Institute of Oceanology, Chinese Academy of Sciences
Article Information
- PU Longjun(濮龙军), WANG Jing(王晶), WANG Yu(王玉), ZUO Jianwei(左建伟), GUO Huarong(郭华荣)
- Computational identification and characterization of microRNAs and their targets in Penaeus monodon
- Chinese Journal of Oceanology and Limnology, 36(3): 853-869
- http://dx.doi.org/10.1007/s00343-018-6348-x
Article History
- Received Dec. 26, 2016
- accepted in principle Feb. 3, 2017
- accepted for publication Feb. 28, 2017
2 Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China
MicroRNAs (miRNAs) are endogenous short noncoding RNAs with 20-23 nt in length that involve in the negative regulation of gene expression by targeting mRNAs for cleavage or translational repression based on the complementarity of the miRNA sequences to the targets (Bartel, 2004; Carthew and Sontheimer, 2009). Accumulating evidences have shown that miRNAs play an important role in the cellular proliferation, differentiation and apoptosis, metabolic process and early embryo development of animals (Alvarez-Garcia and Miska, 2005; Chen, 2005). In eukaryotes, miRNA-encoding genes are widely distributed in the introns and exons of proteinencoding genes or in the intergenic regions (Saini et al., 2007). About fifty percent of the miRNA-encoding genes have independent transcriptional promoter but the others are co-transcribed with protein-encoding genes (Rodriguez et al., 2004; Baskerville and Bartel, 2005). The formation of mature miRNAs is a multistep process. First, in the nucleus, the miRNAencoding genes are usually transcribed by RNA polymerase Ⅱ as a long primary transcript called primary microRNA (pri-miRNA). Then the pri-miRNA forms a hairpin-like secondary structure, which was subsequently processed into precursor miRNA (pre-miRNA) by RNase Ⅲ, Drosha. Second, the pre-miRNAs are exported by exportin-5 into the cytoplasm and further cleaved by another RNase Ⅲ, Dicer into matured 20-23 nt miRNA: miRNA* duplex, and the miRNA* is subsequently degraded. Finally, the remained single-stranded miRNA is incorporated into RNA-induced silencing complex (RISC) which mediates the subsequent posttranscriptional gene silencing (Gregory et al., 2005; Kim, 2005; Davis and Hata, 2009).
Different approaches have been adopted to identify putative miRNAs over the years. The experimental methods including the direct cloning (Kloosterman et al., 2006; Ramachandra et al., 2008), microarray (Liu et al., 2004), Northern blot and in-situ hybridization (Wienholds et al., 2005) are tedious and timeconsuming, and not successful in detecting low abundant miRNAs. Recently, rapid advances in bioinformatics and next generation sequencing (NGS) technology allowed the identification of a great number of additional miRNA candidates in different organisms at low cost and high sensitivity (Bar et al., 2008; Soares et al., 2009). The computational tools developed for miRNAs identification are usually based on the length, high sequence conservation among species, and structural features like hairpin and minimal folding free energy of miRNAs (Li et al., 2010; Gomes et al., 2013). The computational prediction can be used not only in species with complete genome reference sequences, but also in those without reference genomes (Li et al., 2010). For organisms whose complete genome information is not available, the expressed sequence tags (ESTs) and genome survey sequences (GSS) databases can be alternative sequence resources for the prediction of miRNA candidates and their target genes (Zhang et al., 2007; Xu et al., 2012).
With the development of various bioinformatics methods and experimental biological technology, lots of works on the identification, characterization and functions of miRNAs have been done in human Homo sapiens (Berezikov et al., 2005), model animals include mouse Mus musculus (Lagos-Quintana et al., 2002), fly Drosophila melanogaster (Lai et al., 2003), zebrafish Danio rerio (Kloosterman et al., 2006) and nematode Caenorhabditis elegans (Lim et al., 2003), and model plants like thale cress Arabidopsis thaliana (Adai et al., 2005). However, little attention has been paid to the miRNAs in non-model species, especially in aquatic organisms. In the past years, a small amount of miRNAs were identified and validated from big head carp Hypophthalmichthys nobilis and silver carp H. molitrix (Chi et al., 2011), channel catfish Ictalurus punctatus (Xu et al., 2012) and flounder Paralichthys olivaceus (Xie et al., 2011), rainbow trout (Oncorhynchus mykiss) (Yang and He, 2014). In shrimps, seven mature miRNA and five pre-miRNA sequences identified from Marsupenaeus japonicus have been deposited in miRBase (Release 21, June 2014; http://www.mirbase.org/), and these miRNAs were reported to be involved in response to virus infection (Huang et al., 2012; Yang et al., 2012). 239 conserved miRNAs, 14 miRNA* sequences and 20 novel miRNAs were identified by bioinformatics analysis in white shrimp (Litopenaeus vannamei) (Xi et al., 2015). Recently, an interesting work on miRNA editing and its effect on the binding of miRNA to target gene have been reported in Marsupenaeus japonicas, which indicates the important roles of miRNA played in aquatic species (Cui et al., 2015). Penaeus monodon is an economically important aquaculture species with the largest farming area and breeding production in cultured shrimps (Rengpipat et al., 1998). Information on miRNAs and their target genes in P. monodon is also very limited, however, a computational workflow has been developed to identify conserved miRNA genes in the 10 536 unique P. monodon ESTs (Meemak et al., 2013).
