Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Jiaqi(李加琦), MAO Yuze(毛玉泽), JIANG Zengjie(蒋增杰), ZHANG Jihong(张继红), BIAN Dapeng(卞大鹏), FANG Jianguang(方建光)
- Impact of seawater carbonate variables on post-larval bivalve calcification
- Chinese Journal of Oceanology and Limnology, 36(2): 405-413
- http://dx.doi.org/10.1007/s00343-017-6277-0
Article History
- Received Oct. 14, 2016
- accepted in principle Nov. 29, 2016
- accepted for publication Dec. 16, 2016
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 Function Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4 National Engineering and Research Center of Marine Shellfish, Weihai 264316, China
Marine calcifiers, such as corals, coralline algae, and shellfish that produce calcium carbonate are vulnerable to ocean acidification (OA) (Anthony et al., 2008; Kuffner et al., 2008; Pandolfi et al., 2011). The calcification rate of coral reefs (Maier et al., 2009; Jokiel, 2013; Maier et al., 2016) and coralline algae (Kuffner et al., 2008; Semesi et al., 2009b) will decrease under acidified conditions based on the carbon dioxide partial pressure (pCO2) levels predicted at the end of this century. Larval shell growth and hardness of eastern oysters (Crassostrea virginica) decreases under high pCO2 conditions (Anthony et al., 2008). Larval shells in several species of shellfish, such as mussels (Kurihara et al., 2009; Gazeau et al., 2010; Kelly et al., 2016), oysters (Kurihara et al., 2007; Parker et al., 2010; Waldbusser et al., 2016), abalone (Byrne et al., 2011; Crim et al., 2011; Li et al., 2013), and scallops (Talmage and Gobler, 2009; White et al., 2013; Wang et al., 2016) are malformed by OA. Larval (Miller et al., 2009; Talmage and Gobler, 2010) and adult bivalves (Gazeau et al., 2007; Pfister et al., 2016; Ries et al., 2016) will also suffer reduced calcification under the pH conditions predicted for the end of this century. The calcification response by these marine calcifiers to acidified environments is a main issue for OA-related studies to explore. However, the variables regulating calcification rate are controversial.
It is well known that marine calcifiers use HCO3- to deposit their calcareous skeleton by the following reaction: Ca2++2HCO3-→CaCO3+CO2+H2O (Frankignoulle et al., 1995). Therefore, a decline in calcification rates of calcifiers under acidified conditions might be attributed to increased [H+] or a decrease of [CO32-], [HCO3-], or dissolved inorganic carbon (DIC). Some researchers have proposed that calcification rate of coral (Madracis auretenra) responds to variations in bicarbonate concentration rather than carbonate concentration or pH (Jury et al., 2010). They observed a normal or even elevated calcification rate of corals at pH 7.6–7.8 when bicarbonate concentration was > 1 800 μmol/L, but the calcification rate decreased if the bicarbonate concentration was lower under a normal pH condition. Other researchers believe that coral calcification is determined by the availability of the CO32- rather than pH or HCO3- (Kleypas et al., 1999; Silverman et al., 2007; Marubini et al., 2008). Gazeau et al. (2007) reported that the calcification rates of mussels (Mytilus edulis) and Pacific oysters (Crassostrea gigas) decline linearly with increasing pCO2, but they did not differentiate the roles of pH, bicarbonate, carbonate, or DIC concentration when determining the calcification rate.
