Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SHI Limei(施丽梅), HUANG Yaxin(黄亚新), LU Yaping(卢亚萍), CHEN Feizhou(陈非洲), ZHANG Min(张民), YU Yang(于洋), KONG Fanxiang(孔繁翔)
- Stocks and dynamics of particulate and dissolved organic matter in a large, shallow eutrophic lake (Taihu, China) with dense cyanobacterial blooms
- Chinese Journal of Oceanology and Limnology, 36(3): 738-749
- http://dx.doi.org/10.1007/s00343-018-7031-y
Article History
- Received Feb. 8, 2017
- accepted in principle Mar. 18, 2017
- accepted for publication Apr. 20, 2017
2 Biological Experiment Teaching Center, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China
In aquatic environments and especially eutrophic waters, phytoplankton are major components of the seston and play a major role in the biogeochemical cycling of carbon (C), nitrogen (N), and phosphorus (P). In particular, phytoplankton contribute to autochthonous particulate organic matter (POM) through cell proliferation, or cell detritus (Hessen et al., 2003) and to dissolved organic matter (DOM) by direct extracellular release, cellular lysis, or zooplankton sloppy grazing (Nagata, 2000; Zhang et al., 2009). Given that POM is a major substrate for grazers and DOM is an important substrate for microbial communities (Mei et al., 2005), their concentrations greatly influence energy transfer in the food web, and thus control ecosystem structure. However, partitioning of organic matter appears to vary by ecosystem. Respectively, about 89% and 78% of accumulated organic matter were partitioned into POM during Phaeocystis blooms in the Ross Sea or during the exponential growth of coastal diatom blooms in Oregon deck incubations (Carlson et al., 2000; Wetz and Wheeler, 2003). More than 56% of total organic carbon loading was partitioned into DOC with cyanobacterial blooms in Lake Soyang, Korea (Kim et al., 2000).
As the three most important elements of organic matter, C, N, and P can provide basic stoichiometric information on food resources. Lower C:N:P ratios indicate good food quality, while higher C:N:P ratios suggest poor food quality (Sterner et al., 1993; Elser et al., 2001). Thus, the nutrient ratio of organic matter determines the features, roles and fates of organic matter in ecosystems. In fact, the relative proportions of detritus and various phytoplankton species comprising the seston greatly affect the C:N:P ratio (Hecky and Kilham, 1988; Geider and La Roche, 2002; Hessen et al., 2003; Klausmeier et al., 2004). Even for a certain type of phytoplankton, the elemental stoichiometry within a cell is regulated by the biochemical allocation of resources to different growth strategies (Elser et al., 2003; Klausmeier et al., 2004). DOC concentrations with strong impacts on the C:N:P ratio of DOM are greatly influenced by phytoplankton blooms or allochthonous input (Zhang et al., 2009). Therefore, patterns of that ratio can vary geographically towing to variations in temperature, nutrients and phytoplankton composition among various ecosystems (Sterner and Elser, 2002; Martiny et al., 2013). Moreover, the C:N:P ratio patterns may vary seasonally, even within specific ecosystems.
The occurrence of cyanobacterial blooms dramatically influences carbon and nutrient cycling and ecosystem structure in eutrophic lakes worldwide. However, information about the entire seasonal cycle of both POM and DOM in cyanobacterial bloomforming lakes is still lacking. In the present study, we monitored C, N, and P contents in both POM and DOM over a full year in Taihu Lake. This is a typical eutrophic lake, with high concentrations of nitrogen and phosphorus caused by human activities that has long experienced from cyanobacterial blooms (Chen et al., 2003; Qin et al., 2015). There are heavy cyanobacterial blooms in warm seasons, which generally decline in winter. Our objective here was to (1) evaluate the dynamics of concentrations and stoichiometry of C, N and P in POM and DOM in the eutrophic lake, and (2) access key water parameters contributing to the dynamics of POM and DOM.
2 METHOD AND MATERIAL 2.1 Study areaTaihu Lake, which is located between 30°56′- 31°33′N and 119°53′-120°36′E in eastern China, is the third largest lake in the country. With a surface area of 2 338 km2, a mean depth of 1.9 m, and a water residence time of approximately 284 days (Qin et al., 2007), the lake is used as the drinking water source of several cities, such as Shanghai, Suzhou, Wuxi, and Huzhou; serves as an important water resource for irrigation in farming and industry; and is an important recreational and tourist attraction (Qin et al., 2007). Along with the rapid development of the economy, the lake water has been seriously polluted. Presently, the lake as a whole is eutrophic and suffers from cyanobacterial blooms, which are overwhelming dominated by Microcystis spp. (Chen et al., 2003; Ma et al., 2016).
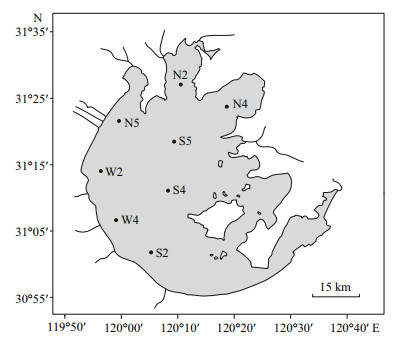
2.2 Sample collection and handlingMonthly samplings were carried out between December 2014 and November 2015 at eight stations in the lake (Fig. 1). Water samples were collected from the surface to a depth of 0.5 m using a 5-L acidcleaned plastic bucket. Because Taihu Lake is shallow and well mixed (McCarthy et al., 2007), sampling of the top 0.5 m water column was considered representative of the entire column. Phytoplankton (mainly composed of cyanobacterial colonies) was collected by towing a phytoplankton net (64 μm mesh) through the cyanobacterial surface bloom from a slowly moving motorboat around the sampling station. The water physical parameters including the temperature, pH and dissolved oxygen (DO) were measured at each sample site using a multiparameter meter (model 6600V2; Yellow Springs Instruments, Yellow Springs, OH, USA). An adequate water sample with a volume of 100-300 mL was filtered through a precombusted (450℃ for 4 h) Whatman GF/F glass fiber filter (nominal pore size 0.7 μm), and the filters and filtrate were used for the determination of POM and DOM, respectively. In addition, 50- 100 mL water samples were filtered through Whatman GF/C glass fiber filters (nominal pore size 1.2 μm), and the filters were used to determine the chlorophyll a (Chl a) concentration. All the filters and filtrates collected in acid washed glass bottles were stored frozen until analysis.
|
| Figure 1 The map of Taihu Lake and sampling locations as indicated |
Chl a was extracted from the filters with 90% acetone and determined using a spectrofluorophotometer (RF-5301PC, Shimadzu, Japan). For the particulate organic carbon (POC) and particulate nitrogen (PN) analysis, the filters were freeze-dried, weighed, and then fumed with concentrated HCl for 4-5 h in a desiccator to remove inorganic carbon. The carbon and nitrogen contents on the filters were measured using a CN elemental analyzer EA3000 (EuroVector, Italy). Particulate phosphorus (PP) was determined by inductively coupled plasma- atomic emission spectrometry (ICP-AES, Prodigy, Teledyne Leeman Labs, Hudson NH, America) after complete digestion with HCl-HNO3-HF-HClO4. The C:N:P molar ratios were obtained as POC:PN:PP from the bulk particulate fraction.
DOC was determined using a TOC analyzer (Teledyne Tekmar, Torch, USA). The concentrations of nutrients in the GF/F filtrate, including ammonium (NH4+), nitrate (NO3-), nitrite (NO2-), and phosphate (PO43-) were analyzed using a continuous flow analyzer (Skalar San++, Netherlands). The concentrations of total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP) were determined using alkaline potassium persulfate digestion (Ebina et al., 1983), followed by spectrophotometric analysis similarly to the determination of NO3- and PO43-. DON and DOP were determined as the differences between TDN and inorganic N (DIN=NH4++NO3-+NO2-), between TDP and inorganic P (PO43-), respectively. The C:N:P molar ratios were obtained as DOC:DON:DOP from the bulk dissolved fraction.
