, Pochon X, Luttringer-Plu L, Vant B N, Hamilton D P, 2014. Recent invader or indicator of environmental change? A phylogenetic and ecological study of Cylindrospermopsis raciborskii in New Zealand. Harmful Algae, 39: 64–74. Doi: 10.1016/j.hal.2014.06.013 |
| Wu S K, Xie P, Liang G D, Wang S B, Liang X M, 2006. Relationships between microcystins and environmental parameters in 30 subtropical shallow lakes along the Yangtze River, China. Freshwater Biology, 51(12): 2 309–2 319. Doi: 10.1111/fwb.2006.51.issue-12 |
| Wu Z X, Shi J Q, Xiao P, Liu Y, Li R H, 2011. Phylogenetic analysis of two cyanobacterial genera Cylindrospermopsis and Raphidiopsis based on multi-gene sequences. Harmful Algae, 10(5): 419–425. Doi: 10.1016/j.hal.2010.05.001 |
| Zhu L T, Chen Y S, Yan R R, Shen T, Jiang L, Wang Y, 2012. Characteristics of precipitation and temperature changes in Beijing city during 1951-2010. Resources Science, 34(7): 1 287–1 297. |
 2018, Vol. 36
2018, Vol. 36