To identify the key factors involved in biomineralization, calcification rates of calcifiers have been measured in manipulated CO2-carbonate systems. Gattuso et al. (1998) manipulated calcium concentration under constant pH and carbonate conditions to adjust the aragonite saturation level of seawater and found that the coral calcification rate increased if the aragonite saturation level was also increased. Langdon et al. (2000) manipulated both calcium and carbonate concentrations to obtain the required aragonite saturation level of seawater and found consistent regulated changes in calcification rate of coral to the manipulated aragonite saturation. Waldbusser et al. (2015) manipulated seawater DIC with mineral acids and bases and reported that shell development of two bivalve larvae, C. gigas, and Mytilus gallo provincialis, are dependent on carbonate saturation state, and not on pCO2 or pH. Another study reported that limiting DIC strongly reduced calcification, despite a high [CO32-] (Thomsen et al., 2015). They believed that mussels utilize [HCO3-] rather than [CO32-] as the inorganic carbon source for biomineralization. To distinguish the key factors determining calcification rate of post-larval shellfish, conditions with decreased pH or lowered DIC were created by bubbling CO2-enriched air or adding a hydrochloride solution to natural seawater. Calcification rates of juvenile blue mussels (M. edulis) and Zhikong scallops (Chlamys farreri) were measured in these different carbonate systems. Significant correlations were observed between calcification rate and DIC/[H+] and [CO32-] in both species. We also found that calcification rate of these two bivalve species increased under elevated pH conditions.
2 MATERIAL AND METHOD 2.1 Animal collection and cultivationAll animals used in the experiment were collected from Sangou Bay, Weihai, China. The shell heights of the Zhikong scallops and blue mussels sampled from the local aquaculture populations were 3.53±0.25 cm and 3.55±0.41 cm, respectively. Individuals were acclimated to experimental conditions for 2 weeks before being used to measure calcification rate. Animals were reared together with Ulva pertusa Kjellman in 1-L beakers placed in an illumination incubator. The incubation temperature was 20℃ and the light-dark photoperiod was 6 min light: 3 min dark. The pH of the experimental seawater was maintained at 8.20±0.10 by adjusting the amount of U. pertusa in the beaker. No aeration was used, as the mollusks obtained sufficient oxygen from the photosynthetic activity of U. pertusa (dissolved oxygen [DO] > 6.5 mg/L). Animals in each beaker were fed about a 50 mL suspension of Platymonas sp. (about 2×106/mL seawater) twice daily.
2.2 Manipulation of the seawater carbonate systemThe pH level or DIC was adjusted in the seawater carbonate system. The seawater was bubbled with CO2-enriched air, and pH was adjusted down to the required level. U. pertusa was added and incubated in the illumination incubator to increase seawater pH. A two-step method was applied to decrease DIC of seawater while the pH remained constant. In the first step, seawater pH was increased by incubating with U. pertusa in the illumination incubator. Then, a mixture of seawater and concentrated hydrochloric acid (1:1) was added to the experimental seawater to reduce pH down to pH 8.2 in the second step. After repeating these steps twice, we acquired experimental seawater with an extremely low DIC level and normal pH (8.2). Experimental seawater with the required DIC level was prepared by mixing seawater with a low level of DIC and natural seawater.
Water temperature and salinity were measured using a combined electrode (YSI ProPlus; YSI, Yellow Springs, OH, USA), and pH was measured using a pH electrode (Thermo Scientific 3-Star, ORION; Waltham, MA, USA). The pH electrode was calibrated daily with buffers traceable to the NIST (NBS) standard. The DO concentration in the experimental seawater was measured using a DO electrode (Multi 3420; WTW, Weilheim, Germany). Total alkalinity (TA) was measured within hours after sampling using an automatic titrator (848 Titrino plus; Metrohm, Riverview, FL, USA).
2.3 Calcification rate measurementWe used the following equation to calculate the calcification rate (Gazeau et al., 2007):
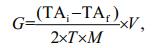
where G is calcification rate (μmol/(g·h)), TAi is initial TA of the experimental seawater (μmol/L), TAf is the final TA of the experimental seawater (μmol/L), T is the experimental duration (h), M is whole wet weight of the experimental animals (g), and V is the volume of the experimental seawater (L).