2.4 Phytoplankton derived carbon, nitrogen and phosphorus compositionTo estimate the contribution of phytoplankton (mainly composed of cyanobacterial colonies) to total POM, C, N and P compositions in the collected phytoplankton were determined as described above. The dry weight of phytoplankton was calculated based on a linear equation describing the relationship between the dry weight of the phytoplankton and the Chl a for Taihu Lake:
Cphytoplankton=0.09CChl a+Pa (Zhang et al., 2011b).
C, N and P derived from phytoplankton were estimated by the dry weight of phytoplankton, multiplied by the relative contents of phytoplankton C, N, and P. The proportion of phytoplankton derived organic carbon (phyC), nitrogen (phyN) and phosphorus (phyP) in total POM was calculated by the contents of C, N, and P of phytoplankton divided by the concentrations of POC, PN, and PP respectively.
2.5 Data analysisRelationships between POM, DOM and water quality variables such as Chl a, temperature, pH, DO, inorganic nitrogen and phosphate were analyzed using Pearson's correlation and regression analyses. The correlations were considered statistically significant at 95% confidence intervals (P < 0.05). Redundancy analyses were performed using the vegan package in R software to identify the major water quality variables affecting C:N:P ratios in POM and DOM.
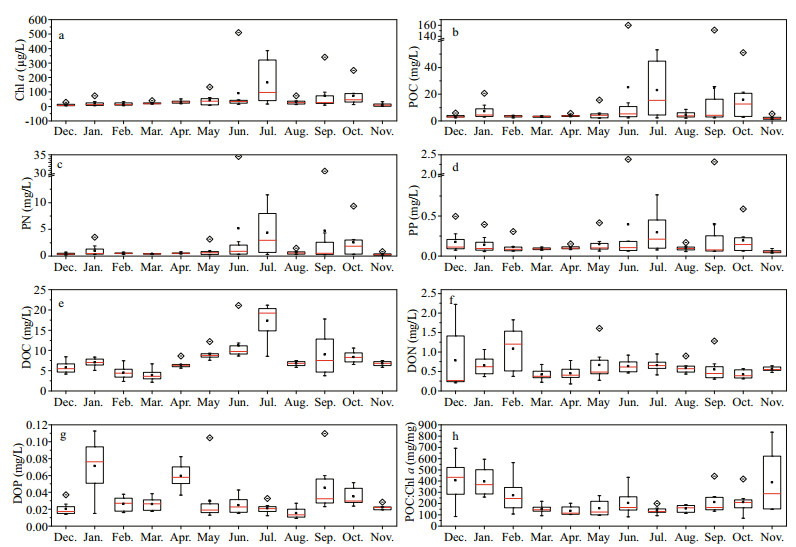
3 RESULT 3.1 Seasonal changes in concentrations of Chl a and phytoplankton derived C, N and PThe Chl a concentration grew in fluctuations from winter to late autumn, with the median values at the total eight stations reaching two peaks at 97.7 μg/L in July and 45.3 μg/L in October, and subsequently dropping to 10.5 μg/L in November, which was similar to the level measured at the beginning of the sampling in December (8.2 μg/L) (Fig. 2a).
|
| Figure 2 Temporal variations in chlorophyll a (Chl a) (a), particulate organic carbon (POC) (b), particulate nitrogen (PN) (c), particulate phosphorus (PP) (d), dissolved organic carbon (DOC) (e), dissolved organic nitrogen (DON) (f), dissolved organic phosphorus (DOP) (g), and POC: Chl a ratio (h) from December 2014 to November 2015 in Taihu Lake Red line, black square and box indicated median, mean, and 25%–75% values at eight stations, respectively. Diamond indicated outliers and whisker indicated the maximum and minimum values. |
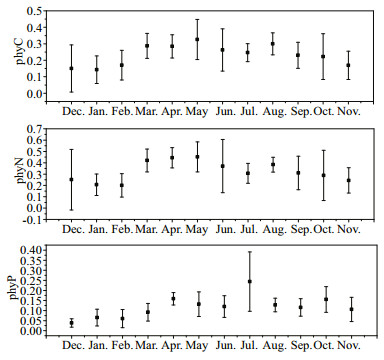
The estimated proportion of phytoplankton (mainly composed of cyanobacterial colonies) derived carbon (phyC), nitrogen (phyN) and phosphorus (phyP) in total POM varied from 15% to 33%, 20% to 45%, 3% to 24% respectively. PhyC and phyN peaked in May, while phyP peaked in July (Fig. 3).
|
| Figure 3 Estimated proportion of phytoplankton (mainly composed of cyanobacterial colonies) derived organic carbon (phyC), nitrogen (phyN), and phosphorus (phyP) in total POM Black square and error bar represent average values and standard deviations at eight stations in Taihu Lake, respectively. |
Although the concentrations of POC, PN, and PP varied widely among different sampling sites, changes in POC were strongly coincident with PN and PP. All POC, PN and PP concentrations in December were extremely low (i.e., median values of 3.05 mg/L, 0.35 mg/L, and 0.11 mg/L, respectively) (Fig. 2b-d). However, coinciding with the increases and peak values of Chl a, all POC, PN and PP values also increased, with two peaks in July and October. A respective of 5-fold, 8.4-fold, and 1.9-fold increase in POC, PN and PP, which corresponded to ∆POC, ∆PN and ∆PP values of approximately 12.31 mg/L, 2.59 mg/L, and 0.10 mg/L, respectively, was observed by comparison of the maximal median value in July to that in December.
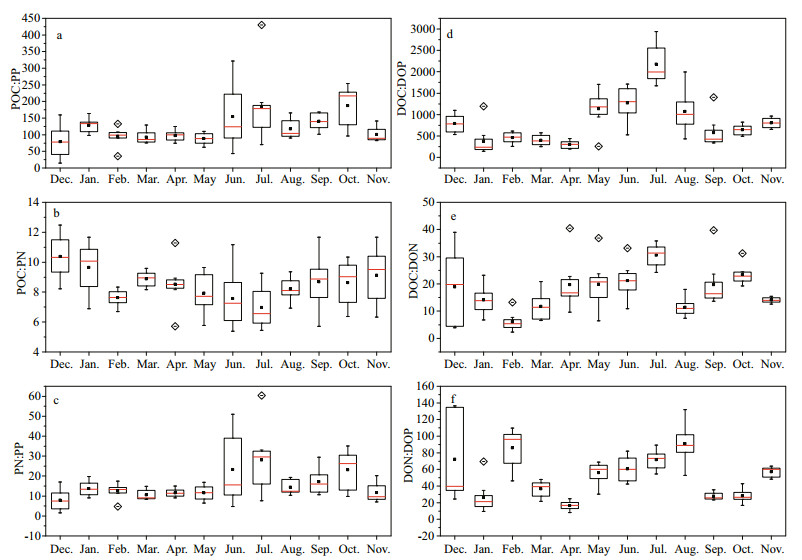
The molar POC:PN ratios fluctuated between 5.4 and 12.5, with a mean value of 8.5 (±1.6); this was slightly higher than the Redfield C:N ratio of 6.6. However, the molar POC:PP ratios spanned a range of nearly 20 times, with an overall mean of 122.1 (±59.8). The mean molar PN:PP ratio was 15.5 (±9.8). Nonetheless, neither the POC:PP nor the PN:PP ratios were statistically different from the corresponding Redfield ratios. The seasonal average molar POC:PP ratios and PN:PP ratios were in the range of 79 to 187 and 8 to 28, respectively, and they exhibited similar seasonal trends (Fig. 4a, c). That is, from December to May, the median molar POC:PP and PN:PP ratios were consistently lower than 100 (except January) and 13, respectively, and then increased along with bloom development (as indicated by the increased Chl a concentrations). The median values of POC:PP and PN:PP showed two peaks in July and October, which coincided with the Chl a concentration peaks; by contrast, the median POC:PN ratio decreased gradually from 10 in December to 7 in July, and then began to increase (Fig. 4b).
|
| Figure 4 Temporal variations in molar C:N:P elemental ratios of particulate and dissolved pools of organic matter collected during the study periods in Taihu Lake POC:PP (a), POC:PN (b), PN:PP (c), DOC:DOP (d), DOC:DON (e), DON:DOP (f). Red line, black square and box indicated median, mean, and 25%–75% values at eight stations, respectively. Diamond indicated outliers and whisker indicated the maximum and minimum values. |
In contrast to the wide spatial variation in POM, the concentrations of DOM among the different sampling sites were quite similar to each other. However, the temporal changes of the DOC, DON, and DOP concentrations were quite varied (Fig. 2e-g). The DOC concentrations increased significantly from winter to summer and reached maximal value in July, which was broadly synchronous with the increase and maximum of Chl a. The ∆DOC was approximately 13.8 mg/L, which corresponded to a 3.5-fold increase of DOC concentrations. However, during the study period, DON tended to fluctuate approximately 0.6 mg/L, whereas the DOP concentrations fluctuated approximately 0.02 mg/L during most months, with three peaks in January, April, and September.