The main purpose of the present study was to identify miRNAs candidates and their potential target genes from the known EST and GSS databases of P. monodon using comparative algorithms. The results obtained could guide the future experimental validations and contribute to the understanding of the roles of these miRNAs in regulating the development and metabolism of P. monodon.
2 MATERIAL AND METHOD 2.1 Cells and cultureThe mammalian Neuro-2a cell, purchased from ATCC cell bank, was a fast-growing mouse neuroblastoma cell line. Neuro-2a cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Life Technologies, USA) supplemented with 10% bovine calf serum (BCS, Life Technologies, USA), and cultured at 37℃ in a 5% CO2 incubator.
2.2 miRNA reference setIn order to increase the accuracy of prediction, only the published miRNAs from the phylum arthropoda, close to shrimps in relationship, were selected as references for the identification of potential miRNA homologies in P. monodon. A total of 2 669 previously identified mature miRNAs and their premiRNAs sequences were obtained from miRNA registry Database (Release 20, June 2013; http://www.mirbase.org/). These miRNAs were defined as a reference set of miRNA sequences. To avoid the redundant or overlapping miRNAs, the repeated sequences of miRNAs within the above reference set were removed by Perl scripts (http://www.perl.org) and the remaining sequences were used as query sequences for BLAST search. These known miRNAs references are from 17 species including shrimp M. japonicas, water flea Daphnia pluex, mosquito Aedes aegypti, butterfly Heliconius melpomene, moth Manduca sexta, mite Tetranychus urticae, aphid Acyrthosiphon pisum, acridid Locusta migratoria, beetle Tribolium castaneum, silkworm Bombyx mori, two ticks of Ixodes scapularis and Rhipicephalus microplus, two bees of Apis mellifera and Nasonia vitripennis, and three flies of Drosophila melanogaster, D. pseudoobscura and D. simulans,
2.3 Data sources of expressed sequence tags (EST), genome survey sequences (GSS) and mRNAs of P. monodonEST, GSS, and mRNA sequences of P. monodon were downloaded from GenBank nucleotide database of National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/). A total of 39 908 EST sequences and 21 124 GSS sequences have been obtained and used as the searching sources for miRNA. A total of 10 331 mRNA sequences were obtained for the searching sources for target genes. To avoid redundancy, the downloaded EST, GSS and mRNA sequences were blasted against each other and the sequences with high similarity (> 98%) were removed.
2.4 Prediction of potential miRNAsThe unique miRNA reference set was used as query sequences to blast against the P. monodon EST and GSS databases. The BLAST search was carried out through BLAST 2.2.28+ (Zhang et al., 2000). The sequences which had less than 3 nt mismatch with query miRNA sequences and more than 16 nucleotides in length were manually chosen as potential P. monodon miRNAs. The blast parameter settings were adjusted as follows: an expect value cut-off of 10, a low-complexity sequence filter, 1 000 descriptions and alignments, and automatically adjusted parameters for short input sequences to improve the accuracy of blast result. Figure 1 showed the flowchart of miRNAs prediction procedure in P. monodon.