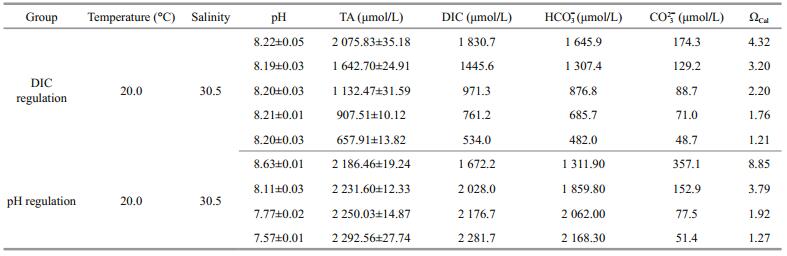
Four to six animals and about 3 g of U. pertusa were placed in a 500-mL beaker filled with 400 mL of seawater and the required carbonate system. The beaker was placed in the illumination incubator with a light-dark photoperiod of 6 min light: 3 min dark. The animals were incubated in the beaker for 1 hour while the amount of U. pertusa was adjusted every 15 min to maintain a stable pH. Before measuring the calcification rate, the incubating seawater was replaced with fresh seawater. During the 2 h incubation for the calcification rate measurement, the amount of U. pertusa was adjusted every 30 min to maintain pH within ±0.1 units. Seawater that was used to measure pH, salinity, and TA was sampled before and immediately after the incubation. Mean pH, TA, water temperature, and salinity were used to calculate the pCO2, [HCO3-], [CO32-], DIC, calcite, and aragonite saturation values (Table 1) using CO2SYS (Pierrot et al., 2006). Four replicate groups were employed for the calcification rate measurements of each species.
|
The correlation coefficient between carbonate variables and the calcification rates of blue mussels and Zhikong scallops and its significance was calculated using Pearson's correlation analysis and SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). A two-tailed procedure was used to test the significance of the correlation coefficient, and a P < 0.05 was considered significant for all tests.
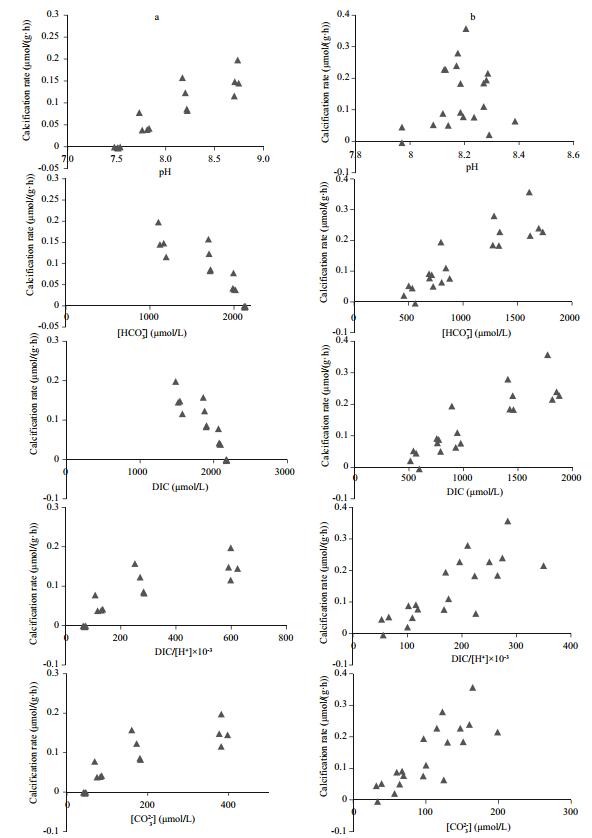
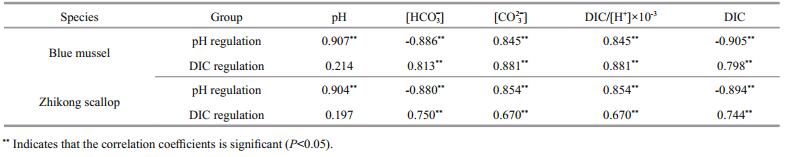
3 RESULTCalcification rate of juvenile blue mussels was associated with the seawater carbonate system (Fig. 1). However, different results were found between the two different carbonate systems. Positive correlations were observed between calcification rate of juvenile blue mussels and pH, DIC/[H+] or [CO32-], whereas negative correlations were observed between calcification rate and DIC or [HCO3-] during incubations under high or low pH conditions (Fig. 1, column a). Positive correlations were observed between calcification rate and DIC, [HCO3-], DIC/ [H+], and [CO32-] during the incubation with low DIC and normal pH, whereas no correlation was detected between calcification rate and pH (Fig. 1, column b).It was clear that the calcification rate of juvenile mussels was positively correlated (P < 0.05) with and DIC/[H+] and [CO32-] in both carbonate systems (Table 2).