On average, the molar DOC:DON:DOP ratio of Taihu Lake was 832:52:1, which was substantially higher than the Redfield ratio (106:16:1). Both molar DOC:DOP and molar DOC:DON ratios increased greatly from spring to summer and peaked in July, reaching maximal average values of 2 175 and 30, respectively (Fig. 4d-f). This coincided with the maximal Chl a concentrations. However, the temporal changes in DON:DOP were quite different from the temporal changes in DOC:DON and DOC:DOP. There were significant increases in the DON:DOP ratio in February, August and November, and the latter two months occurred directly after the increase of Chl a.
3.4 Relationships between POM, DOM, and water quality variablesThe POC:Chl a ratio (mg/mg) varied seasonally and spanned from 69 to 692 (Fig. 2h). The POC:Chl a ratio peaked in December (with a median value of 434), decreased thereafter until April, and then increased gradually from May to September. The median POC:Chl a ratio displayed a pattern of spring < summer < autumn < winter, with median values in the ranges of 115-146, 131-165, 166-212, and 246-434, respectively.
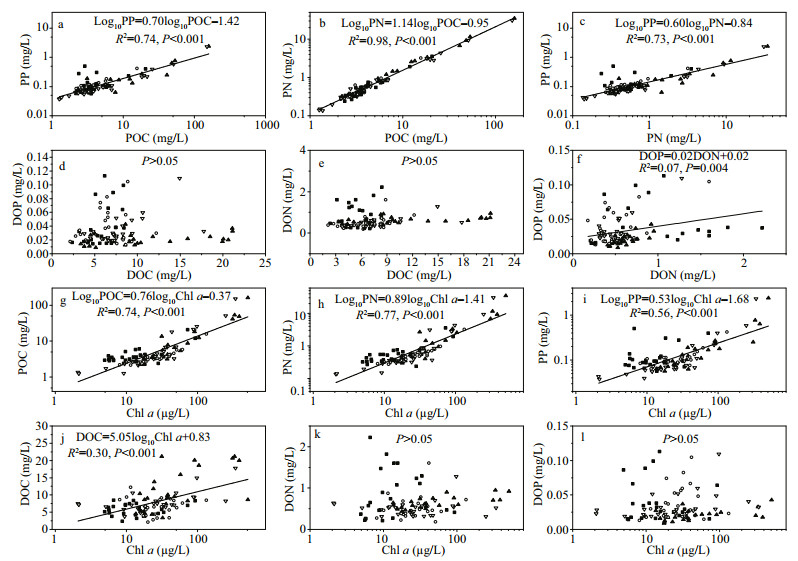
Regression analyses revealed that significant relationships (P < 0.001 in all regressions) were observed for POC versus PN, POC versus PP, and PN versus PP with R2 values of 0.98, 0.74, and 0.73, respectively (Fig. 5a-c). Despite this, significant correlation relationships (P < 0.001 in all regressions) were also observed for POC versus Chl a (R2=0.73), PN versus Chl a (R2=0.76), and PP versus Chl a (R2=0.55) (Fig. 5g-i). No significant relationships were found for DOC versus DON and DOC versus DOP (P>0.05), while only a weak correlation was observed for DON versus DOP (R2=0.007, P=0.004) (Fig. 5d-f). There was a significant correlation between DOC and Chl a (R2=0.30, P < 0.001), but DON and DOP were not correlated with Chl a (P>0.05) (Fig. 5j-l).
|
| Figure 5 Correlations among C, N, P in POM and DOM, and scatterplots of Chl a and POC, PN, PP, DOC, DON, and DOP Note logarithmic scale of POC, PN, PP and Chl a. Samples in winter, spring, summer and autumn were indicated by solid square, open circle, solid up triangle, and open down triangle, respectively. |
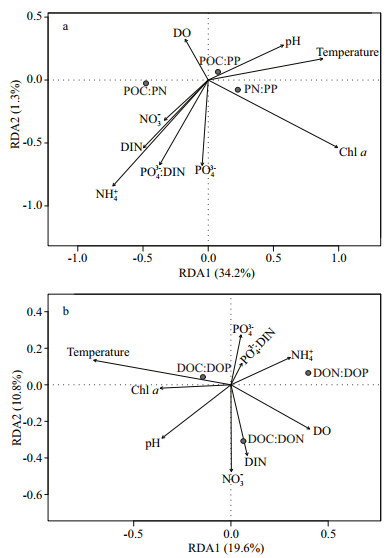
Results of the redundancy analysis illustrated that the measured water quality variables explained 34% of the variations of C:N:P ratios in POM. Chl a was the most significant explanatory variable (adjusted R2=0.21, P=0.002) (Fig. 6a). The water quality variables explained 30.4% of the variations of C:N:P ratios in DOM. Temperature was the most significant explanatory variable (adjusted R2=0.14, P=0.002), followed by NO3- (adjusted R2=0.04, P=0.036) and pH (adjusted R2=0.03, P=0.028) (Fig. 6b).
|
| Figure 6 Redundancy analysis biplot showing composition of C:N:P ratios of POM (a) and DOM (b) in relation to water quality variables |
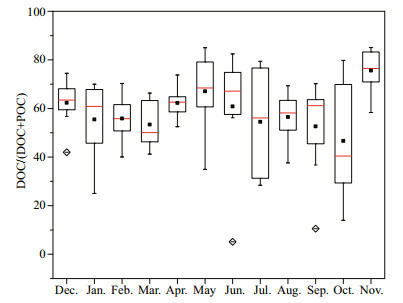
In Taihu Lake, both the POC and DOC increased from low values in winter to their maximal values in July. These increases occurred with the increasing Chl a concentration. The ∆POC was almost equal to ∆DOC, indicating that the cyanobacterial bloom contributed relatively equally to POC and DOC. Aside from the two lowest DOC:(DOC+POC) ratios of 5% and 10% in the samples (which had extremely high POC values, as described in 3.2), most of the DOC:(DOC+POC) ratios ranged widely from 14% to 85% (Fig. 7), indicating large spatial-temporal variance. However, the seasonal median values of the DOC:(DOC+POC) ratio were in the range of 40% to 76%, among which only the value in October was lower than 50%. Overall, the partitioning of total organic C into DOC was a little higher than into POC, with overall median values of 62%.
|
| Figure 7 Partitioning of DOC in total organic carbon in Taihu Lake Red line, black square and box indicated median, mean, and 25%–75% values at eight stations, respectively. Diamond indicated outliers and whisker indicated the maximum and minimum values. |
During the study period, the Chl a concentration increased from winter to late autumn, with median values of the eight sampling sites varying from 8.2 to 97.7 μg/L. According to the threshold of visible surface cyanobacterial blooms in Taihu Lake, defined as Chl a concentration 20 μg/L(Qin et al., 2015), the Chl a concentrations we determined verified that cyanobacterial blooms occurred from April through October, and were most serious in July.
4.1 POM concentrations and contribution of phytoplankton to total POMThe total mean POC concentration in Taihu Lake was similar to those of the shallow lake Kyoga and Prairie Lake 885 (Hecky et al., 1993), which also have high algal biomass. The changes in POC, PN and PP were significantly correlated to each other, indicating the tight coupling of C, N, and P in POM. Moreover, all of their concentrations were significantly correlated with Chl a, which suggested that the POM pool was mainly influenced by phytoplankton cell constituents during the study period. In addition, the sharp increase of POC concentrations in summer and autumn (July and October) coincided with the peak values of Chl a, indicating the cyanobacterial blooms contributed greatly to the increase in POC.