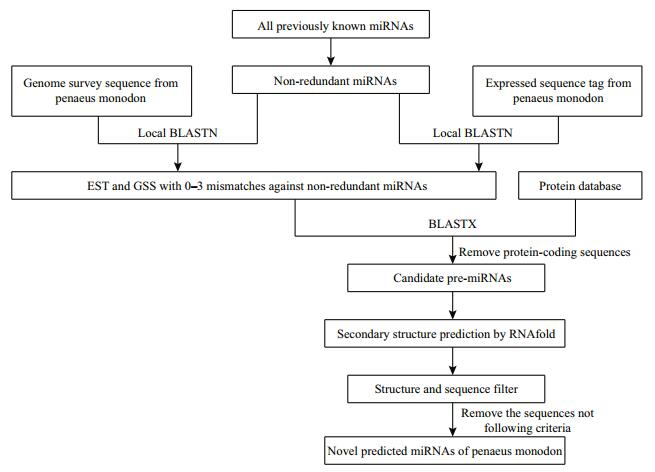
|
| Figure 1 Flowchart of miRNAs prediction procedure in P. monodon |
All the predicted mature miRNA sequences along with their 200 bp upstream and 200 bp downstream flanking sequences were selected from the P. monodon ETS and GSS databases and subjected to RNAfold web server (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) to predict and generate the secondary structure with the default parameters (Hofacker, 2003). In each case, we chose the lowest-energy secondary structure as the target architecture for the next validation. Only the sequences which fit the following strict criteria were regarded as the potential miRNAs and pre-miRNAs in P. monodon: (1) the potential miRNA sequence did not contain more than 3 nt mismatches with the query homology; (2) the selected pre-miRNA could be folded into stem-loop hairpin secondary structure and the mature miRNA sequence was not located in the terminal loop of the hairpin structure; (3) the mismatches between miRNA and the complementary sequence (miRNA*) was less than 6 nt; (4) the potential mature miRNAs should be located in the same arm of the stem-loop hairpin structure with their known homologs; (5) the potential pre-miRNA had higher minimal folding free energy index (MFEI) than other non-miRNAs. MFEI was calculated by the following formula: MFEI=[(minimal folding free energy/length of RNA sequence)×100]/ (G+C)%; (6) the potential miRNA sequence could not contain large loops or breaks in microRNA: microRNA* duplex (Yin et al., 2008; Wang et al., 2012). Finally, all the selected pre-miRNA could be folded into stem-loop hairpin secondary structure and have higher minimal folding free energy index (MFEI) than other non-miRNAs. Thus the length of the actual miRNAs precursor might be longer than what is presented here.
2.6 Phylogenetic analysis of the identified pmomiRNAsBoth the pre-miRNAs and mature miRNAs sequences are highly conserved among different animal species, thus homology analysis of the identified pmo-miRNAs can reveal their evolutionary relationships. The identified mature pmo-miRNAs and pre-pmo-miRNAs were aligned with the known miRNAs and pre-miRNAs from the same miRNA family but different species, respectively, and the corresponding evolutionary trees were generated by maximum likelihood (ML) method (Strimmer and von Haeseler, 1996) following 1 000 bootstrapped replicates and other default parameters. All the analyses were performed on the MEGA 5.2 software (Tamura et al., 2011).
2.7 Prediction of the pmo-miRNA targetsThe widely used computational algorithm of miRanda was employed in this study to predict the potential target genes of pmo-miRNA (John et al., 2004; Griffiths-Jones et al., 2006). To minimize false positives, two sets of criteria were used for the screening of pmo-miRNA targets and only the target sites predicted by both sets of criteria were accepted as the potential target genes. One criteria were set as followed: (1) S, the sum of single-residue-pair match scores over the alignment, ≥140; (2) ΔG, the free energy of duplex formation, < -17 kcal/mol. The other criteria were set as followed: (1) the number of mismatches between the predicted miRNA and complementary target sequence was not beyond four (G-U base pair was counted as 0.5 mismatches); (2) two consecutive mismatches did not exist in the miRNA: target duplex; (3) mismatches occurred in the first 9 bp of the 5' end of miRNA in the miRNA: target duplex were not more than one; (4) positions 10 and 11 (numbered from the 5' end of miRNA) in the miRNA : target duplex, which were considered as the cleavage sites, should not contain any mismatch; and (5) no gap appeared in the miRNA: target duplex (Wang et al., 2012). Target sequences filtered by the above criteria were further filtered by BLASTX search program to remove the targets without significant similarity and then the remaining target sequences were subjected to functional assignment.