|
| Figure 1 Plot of seawater pH, [HCO3-], [CO32-], dissolved inorganic carbon (DIC), DIC/[H+], and [HCO3-]/[H+] vs. calcification rate of blue mussels Seawater carbonate parameters were adjusted by changing the pH (column a) or DIC (column b). |
|
Similar results were found in Zhikong scallops (Fig. 2). Positive correlations were observed between calcification rate of Zhikong scallops and pH, DIC/ [H+], and [CO32-], whereas negative correlations were observed between calcification rate and DIC and [HCO3-] under low and high pH conditions (Fig. 1, column a). Positive correlations were detected between calcification rate and DIC, [HCO3-], DIC/ [H+], and [CO32-], whereas no significant correlation was observed between calcification rate and pH in seawater with a low DIC level and normal pH (Fig. 1, column b). Only DIC/[H+] and [CO32-] showed a positive dominant effect (P < 0.05) when determining calcification rate of juvenile Zhikong scallops in both carbonate systems (Table 2).

|
| Figure 2 Plot of seawater pH, [HCO3-], [CO32-], dissolve inorganic carbon (DIC), DIC/[H+], and [HCO3-]/[H+] vs. calcification rate of Zhikong scallops Seawater carbonate parameters were adjusted by changing pH (column a) or DIC (column b). |
It has been reported that ocean surface seawater pH will drop to about 7.7 (IPCC, 2007) by the end of this century. Marine calcifiers, such as mollusks, may face a stressful future as it will be more difficult for them to generate calcareous shells (Gazeau et al., 2007). It seems that the decrease in calcification rate under acidified conditions is directly attributed to higher [H+]. However, higher [H+] changes the seawater carbonate system, which may play a determining role in biological calcium carbonate deposition. Therefore, it is important to isolate the main factor from acidified seawater that is responsible for the decrease in calcification rate. We measured calcification rate of blue mussels and Zhikong scallops under conditions created in different carbonate systems. The results support that the calcifying ability of post-larval shellfish was correlated with [HCO3-]/[H+] or [CO32-] rather than pH, DIC, or [HCO3-].
Experimental animals continuously released CO2 into seawater during the calcification rate measurement. If this CO2 is absorbed, experimental seawater pH would decrease. Thus, we introduced U. pertusa into the incubation system to remove the CO2. However, this raised concern about the accuracy of the calcification measurement, as the seawater carbonate system would change in response to the photosynthetic activity of U. pertusa. The carbonate system variables and pH of the experimental seawater changed in response to the photosynthetic activity of U. pertusa, but the TA of seawater remained unchanged (Table 3). As a result, adding U. pertusa to the incubation system did not affect the calcification rate calculation.