The POC to Chl a (POC:Chl a) ratio, which represents the relative proportion of particulate detritus, is also usually used as a marker of the quality of particulate organic matter (Parsons et al., 1977). That is, a POC:Chl a value lower than 200 indicates a greater contribution of "fresh" living algae, while a value higher than 200 is usually considered to indicate detrital or degraded organic matter (Parsons et al., 1977; Cifuentes et al., 1988). The seasonal changing trend of the POC:Chl a ratios in Taihu Lake was similar to a parabola, which was similar to that observed in Lake Kinneret (Berman and Pollingher, 1974), Jiaozhou Bay in China (Lü et al., 2009), and also observed during a phytoplankton bloom induced by in situ iron enrichment in the western subarctic Pacific (Yoshimura et al., 2009). In summary, newly formed organic matter of predominantly planktonic origin (seston with a POC:Chl a ratio lower than 200) was found during March and September, whereas most of the detrital or degraded organic matter (seston with a POC:Chl a ratio higher than 200) was observed during late autumn and winter.
It was difficult to directly separate phytoplankton from seston including detritus, and zooplankton, etc. Here we estimated contribution of phytoplankton (mainly composed of cyanobacterial colonies) to total POM based on the C, N, and P contents of phytoplankton collected from Meiliang Bay (N2 in Fig. 1) and the evaluated dry weight of phytoplankton according to Zhang (Zhang et al., 2011b). The proportion of phytoplankton derived organic matter in total POM were higher during bloom season than late autumn and winter, coinciding with that estimated based on values of the POC:Chl a ratio.
4.2 C:N:P ratio in POMThe average C:N:P ratio of 122:16:1 in POM in Taihu Lake was similar to that of 137:18:1 in warm, nutrient-rich upwelling zones (Martiny et al., 2013). Because the C:N ratio in POM, which is often indicative of the predominant source of organic matter in a system, and that of phytoplankton origin ranged from 7.7 to 10.1 (Meyers, 1994), the average POC:PN ratios (8.5) in Taihu Lake also indicated the major contribution of phytoplankton to POM. This was consistent with the redundancy analysis result that Chl a was the most significant explanatory factor for the variance of C:N:P ratio in POM.
The average value of POC:PN:PP increased from 97:11:1 during winter and spring to 157:21:1 during June to October, when there were large cyanobacterial blooms. This did not correspond with seasonal changes of POC:Chl a, a result similar to that observed by Hessen (Hessen et al., 2005), which was because POM was mainly composed of phytoplankton. There are three possible explanations for the lower values of POC:PP and PN:PP in winter and spring. One may be due to luxury consumption and storage of phosphate by phytoplankton in winter, as autotrophs accumulate P while photosynthetic activity is low (Hessen et al., 2005). The second possible reason was that phytoplankton proliferated rapidly along with the rising temperature during spring (Kong and Gao, 2005). Fast-growing phytoplankton synthesize large amount of P-rich ribosomal RNA, allocating resources toward the production of growth machinery by reducing their N:P ratio (Elser et al., 2000; Arrigo, 2005; Hillebrand et al., 2013). Lastly, the phytoplankton community was dominated by diatoms in late winter and early spring (data not shown). Diatoms generally have lower C:P, and N:P ratios, whereas cyanobacteria have higher C:P and N:P ratios (Arrigo et al., 1999; Bertilsson et al., 2003). Thus, changes in the phytoplankton community may be the third reason for the variation of the C:N:P ratios in the seston. Given that POM is a substrate for grazers (Mei et al., 2005), the nutrient ratio of POM can provide basic stoichiometric information about the food resources. Thus these POM of different quality may influence community structure of zooplankton through a bottom-up manner (Elser et al., 1998; Cross et al., 2007).
4.3 DOM concentrations and contributions of cyanobacterial bloomsTaihu Lake had a strong gradient of DOC (2.1- 21.1 mg/L) that varied by over 10-fold over the course of the observation period; this DOC gradient was comparable to the typical DOC concentration of 3-34 mg/L in eutrophic lakes (Thurman, 1985). The DOC concentration, which increased after winter and reached a maximum in July, when there was a dense cyanobacterial bloom, was similar to the high DOC concentrations during cyanobacterial blooms observed in Lake Soyang in Korea (Kim et al., 2000), and in Lake Kasumigaura and Lake Suwa in Japan (Hama and Handa, 1983; Fukushima et al., 1996). These results indicated that the cyanobacterial blooms introduced substantial amounts of DOC into water column. However, in contrast to the synchronous increase of POM and DOC along with Chl a, both DON and DOP fluctuated independently. In the case of a freshwater lake, much of DON is often from terrestrial leaching and runoff, consisting mainly of humic substances and atmospheric deposition (Berman and Bronk, 2003). DOM from surrounding rivers may also contribute to the DOM in Taihu Lake (Zhang et al., 2011a). Furthermore, the fact that both DON and DOP can be assimilated and utilized by cyanobacteria under certain conditions (Glibert et al., 2004; Davis et al., 2010; Shi et al., 2011) may have also led to their fluctuations.
4.4 C:N:P ratio in DOMOn average, the C:N:P ratio of 832:52:1 for bulk DOM in Taihu Lake was lower than that of the ultraoligotrophic freshwater Lake Puma Yumco located in the pre-Himalayas of Tibet in China (2100:140:1) (Mitamura et al., 2003) and the mesotrophic Lake Biwa (1978:147:1) (Kim et al., 2006). However, it was higher than the average ratio of C:N:P in the DOM (374:22:1) of the n-limited, cyanobacteria-dominated East/Japan Sea (Kim and Kim, 2013). This suggests that the C:N:P ratios in DOM varied among different ecosystems.
Both C:P and C:N increased from spring until July, reaching maximum average values of 2 175 and 30, respectively. These maxima coincided with the maximum Chl a concentration, suggesting that the newly produced DOM was quite C-rich and deficient in both nitrogen and phosphorus (Norrman et al., 1995; Williams, 1995; Søndergaard et al., 2000). Among the environmental factors determined, temperature, NO3-, and pH contributed to the variance of C:N:P in DOM. Given that temperature greatly affects carbon excretion from cyanobacteria (Zlotnik and Dubinsky, 1989), the rising temperature leading to increased DOC may be one reason for the high C:P and C:N ratios in DOM. NO3- may also influence the metabolism of cyanobacteria, thereby affecting DOC excretion (Huang et al., 2007). Moreover, DOM and NO3- are closely coupled (Zhang et al., 2014), whereas pH influences the mineralization of DOM (Roth et al., 2013). Thus, both NO3- and pH had a role in DOM composition. Furthermore, in situ preferential remineralization of the n- and/or P-rich compounds (Hopkinson et al., 1997, 2002) may be another reason for the high C:N:P ratios in the bulk DOM.
However, as the cyanobacterial bloom proceeded, there was a decrease of both DOC:DON and DOC:DOP after their maximum values in July. Similar to this study, substantial evidence from previous studies (and the references therein) suggests that production of the DOC fraction may be exceeding decomposition during summer bloom months, while verse visa in the autumn (Williams, 1995). Because cyanobacteria-derived DOC is biodegradable and is preferentially utilized by bacteria (Ye et al., 2015), the decreased C:N and C:P ratios of DOM from summer to autumn in Taihu Lake indicated this DOM appears to be rapidly assimilated. Thus, this autochthonous organic matter appears to be an important carbon and energy source for the food web.
4.5 Partitioning of POC and DOC in Taihu LakeIn Taihu Lake, the partitioning of total organic carbon into DOC varied from 40% to 76% and tended to fluctuate by approximately 62%. Thus, the partitioning of total organic carbon into DOC was slightly higher than that of POC. This is unlike the Ross Sea with occurrence of Phaeocystis blooms or deck incubations of coastal diatom blooms in Oregon, in which 89% and 78% of the accumulated organic matter were partitioned into POM, respectively (Carlson et al., 2000; Wetz and Wheeler, 2003). However, it was similar to those in Lake Soyang in Korea, a deep reservoir with cyanobacterial blooms, in which monthly DOC loading comprises more than 56% of the TOC loading (Kim et al., 2000). In addition, along with the increase of Chl a concentration from a low level in winter to a maximum level in July, ∆POC was comparable to ∆DOC, indicating that the cyanobacterial bloom contributed nearly equally to POC and DOC. These results further revealed the influence of phytoplankton community structure on the differential partitioning of organic carbon within various water systems (Carlson et al., 2000).