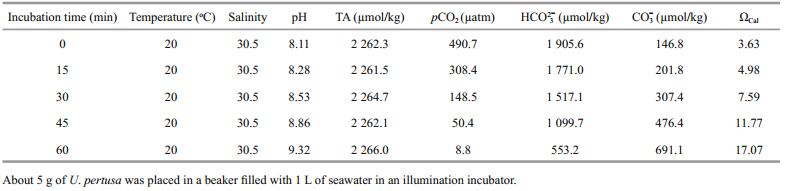
|
Several studies have investigated the dominant variables in carbonate systems while calcium carbonate deposition rate was determined in marine calcifiers. Jury et al., (2010) reported that calcification rate of coral (Madracis auretenra) responds to variations in bicarbonate concentration rather than carbonate concentration. In contrast, Jokiel (2013) found that the calcification rates of the coral Porites rus and the crustose coralline algae (CCA) Hydrolithon onkodes are correlated with the DIC/[H+] and [CO32-]. Moreover, Gattuso et al. (1998) manipulated a carbonate chemistry system by adjusting calcium concentration and found that calcification rate of the coral Stylophora pistillata was affected by carbonate saturation state. Waldbusser et al. (2015) reported that larval bivalve shell development and growth are dependent on seawater carbonate saturation state, but not on pCO2 or pH. They did not discuss the correlation between calcification rate of larval bivalves with DIC/ [H+]. Jokiel (2013) reported that calcification of coral reefs is driven by the DIC/[H+] ratio and that [CO32-] has no physiological relevance. However, the carbonate saturation state or [CO32-] in natural seawater is tightly correlated with the DIC/[H+] ratio, and carbonate saturation state is determined by [CO32-]. Therefore, it is difficult to determine whether [CO32-] or the DIC/[H+] ratio is the key variable when calculating calcification rate. Our findings support that calcification rates of post-larval blue mussels and Zhikong scallops are correlated with [CO32-] and DIC/ [H+] ratio rather than DIC, [HCO3-], or pH.
It is believed that [HCO3-] is the inorganic carbon source for biomineralization. However, Putron et al. (2011) reported that two species of coral exhibit the same positive response to increasing [CO32-], but not to total DIC or [HCO3-]. We also found that calcification rate of shellfish did not follow changes in DIC or [HCO3-]. To exclude [HCO3-] or DIC as a sole variable when determining calcification rate of shellfish, we increased seawater pH to decrease [HCO3-] and DIC, and then measured the calcification rate of the two species of shellfish. However, the calcification rates of these shellfish increased under the high pH condition and significantly increased calcifying ability was observed in blue mussels and Zhikong scallops. It must be pointed out that [CO32-] and the DIC/[H+] ratio increased under this high pH condition. Therefore, this result demonstrates that calcification rate of post-larval shellfish is not dependent on [HCO3-], but also supports the conclusion that [CO32-] and the DIC/[H+] ratio are dominant variables when determining calcification rate.
Several biological processes of calcifying organisms are stressed under acidified conditions (Orr et al., 2005). We also found that biological calcification of bivalves is susceptible to seawater acidification. No signs of acclimation to a saturated state by a coral reef have been observed, as no significant difference in calcification rate was observed between short-term and long-term incubation (Pandolfi et al., 2011). This result reminds us that some species of marine calcifiers may lack the capacity to generate a calcium carbonate skeleton under acidified conditions even after long-term acclimation. These calcifying organisms may face severe challenges in the acidified future. However, blue mussels generated a calcareous shell under extremely low levels of carbonate saturation in a long-term incubation experiment (Thomsen and Melzner, 2010). This finding is markedly different from short-term research (White et al., 2013), in which blue mussels nearly lost their calcifying ability under similar conditions to that of the long-term incubation. It seems that some bivalves may possess the mechanisms to acclimate to low [CO32-] or DIC/ [H+] conditions. As a result, more studies are required to understand the response of shellfish calcification to the seawater carbonate system.
This study may provide some novel insight about the benefit that shellfish can acquire in integrated shellfish-algae aquaculture. Algae are helpful for producing oxygen and absorbing waste in integrated aquaculture systems (Mao et al., 2009; Tang et al., 2011; Chopin et al., 2012). Algae may also be helpful in creating a comfortable carbonate environment for shellfish to calcify. Algae provide oxygen, absorb nutrients, and are capable of adjusting the carbonate system. Seawater pH in a low water exchange bay can increase from 7.9 to 8.9 because of the photosynthetic activity of seaweeds (Semesi et al., 2009a), and a high seaweed biomass can easily elevate the pH of seawater in confined tidal pools (Clements and Chopin, 2016). This higher seawater pH would then increase the level of [CO32-] or the DIC/[H+] ratio, which is beneficial for biomineralization of shellfish. Therefore, algae can help enhance the calcium carbonate deposition efficiency of shellfish in an integrated aquaculture system.