5 CONCLUSIONOur findings that POC, PN, PP, and DOC were significantly and positively correlated with Chl a indicated the substantial contribution of cyanobacterial blooms to POM and DOC in Taihu Lake. Moreover, the partitioning of total organic carbon into DOC was slightly higher than POC. Compared to winter and spring, elevated C:P and N:P ratios in POM and C:P and C:N ratios in DOM were observed during the dense cyanobacterial blooms in July. These ratios were representative of the algal production of C-rich POM and DOM. However, their subsequent decreases indicated that these C-rich POM and DOM can be easily assimilated. Furthermore, redundancy analysis revealed that Chl a explained most of the variations of C:N:P ratios in POM, whereas temperature, NO- 3, and pH were significant explanatory factors for the variations of C:N:P ratios in DOM. The present study provides basic information on the food web, and may be helpful for understanding ecosystem structure in these eutrophic lakes. Future studies are needed of the mechanisms as to how the cyanobacterial bloom derived organic matter is cycled in the food web.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENTWe thank the anonymous reviewers for their valuable comments in the previous version of this manuscript. We thank Taihu Laboratory for Lake Ecosystem Research (TLLER) for providing the monitoring data of phytoplankton. We appreciate CHEN Chao for his help for sample collection in the field.
Arrigo K R, Robinson D H, Worthen D L, Dunbar R B, DiTullio G R, VanWoert M, Lizotte M P. 1999. Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science, 283(5400): 365-367. DOI:10.1126/science.283.5400.365 |
Arrigo K R. 2005. Marine microorganisms and global nutrient cycles. Nature, 437(7057): 349-355. DOI:10.1038/nature04159 |
Berman T, Bronk D A. 2003. Dissolved organic nitrogen:a dynamic participant in aquatic ecosystems. Aquat.Microb. Ecol., 31(3): 279-305. |
Berman T, Pollingher U. 1974. Annual and seasonal variations of phytoplankton, chlorophyll, and photosynthesis in Lake Kinncret. Limnol. Oceanogr., 19(1): 31-55. DOI:10.4319/lo.1974.19.1.0031 |
Bertilsson S, Berglund O, Karl D M, Chisholm S W. 2003. Elemental composition of marine Prochlorococcus and Synechococcus:implications for the ecological stoichiometry of the sea. Limnol. Oceanogr., 48(5): 1721-1731. DOI:10.4319/lo.2003.48.5.1721 |
Carlson C A, Hansell D A, Peltzer E T, Smith W O Jr. 2000. Stocks and dynamics of dissolved and particulate organic matter in the southern Ross Sea, Antarctica. Deep Sea Res. Part Ⅱ Top. Stud. Oceanogr., 47(15-16): 3201-3225. DOI:10.1016/S0967-0645(00)00065-5 |
Chen Y W, Qin B Q, Teubner K, Dokulil M T. 2003. Long-term dynamics of phytoplankton assemblages:Microcystisdomination in Taihu Lake, a large shallow lake in China. J. Plankton Res., 25(4): 445-453. DOI:10.1093/plankt/25.4.445 |
Cifuentes L A, Sharp J H, Fogel M L. 1988. Stable carbon and nitrogen isotope biogeochemistry in the Delaware estuary. Limnol. Oceanogr., 33(5): 1102-1115. DOI:10.4319/lo.1988.33.5.1102 |
Cross W F, Wallace J B, Rosemond A D. 2007. Nutrient enrichment reduces constraints on material flows in a detritus-based food web. Ecology, 88(10): 2563-2575. DOI:10.1890/06-1348.1 |
Davis T W, Harke M J, Marcoval M A, Goleski J, OranoDawson C, Berry D L, Gobler C J. 2010. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat. Microb. Ecol., 61(2): 149-162. DOI:10.3354/ame01445 |
Ebina J, Tsutsui T, Shirai T. 1983. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res., 17(12): 1721-1726. DOI:10.1016/0043-1354(83)90192-6 |
Elser J J, Chrzanowski T H, Sterner R W, Mills K H. 1998. Stoichiometric constraints on food-web dynamics:a whole-lake experiment on the Canadian Shield. Ecosystems, 1(1): 120-136. DOI:10.1007/s100219900009 |
Elser J J, Hayakawa K, Urabe J. 2001. Nutrient limitation reduces food quality for zooplankton:Daphnia response to seston phosphorus enrichment. Ecology, 82(3): 898-903. DOI:10.1890/0012-9658(2001)082[0898:NLRFQF]2.0.CO;2 |
Elser J J, Sterner R W, Gorokhova E, Fagan W F, Markow T A, Cotner J B, Harrison J F, Hobbie S E, Odell G M, Weider L W. 2000. Biological stoichiometry from genes to ecosystems. Ecol. Lett., 3(6): 540-550. DOI:10.1046/j.1461-0248.2000.00185.x |
Elser J, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner R W. 2003. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett., 6(10): 936-943. DOI:10.1046/j.1461-0248.2003.00518.x |
Fukushima T, Park J C, Imai A, Matsushige K. 1996. Dissolved organic carbon in a eutrophic lake; dynamics, biodegradability and origin. Aquat. Sci., 58(2): 139-157. DOI:10.1007/BF00877112 |
Geider R, La Roche J. 2002. Redfield revisited:variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol., 37(1): 1-17. DOI:10.1017/S0967026201003456 |
Glibert P M, Heil C A, Hollander D, Revilla M, Hoare A, Alexander J, Murasko S. 2004. Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Prog.Ser., 280: 73-83. DOI:10.3354/meps280073 |
Hama T, Handa N. 1983. The seasonal variation of organic constituents in a eutrophic lake, Lake Suwa, Japan. Part Ⅱ. Dissolved organic matter. Arch. Hydrobiol., 98(4): 443-462. |
Hecky R E, Campbell P, Hendzel L L. 1993. The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol. Oceanogr., 38(4): 709-724. DOI:10.4319/lo.1993.38.4.0709 |
Hecky R E, Kilham P. 1988. Nutrient limitation of phytoplankton in freshwater and marine environments:a review of recent evidence on the effects of enrichment. Limnol. Oceanogr., 33(4 Part 2): 796-822. |
Hessen D O, Andersen T, Brettum P, Faafeng B A. 2003. Phytoplankton contribution to sestonic mass and elemental ratios in lakes:implications for zooplankton nutrition. Limnol. Oceanogr., 48(3): 1289-1296. DOI:10.4319/lo.2003.48.3.1289 |
Hessen D O, Van Donk E, Gulati R. 2005. Seasonal seston stoichiometry:effects on zooplankton in cyanobacteriadominated lakes. J. Plankton Res., 27(5): 449-460. DOI:10.1093/plankt/fbi018 |
Hillebrand H, Steinert G, Boersma M, Malzahn A, Meunier C L, Plum C, Ptacnik R. 2013. Goldman revisited:fastergrowing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol. Oceanogr., 58(6): 2076-2088. DOI:10.4319/lo.2013.58.6.2076 |
Hopkinson C S Jr, Vallino J J, Nolin A. 2002. Decomposition of dissolved organic matter from the continental margin. Deep Sea Res. Part Ⅱ Top. Stud. Oceanogr., 49(20): 4461-4478. DOI:10.1016/S0967-0645(02)00125-X |
Hopkinson C S, Fry B, Nolin A L. 1997. Stoichiometry of dissolved organic matter dynamics on the continental shelf of the northeastern U. S.A. Cont. Shelf Res., 17(5): 473-489. DOI:10.1016/S0278-4343(96)00046-5 |
Huang W J, Lai C H, Cheng Y L. 2007. Evaluation of extracellular products and mutagenicity in cyanobacteria cultures separated from a eutrophic reservoir. Sci. Total Environ., 377(2-3): 214-223. DOI:10.1016/j.scitotenv.2007.01.075 |
Kim B, Choi K, Kim C, Lee U H, Kim Y H. 2000. Effects of the summer monsoon on the distribution and loading of organic carbon in a deep reservoir, Lake Soyang, Korea. Water Res., 34(14): 3495-3504. DOI:10.1016/S0043-1354(00)00104-4 |
Kim C, Nishimura Y, Nagata T. 2006. Role of dissolved organic matter in hypolimnetic mineralization of carbon and nitrogen in a large, monomictic lake. Limnol.Oceanogr., 51(1): 70-78. DOI:10.4319/lo.2006.51.1.0070 |
Kim T H, Kim G. 2013. Factors controlling the C:N:P stoichiometry of dissolved organic matter in the N-limited, cyanobacteria-dominated East/Japan Sea. J. Mar. Syst., 115-116: 1-9. DOI:10.1016/j.jmarsys.2013.01.002 |
Klausmeier C A, Litchman E, Daufresne T, Levin S A. 2004. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature, 429(6988): 171-174. DOI:10.1038/nature02454 |
Kong F X, Gao G. 2005. Hypothesis on cyanobacteria bloomforming mechanism in large shallow eutrophic lakes. Acta Ecol. Sin., 25(3): 589-595. |
Lü S G, Wang X C, Han B P. 2009. A field study on the conversion ratio of phytoplankton biomass carbon to chlorophyll-a in Jiaozhou Bay, China. Chin. J. Oceanol.Limnol., 27(4): 793-805. DOI:10.1007/s00343-009-9221-0 |
Ma J R, Qin B Q, Paerl H W, Brookes J D, Hall N S, Shi K, Zhou Y Q, Guo J S, Li Z, Xu H, Wu T F, Long S X. 2016. The persistence of cyanobacterial (Microcystis spp. )blooms throughout winter in Taihu Lake, China. Limnol.Oceanogr., 61(2): 711-722. |
Martiny A C, Pham C T A, Primeau F W, Vrugt J A, Moore J K, Levin S A, Lomas M W. 2013. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci., 6(4): 279-283. DOI:10.1038/ngeo1757 |
McCarthy M J, Lavrentyev P J, Yang L Y, Zhang L, Chen Y W, Qin B Q, Gardner W S. 2007. Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, wellmixed, eutrophic lake (Taihu Lake, China). Hydrobiologia, 581(1): 195-207. DOI:10.1007/s10750-006-0496-2 |
Mei Z P, Legendre L, Tremblay J É, Miller L A, Gratton Y, Lovejoy C, Yager P L, Gosselin M. 2005. Carbon to nitrogen (C:N) stoichiometry of the spring-summer phytoplankton bloom in the North Water Polynya (NOW). Deep Sea Res. Part Ⅰ Oceanogr. Res. Pap., 52(12): 2301-2314. DOI:10.1016/j.dsr.2005.07.001 |
Meyers P A. 1994. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol., 114(3-4): 289-302. DOI:10.1016/0009-2541(94)90059-0 |
Mitamura O, Seike Y, Kondo K, Goto N, Anbutsu K, Akatsuka T, Kihira M, Tsering T Q, Nishimura T M. 2003. First investigation of ultraoligotrophic alpine Lake Puma Yumco in the pre-Himalayas, China. Limnology, 4(3): 167-175. DOI:10.1007/s10201-003-0101-6 |
Nagata T. 2000. Production mechanisms of dissolved organic matter. In: Kirchman D L ed. Microbial Ecology of the Oceans. Wiley-Liss, New York.
|
Norrman B, Zwelfel U L, Hopkinson C S Jr, Brian F. 1995. Production and utilization of dissolved organic carbon during an experimental diatom bloom. Limnol. Oceanogr., 40(5): 898-907. DOI:10.4319/lo.1995.40.5.0898 |
Parsons T R, Takahashi M, Hargrave B. 1977. Biological Oceanographic Processes. 2nd edn. Pergamon Press, New York. p. 332.
|
Qin B Q, Li W, Zhu G W, Zhang Y L, Wu T F, Gao G. 2015. Cyanobacterial bloom management through integrated monitoring and forecasting in large shallow eutrophic Taihu Lake (China). J. Hazard. Mater., 287: 356-363. DOI:10.1016/j.jhazmat.2015.01.047 |
Qin B Q, Xu P Z, Wu Q L, Luo L C, Zhang Y L. 2007. Environmental issues of lake Taihu, China. Hydrobiologia, 581(1): 3-14. DOI:10.1007/s10750-006-0521-5 |
Roth V N, Dittmar T, Gaupp R, Gleixner G. 2013. Latitude and pH driven trends in the molecular composition of DOM across a north south transect along the Yenisei River. Geochim. Cosmochim. Acta, 123: 93-105. DOI:10.1016/j.gca.2013.09.002 |
Shi X L, Qian S Q, Kong F X, Zhang M, Yu Y. 2011. Differences in growth and alkaline phosphatase activity between Microcystis aeruginosa and Chlorella pyrenoidosa in response to media with different organic phosphorus. J.Limnol., 70(1): 21-25. DOI:10.4081/jlimnol.2011.21 |
Søndergaard M, Williams P J L B, Cauwet G, Riemann B, Robinson C, Terzic S, Woodward E M S, Worm J. 2000. Net accumulation and flux of dissolved organic carbon and dissolved organic nitrogen in marine plankton communities. Limnol. Oceanogr., 45(5): 1097-1111. DOI:10.4319/lo.2000.45.5.1097 |
Sterner R W, Elser J J. 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton.
|
Sterner R W, Hagemeier D D, Smith W L, Smith R F. 1993. Phytoplankton nutrient limitation and food quality for Daphnia. Limnol. Oceanogr., 38(4): 857-871. DOI:10.4319/lo.1993.38.4.0857 |
Thurman E M. 1985. Organic Geochemistry of Natural Waters. Springer, Netherlands.