5 CONCLUSIONOur findings show that calcification rates of post-larval blue mussels and Zhikong scallops are correlated with [CO32-] and the DIC/[H+] ratio rather than DIC, [HCO3-], or pH. However, the calcification rate differed in short-term and long-term experiments. As a result, more studies are required to understand the response of shellfish calcification to the seawater carbonate system.
6 ACKNOWLEDGEMENTWe thank Mr. James Yang Xie from Hong Kong Baptist University (HKBU) for editing our manuscript.
| Anthony K R N, Kline D I, Diaz-Pulido G, Dove S, HoeghGuldberg O, 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences of the United States of America, 105(45): 17442–17446. Doi: 10.1073/pnas.0804478105 |
| Byrne M, Ho M, Wong E, Soars N A, Selvakumaraswamy P, Shepard-Brennand H, Dworjanyn S A, Davis A R, 2011. Unshelled abalone and corrupted urchins:development of marine calcifiers in a changing ocean. Proceedings of the Royal Society B:Biological Sciences, 278(1716): 2376–2383. Doi: 10.1098/rspb.2010.2404 |
| Chopin T, Cooper J A, Reid G, Cross S, Moore C, 2012. Openwater integrated multi-trophic aquaculture:environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Reviews in Aquaculture, 4(4): 209–220. Doi: 10.1111/raq.2012.4.issue-4 |
| Clements J C, Chopin T, 2016. Ocean acidification and marine aquaculture in North America:potential impacts and mitigation strategies. Reviews in Aquaculture, 56(3): 182–196. |
| Crim R N, Sunday J M, Harley C D G, 2011. Elevated seawater CO2 concentrations impair larval development and reduce larval survival in endangered northern abalone (Haliotis kamtschatkana). Journal of Experimental Marine Biology and Ecology, 400(1-2): 272–277. Doi: 10.1016/j.jembe.2011.02.002 |
| de Putron S J, McCorkle D C, Cohen A L, Dillon A B, 2011. The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals. Coral Reefs, 30(2): 321–328. Doi: 10.1007/s00338-010-0697-z |
| Frankignoulle M, Pichon M, Gattuso J P. 1995. Aquatic calcification as a source of carbon dioxide. In: Beran M A ed. Carbon Sequestration in the Biosphere: Processes and Prospects. Springer, Berlin Heidelberg. p. 265-271. |
| Gattuso J P, Frankignoulle M, Bourge I, Romaine S, Buddemeier R W, 1998. Effect of calcium carbonate saturation of seawater on coral calcification. Global and Planetary Change, 18(1-2): 37–46. Doi: 10.1016/S0921-8181(98)00035-6 |
| Gazeau F, Gattuso J P, Dawber C, Pronker A E, Peene F, Peene J, Heip C H R, Middelburg J J, 2010. Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences, 7(7): 2051–2060. Doi: 10.5194/bg-7-2051-2010 |
| Gazeau F, Quiblier C, Jansen J M, Gattuso J P, Middelburg J J, Heip C H R, 2007. Impact of elevated CO2 on shellfish calcification. Geophysical Research Letters, 34(7): L07603. |
| IPCC. 2007. Climate Change 2007: The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, UK and New York, USA. |
| Jokiel P L, 2013. Coral reef calcification:carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification. Proceedings of the Royal Society B:Biological Sciences, 280(1764): 20130031. Doi: 10.1098/rspb.2013.0031 |
| Jury C P, Whitehead R F, Szmant A M, 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (=Madracis mirabilis sensu Wells, 1973):bicarbonate concentrations best predict calcification rates. Global Change Biology, 16(5): 1632–1644. Doi: 10.1111/gcb.2010.16.issue-5 |