|
Wetz M S, Wheeler P A. 2003. Production and partitioning of organic matter during simulated phytoplankton blooms. Limnol. Oceanogr., 48(5): 1808-1817. DOI:10.4319/lo.2003.48.5.1808 |
Williams P J L B. 1995. Evidence for the seasonal accumulation of carbon-rich dissolved organic material, its scale in comparison with changes in particulate material and the consequential effect on net C/N assimilation ratios. Mar.Chem., 51(1): 17-29. DOI:10.1016/0304-4203(95)00046-T |
Ye L L, Wu X D, Liu B, Yan D Z, Kong F X. 2015. Dynamics and sources of dissolved organic carbon during phytoplankton bloom in hypereutrophic Taihu Lake (China). Limnologica, 54: 5-13. DOI:10.1016/j.limno.2015.05.003 |
Yoshimura T, Ogawa H, Imai K, Aramaki T, Nojiri Y, Nishioka J, Tsuda A. 2009. Dynamics and elemental stoichiometry of carbon, nitrogen, and phosphorus in particulate and dissolved organic pools during a phytoplankton bloom induced by in situ iron enrichment in the western subarctic Pacific (SEEDS-Ⅱ). Deep Sea Res. Part Ⅱ Top. Stud.Oceanogr., 56(26): 2863-2874. DOI:10.1016/j.dsr2.2009.06.011 |
Zhang Y L, Gao G, Shi K, Niu C, Zhou Y Q, Qin B Q, Liu X H. 2014. Absorption and fluorescence characteristics of rainwater CDOM and contribution to Taihu Lake, China. Atmos. Environ., 98: 483-491. DOI:10.1016/j.atmosenv.2014.09.038 |
Zhang Y L, van Dijk M A, Liu M L, Zhu G W, Qin B Q. 2009. The contribution of phytoplankton degradation to chromophoric dissolved organic matter (CDOM) in eutrophic shallow lakes:field and experimental evidence. Water Res., 43(18): 4685-4697. DOI:10.1016/j.watres.2009.07.024 |
Zhang Y L, Yin Y, Liu X H, Shi Z Q, Feng L Q, Liu M L, Zhu G W, Gong Z J, Qin B Q. 2011a. Spatial-seasonal dynamics of chromophoric dissolved organic matter in Taihu Lake, a large eutrophic, shallow lake in China. Org.Geochem., 42(5): 510-519. DOI:10.1016/j.orggeochem.2011.03.007 |
Zhang Y L, Yin Y, Zhang E L, Zhu G W, Liu M L, Feng L Q, Qin B Q, Liu X H. 2011b. Spectral attenuation of ultraviolet and visible radiation in lakes in the Yunnan Plateau, and the middle and lower reaches of the Yangtze River, China. Photochem. Photobiol. Sci., 10(4): 469-482. DOI:10.1039/C0PP00270D |
Zlotnik I, Dubinsky Z. 1989. The effect of light and temperature on doc excretion by phytoplankton. Limnol. Oceanogr., 34(5): 831-839. DOI:10.4319/lo.1989.34.5.0831 |
| Arrigo K R, Robinson D H, Worthen D L, Dunbar R B, DiTullio G R, VanWoert M, Lizotte M P, 1999. Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science, 283(5400): 365–367. Doi: 10.1126/science.283.5400.365 |
| Arrigo K R, 2005. Marine microorganisms and global nutrient cycles. Nature, 437(7057): 349–355. Doi: 10.1038/nature04159 |
| Berman T, Bronk D A, 2003. Dissolved organic nitrogen:a dynamic participant in aquatic ecosystems. Aquat.Microb. Ecol., 31(3): 279–305. |
| Berman T, Pollingher U, 1974. Annual and seasonal variations of phytoplankton, chlorophyll, and photosynthesis in Lake Kinncret. Limnol. Oceanogr., 19(1): 31–55. Doi: 10.4319/lo.1974.19.1.0031 |
| Bertilsson S, Berglund O, Karl D M, Chisholm S W, 2003. Elemental composition of marine Prochlorococcus and Synechococcus:implications for the ecological stoichiometry of the sea. Limnol. Oceanogr., 48(5): 1721–1731. Doi: 10.4319/lo.2003.48.5.1721 |
| Carlson C A, Hansell D A, Peltzer E T, Smith W O Jr, 2000. Stocks and dynamics of dissolved and particulate organic matter in the southern Ross Sea, Antarctica. Deep Sea Res. Part Ⅱ Top. Stud. Oceanogr., 47(15-16): 3201–3225. Doi: 10.1016/S0967-0645(00)00065-5 |
| Chen Y W, Qin B Q, Teubner K, Dokulil M T, 2003. Long-term dynamics of phytoplankton assemblages:Microcystisdomination in Taihu Lake, a large shallow lake in China. J. Plankton Res., 25(4): 445–453. Doi: 10.1093/plankt/25.4.445 |
| Cifuentes L A, Sharp J H, Fogel M L, 1988. Stable carbon and nitrogen isotope biogeochemistry in the Delaware estuary. Limnol. Oceanogr., 33(5): 1102–1115. Doi: 10.4319/lo.1988.33.5.1102 |
| Cross W F, Wallace J B, Rosemond A D, 2007. Nutrient enrichment reduces constraints on material flows in a detritus-based food web. Ecology, 88(10): 2563–2575. Doi: 10.1890/06-1348.1 |
| Davis T W, Harke M J, Marcoval M A, Goleski J, OranoDawson C, Berry D L, Gobler C J, 2010. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat. Microb. Ecol., 61(2): 149–162. Doi: 10.3354/ame01445 |
| Ebina J, Tsutsui T, Shirai T, 1983. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res., 17(12): 1721–1726. Doi: 10.1016/0043-1354(83)90192-6 |
| Elser J J, Chrzanowski T H, Sterner R W, Mills K H, 1998. Stoichiometric constraints on food-web dynamics:a whole-lake experiment on the Canadian Shield. Ecosystems, 1(1): 120–136. Doi: 10.1007/s100219900009 |
| Elser J J, Hayakawa K, Urabe J, 2001. Nutrient limitation reduces food quality for zooplankton:Daphnia response to seston phosphorus enrichment. Ecology, 82(3): 898–903. Doi: 10.1890/0012-9658(2001)082[0898:NLRFQF]2.0.CO;2 |
| Elser J J, Sterner R W, Gorokhova E, Fagan W F, Markow T A, Cotner J B, Harrison J F, Hobbie S E, Odell G M, Weider L W, 2000. Biological stoichiometry from genes to ecosystems. Ecol. Lett., 3(6): 540–550. Doi: 10.1046/j.1461-0248.2000.00185.x |
| Elser J, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner R W, 2003. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett., 6(10): 936–943. Doi: 10.1046/j.1461-0248.2003.00518.x |
| Fukushima T, Park J C, Imai A, Matsushige K, 1996. Dissolved organic carbon in a eutrophic lake; dynamics, biodegradability and origin. Aquat. Sci., 58(2): 139–157. Doi: 10.1007/BF00877112 |
| Geider R, La Roche J, 2002. Redfield revisited:variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol., 37(1): 1–17. Doi: 10.1017/S0967026201003456 |
| Glibert P M, Heil C A, Hollander D, Revilla M, Hoare A, Alexander J, Murasko S, 2004. Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Prog.Ser., 280: 73–83. Doi: 10.3354/meps280073 |
| Hama T, Handa N, 1983. The seasonal variation of organic constituents in a eutrophic lake, Lake Suwa, Japan. Part Ⅱ. Dissolved organic matter. Arch. Hydrobiol., 98(4): 443–462. |
| Hecky R E, Campbell P, Hendzel L L, 1993. The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol. Oceanogr., 38(4): 709–724. Doi: 10.4319/lo.1993.38.4.0709 |
| Hecky R E, Kilham P, 1988. Nutrient limitation of phytoplankton in freshwater and marine environments:a review of recent evidence on the effects of enrichment. Limnol. Oceanogr., 33(4 Part 2): 796–822. |
| Hessen D O, Andersen T, Brettum P, Faafeng B A, 2003. Phytoplankton contribution to sestonic mass and elemental ratios in lakes:implications for zooplankton nutrition. Limnol. Oceanogr., 48(3): 1289–1296. Doi: 10.4319/lo.2003.48.3.1289 |
| Hessen D O, Van Donk E, Gulati R, 2005. Seasonal seston stoichiometry:effects on zooplankton in cyanobacteriadominated lakes. J. Plankton Res., 27(5): 449–460. Doi: 10.1093/plankt/fbi018 |
| Hillebrand H, Steinert G, Boersma M, Malzahn A, Meunier C L, Plum C, Ptacnik R, 2013. Goldman revisited:fastergrowing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol. Oceanogr., 58(6): 2076–2088. Doi: 10.4319/lo.2013.58.6.2076 |
| Hopkinson C S Jr, Vallino J J, Nolin A, 2002. Decomposition of dissolved organic matter from the continental margin. Deep Sea Res. Part Ⅱ Top. Stud. Oceanogr., 49(20): 4461–4478. Doi: 10.1016/S0967-0645(02)00125-X |