| Kelly M W, Padilla-Gamiño J L, Hofmann G E, 2016. High pCO2 affects body size, but not gene expression in larvae of the California mussel (Mytilus californianus). ICES Journal of Marine Science, 73(3): 962–969. Doi: 10.1093/icesjms/fsv184 |
| Kleypas J A, Buddemeier R W, Archer D, Gattuso J P, Langdon C, Opdyke B N, 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science, 284(5411): 118–120. Doi: 10.1126/science.284.5411.118 |
| Kuffner I B, Andersson A J, Jokiel P L, Rodgers K S, Mackenzie F T, 2008. Decreased abundance of crustose coralline algae due to ocean acidification. Nature Geoscience, 1(2): 114–117. Doi: 10.1038/ngeo100 |
| Kurihara H, Asai T, Kato S, Ishimatsu A, 2009. Effects of elevated pCO2on early development in the mussel Mytilus galloprovincialis. Aquatic Biology, 4(3): 225–233. |
| Kurihara H, Kato S, Ishimatsu A, 2007. Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquatic Biology, 1(1): 91–98. |
| Langdon C, Takahashi T, Sweeney C, Chipman D, Goddard J, Marubini F, Aceves H, Barnett H, Atkinson M J, 2000. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochemical Cycles, 14(2): 639–654. Doi: 10.1029/1999GB001195 |
| Li J Q, Jiang Z J, Zhang J H, Qiu J W, Du M R, Bian D P, Fang J G, 2013. Detrimental effects of reduced seawater pH on the early development of the Pacific abalone. Marine Pollution Bulletin, 74(1): 320–324. Doi: 10.1016/j.marpolbul.2013.06.035 |
| Maier C, Hegeman J, Weinbauer M G, Gattuso J P, 2009. Calcification of the cold-water coral Lophelia pertusa, under ambient and reduced pH. Biogeosciences, 6(8): 1671–1680. Doi: 10.5194/bg-6-1671-2009 |
| Maier C, Popp P, Sollfrank N, Weinbauer M G, Wild C, Gattuso J P, 2016. Effects of elevated pCO2 and feeding on net calcification and energy budget of the Mediterranean cold-water coral Madrepora oculata. The Journal of Experimental Biology, 219(20): 3208–3217. Doi: 10.1242/jeb.127159 |
| Mao Y Z, Yang H S, Zhou Y, Ye N H, Fang J G, 2009. Potential of the seaweed Gracilaria lemaneiformis for integrated multi-trophic aquaculture with scallop Chlamys farreri in North China. Journal of Applied Phycology, 21(6): 649–656. Doi: 10.1007/s10811-008-9398-1 |
| Marubini F, Ferrier-Pagès C, Furla P, Allemand D, 2008. Coral calcification responds to seawater acidification:a working hypothesis towards a physiological mechanism. Coral Reefs, 27(3): 491–499. Doi: 10.1007/s00338-008-0375-6 |
| Miller A W, Reynolds A C, Sobrino C, Riedel G F, 2009. Shellfish face uncertain future in high CO2 world:influence of acidification on oyster larvae calcification and growth in estuaries. PLoS One, 4(5): e5661. Doi: 10.1371/journal.pone.0005661 |
| Orr J C, Fabry V J, Aumont O, Bopp L, Doney S C, Feely R A, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key R M, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar R G, Plattner G K, Rodgers K B, Sabine C L, Sarmiento J L, Schlitzer R, Slater R D, Totterdell I J, Weirig M F, Yamanaka Y, Yool A, 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437(7059): 681–686. Doi: 10.1038/nature04095 |
| Pandolfi J M, Connolly S R, Marshall D J, Cohen A L, 2011. Projecting coral reef futures under global warming and ocean acidification. Science, 333(6041): 418–422. Doi: 10.1126/science.1204794 |
| Parker L M, Ross P M, O'Connor W A, 2010. Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Marine Biology, 157(11): 2435–2452. Doi: 10.1007/s00227-010-1508-3 |
| Pfister C A, Roy K, Wootton J T, McCoy S J, Paine R T, Suchanek T H, Sanford E, 2016. Historical baselines and the future of shell calcification for a foundation species in a changing ocean. Proceedings of the Royal Society B:Biological Sciences, 283(1832): 20160392. Doi: 10.1098/rspb.2016.0392 |
| Pierrot D, Lewis E, Wallace D W R. 2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, TN. |
| Ries J B, Ghazaleh M N, Connolly B, Westfield I, Castillo K D, 2016. Impacts of seawater saturation state (ΩA=0.4-4.6) and temperature (10, 25℃) on the dissolution kinetics of whole-shell biogenic carbonates. Geochimica et Cosmochimica Acta, 192: 318–337. Doi: 10.1016/j.gca.2016.07.001 |
| Semesi I S, Beer S, Björk M, 2009a. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Marine Ecology Progress Series, 382: 41–47. Doi: 10.3354/meps07973 |
| Semesi I S, Kangwe J, Björk M, 2009b. Alterations in seawater pH and CO2 affect calcification and photosynthesis in the tropical coralline alga, Hydrolithon sp.(Rhodophyta). Estuarine, Coastal and Shelf Science, 84(3): 337–341. Doi: 10.1016/j.ecss.2009.03.038 |
| Silverman J, Lazar B, Erez J, 2007. Effect of aragonite saturation, temperature, and nutrients on the community calcification rate of a coral reef. Journal of Geophysical Research:Oceans, 112(C5): C05004. |
| Talmage S C, Gobler C J, 2009. The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters(Crassostrea virginica). Limnology and Oceanography, 54(6): 2072–2080. Doi: 10.4319/lo.2009.54.6.2072 |
| Talmage S C, Gobler C J, 2010. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proceedings of the National Academy of Sciences of the United States of America, 107(40): 17246–17251. Doi: 10.1073/pnas.0913804107 |
| Tang Q S, Zhang J H, Fang J G, 2011. Shellfish and seaweed mariculture increase atmospheric CO2 absorption by coastal ecosystems. Marine Ecology Progress Series, 424: 97–105. Doi: 10.3354/meps08979 |
| Thomsen J, Haynert K, Wegner K M, Melzner F, 2015. Impact of seawater carbonate chemistry on the calcification of marine bivalves. Biogeosciences, 12(14): 4209–4220. Doi: 10.5194/bg-12-4209-2015 |
| Thomsen J, Melzner F, 2010. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Marine Biology, 157(12): 2667–2676. Doi: 10.1007/s00227-010-1527-0 |
| Waldbusser G G, Gray M W, Hales B, Langdon C J, Haley B A, Gimenez I, Smith S R, Brunner E L, Hutchinson G, 2016. Slow shell building, a possible trait for resistance to the effects of acute ocean acidification. Limnology and Oceanography, 61(6): 1969–1983. Doi: 10.1002/lno.10348 |
| Waldbusser G G, Hales B, Langdon C J, Haley B A, Schrader P, Brunner E L, Gray M W, Miller C A, Gimenez I, 2015. Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat. Climate Chang, 5(3): 273–280. Doi: 10.1038/nclimate2479 |
| Wang W M, Liu G X, Zhang T W, Chen H J, Tang L, Mao X W, 2016. Effects of elevated seawater pCO2 on early development of scallop Argopecten irradias (Lamarck, 1819). Journal of Ocean University of China, 15(6): 1073–1079. Doi: 10.1007/s11802-016-3146-y |
| White M M, McCorkle D C, Mullineaux L S, Cohen A L, 2013. Early exposure of bay scallops (Argopecten irradians) to high CO2 causes a decrease in larval shell growth. PLoS One, 8(4): e61065. Doi: 10.1371/journal.pone.0061065 |
 2018, Vol. 36
2018, Vol. 36