| Hopkinson C S, Fry B, Nolin A L, 1997. Stoichiometry of dissolved organic matter dynamics on the continental shelf of the northeastern U. S.A. Cont. Shelf Res., 17(5): 473–489. Doi: 10.1016/S0278-4343(96)00046-5 |
| Huang W J, Lai C H, Cheng Y L, 2007. Evaluation of extracellular products and mutagenicity in cyanobacteria cultures separated from a eutrophic reservoir. Sci. Total Environ., 377(2-3): 214–223. Doi: 10.1016/j.scitotenv.2007.01.075 |
| Kim B, Choi K, Kim C, Lee U H, Kim Y H, 2000. Effects of the summer monsoon on the distribution and loading of organic carbon in a deep reservoir, Lake Soyang, Korea. Water Res., 34(14): 3495–3504. Doi: 10.1016/S0043-1354(00)00104-4 |
| Kim C, Nishimura Y, Nagata T, 2006. Role of dissolved organic matter in hypolimnetic mineralization of carbon and nitrogen in a large, monomictic lake. Limnol.Oceanogr., 51(1): 70–78. Doi: 10.4319/lo.2006.51.1.0070 |
| Kim T H, Kim G, 2013. Factors controlling the C:N:P stoichiometry of dissolved organic matter in the N-limited, cyanobacteria-dominated East/Japan Sea. J. Mar. Syst., 115-116: 1–9. Doi: 10.1016/j.jmarsys.2013.01.002 |
| Klausmeier C A, Litchman E, Daufresne T, Levin S A, 2004. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature, 429(6988): 171–174. Doi: 10.1038/nature02454 |
| Kong F X, Gao G, 2005. Hypothesis on cyanobacteria bloomforming mechanism in large shallow eutrophic lakes. Acta Ecol. Sin., 25(3): 589–595. |
| Lü S G, Wang X C, Han B P, 2009. A field study on the conversion ratio of phytoplankton biomass carbon to chlorophyll-a in Jiaozhou Bay, China. Chin. J. Oceanol.Limnol., 27(4): 793–805. Doi: 10.1007/s00343-009-9221-0 |
| Ma J R, Qin B Q, Paerl H W, Brookes J D, Hall N S, Shi K, Zhou Y Q, Guo J S, Li Z, Xu H, Wu T F, Long S X, 2016. The persistence of cyanobacterial (Microcystis spp. )blooms throughout winter in Taihu Lake, China. Limnol.Oceanogr., 61(2): 711–722. |
| Martiny A C, Pham C T A, Primeau F W, Vrugt J A, Moore J K, Levin S A, Lomas M W, 2013. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci., 6(4): 279–283. Doi: 10.1038/ngeo1757 |
| McCarthy M J, Lavrentyev P J, Yang L Y, Zhang L, Chen Y W, Qin B Q, Gardner W S, 2007. Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, wellmixed, eutrophic lake (Taihu Lake, China). Hydrobiologia, 581(1): 195–207. Doi: 10.1007/s10750-006-0496-2 |
| Mei Z P, Legendre L, Tremblay J É, Miller L A, Gratton Y, Lovejoy C, Yager P L, Gosselin M, 2005. Carbon to nitrogen (C:N) stoichiometry of the spring-summer phytoplankton bloom in the North Water Polynya (NOW). Deep Sea Res. Part Ⅰ Oceanogr. Res. Pap., 52(12): 2301–2314. Doi: 10.1016/j.dsr.2005.07.001 |
| Meyers P A, 1994. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol., 114(3-4): 289–302. Doi: 10.1016/0009-2541(94)90059-0 |
| Mitamura O, Seike Y, Kondo K, Goto N, Anbutsu K, Akatsuka T, Kihira M, Tsering T Q, Nishimura T M, 2003. First investigation of ultraoligotrophic alpine Lake Puma Yumco in the pre-Himalayas, China. Limnology, 4(3): 167–175. Doi: 10.1007/s10201-003-0101-6 |
| Nagata T. 2000. Production mechanisms of dissolved organic matter. In: Kirchman D L ed. Microbial Ecology of the Oceans. Wiley-Liss, New York. |
| Norrman B, Zwelfel U L, Hopkinson C S Jr, Brian F, 1995. Production and utilization of dissolved organic carbon during an experimental diatom bloom. Limnol. Oceanogr., 40(5): 898–907. Doi: 10.4319/lo.1995.40.5.0898 |
| Parsons T R, Takahashi M, Hargrave B. 1977. Biological Oceanographic Processes. 2nd edn. Pergamon Press, New York. p. 332. |
| Qin B Q, Li W, Zhu G W, Zhang Y L, Wu T F, Gao G, 2015. Cyanobacterial bloom management through integrated monitoring and forecasting in large shallow eutrophic Taihu Lake (China). J. Hazard. Mater., 287: 356–363. Doi: 10.1016/j.jhazmat.2015.01.047 |
| Qin B Q, Xu P Z, Wu Q L, Luo L C, Zhang Y L, 2007. Environmental issues of lake Taihu, China. Hydrobiologia, 581(1): 3–14. Doi: 10.1007/s10750-006-0521-5 |
| Roth V N, Dittmar T, Gaupp R, Gleixner G, 2013. Latitude and pH driven trends in the molecular composition of DOM across a north south transect along the Yenisei River. Geochim. Cosmochim. Acta, 123: 93–105. Doi: 10.1016/j.gca.2013.09.002 |
| Shi X L, Qian S Q, Kong F X, Zhang M, Yu Y, 2011. Differences in growth and alkaline phosphatase activity between Microcystis aeruginosa and Chlorella pyrenoidosa in response to media with different organic phosphorus. J.Limnol., 70(1): 21–25. Doi: 10.4081/jlimnol.2011.21 |
| Søndergaard M, Williams P J L B, Cauwet G, Riemann B, Robinson C, Terzic S, Woodward E M S, Worm J, 2000. Net accumulation and flux of dissolved organic carbon and dissolved organic nitrogen in marine plankton communities. Limnol. Oceanogr., 45(5): 1097–1111. Doi: 10.4319/lo.2000.45.5.1097 |
| Sterner R W, Elser J J. 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton. |
| Sterner R W, Hagemeier D D, Smith W L, Smith R F, 1993. Phytoplankton nutrient limitation and food quality for Daphnia. Limnol. Oceanogr., 38(4): 857–871. Doi: 10.4319/lo.1993.38.4.0857 |
| Thurman E M. 1985. Organic Geochemistry of Natural Waters. Springer, Netherlands. |
| Wetz M S, Wheeler P A, 2003. Production and partitioning of organic matter during simulated phytoplankton blooms. Limnol. Oceanogr., 48(5): 1808–1817. Doi: 10.4319/lo.2003.48.5.1808 |
| Williams P J L B, 1995. Evidence for the seasonal accumulation of carbon-rich dissolved organic material, its scale in comparison with changes in particulate material and the consequential effect on net C/N assimilation ratios. Mar.Chem., 51(1): 17–29. Doi: 10.1016/0304-4203(95)00046-T |
| Ye L L, Wu X D, Liu B, Yan D Z, Kong F X, 2015. Dynamics and sources of dissolved organic carbon during phytoplankton bloom in hypereutrophic Taihu Lake (China). Limnologica, 54: 5–13. Doi: 10.1016/j.limno.2015.05.003 |
| Yoshimura T, Ogawa H, Imai K, Aramaki T, Nojiri Y, Nishioka J, Tsuda A, 2009. Dynamics and elemental stoichiometry of carbon, nitrogen, and phosphorus in particulate and dissolved organic pools during a phytoplankton bloom induced by in situ iron enrichment in the western subarctic Pacific (SEEDS-Ⅱ). Deep Sea Res. Part Ⅱ Top. Stud.Oceanogr., 56(26): 2863–2874. Doi: 10.1016/j.dsr2.2009.06.011 |
| Zhang Y L, Gao G, Shi K, Niu C, Zhou Y Q, Qin B Q, Liu X H, 2014. Absorption and fluorescence characteristics of rainwater CDOM and contribution to Taihu Lake, China. Atmos. Environ., 98: 483–491. Doi: 10.1016/j.atmosenv.2014.09.038 |
| Zhang Y L, van Dijk M A, Liu M L, Zhu G W, Qin B Q, 2009. The contribution of phytoplankton degradation to chromophoric dissolved organic matter (CDOM) in eutrophic shallow lakes:field and experimental evidence. Water Res., 43(18): 4685–4697. Doi: 10.1016/j.watres.2009.07.024 |
| Zhang Y L, Yin Y, Liu X H, Shi Z Q, Feng L Q, Liu M L, Zhu G W, Gong Z J, Qin B Q, 2011a. Spatial-seasonal dynamics of chromophoric dissolved organic matter in Taihu Lake, a large eutrophic, shallow lake in China. Org.Geochem., 42(5): 510–519. Doi: 10.1016/j.orggeochem.2011.03.007 |
| Zhang Y L, Yin Y, Zhang E L, Zhu G W, Liu M L, Feng L Q, Qin B Q, Liu X H, 2011b. Spectral attenuation of ultraviolet and visible radiation in lakes in the Yunnan Plateau, and the middle and lower reaches of the Yangtze River, China. Photochem. Photobiol. Sci., 10(4): 469–482. Doi: 10.1039/C0PP00270D |
| Zlotnik I, Dubinsky Z, 1989. The effect of light and temperature on doc excretion by phytoplankton. Limnol. Oceanogr., 34(5): 831–839. Doi: 10.4319/lo.1989.34.5.0831 |
 2018, Vol. 36
2018, Vol. 36