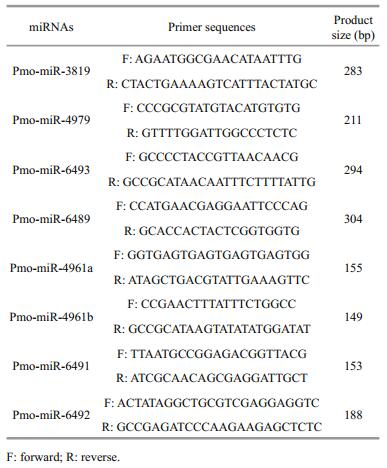
2.8 PCR validation of the pmo-miRNAsTo validate the computationally identified pmomiRNAs, polymerase chain reaction (PCR) was performed to isolate each of the predicted pmomiRNAs from the cDNA templates of P. monodon. The primers for amplifying miRNAs were designed according to the primary sequence and located in the each flank of mature miRNAs. Primers used for amplifying the 8 pmo-miRNAs are listed in Table 1.
Total RNA was extracted from the shrimp muscle using Trizol reagent (TransGen, Beijing, China) and then digested with RNase-free Dnase Ⅰ (TaKaRa, Otsu, Japan) in the presence of RNase inhibitor (TaKaRa, Otsu, Japan) according to the manufacturer's protocol. First-stranded cDNA was synthesized in 20 μL reaction volume by ReverTra Ace qPCR RT Kit (Toyobo, Japan) with 2 μg of total RNA as template and 1 μL oligo (dT) as primer. PCR was performed in 20 μL total volume consisting of 2.0 μL 10× PCR buffer (with Mg2+), 1.6 μL 2.5 mmol/L dNTPs, 1.0 μL 10 μmol/L forward primer, 1.0 μL 10 μmol/L reverse primer, 0.2 μL 5 IU/μL Taq DNA polymerase, 1 μL cDNA template and 13.2 μL ddH2O. One cycle of 94℃ for 10 min, 32 cycles of 94℃ for 45 s, 50–60℃ annealing for 45 s and 72℃ for 50 s, followed by one cycle of 72℃ for 10 min. The PCR results were analyzed by 1.5% agarose gel electrophoresis.
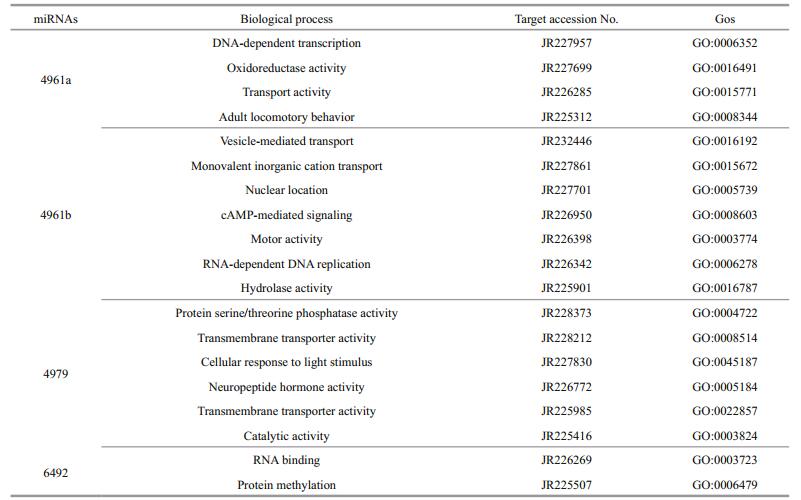
2.9 GO and KEGG analysisTo further understand the functions and metabolic pathway of target genes, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed using Blast2go suit (Ashburner et al., 2000; Kanehisa and Goto, 2000; Conesa et al., 2005; Götz et al., 2008). First, all the target genes were searched against non-redundant (nr) database and annotated by BLASTX with the e-value of 1e-6 (Altschul et al., 1997). The functional category for each target gene was annotated according to the best hits results generated in the mapping of Gene Ontology (GO) terms with default parameters. The metabolic pathway participated by each target gene was analyzed by searching KEGG database.
2.10 Experimental validation of the targeting activity of 3 pmo-miRNAs to their target genesDual luciferase reporter gene assay system (Galen Biopharm International Co. Ltd., Beijing, China) was used to experimentally validate the targeting activity of 3 PCR-confirmed pmo-miRNAs to their predicted target genes. Based on the computational prediction results mentioned above, three pmo-miRNAs and their corresponding two target genes each were randomly selected as followed: miR-4961a and its target genes of JR227957 and JR227699, miR-4961b and its target genes of JR227701 and JR226950, miR- 4979 and its target genes of JR226772 and JR225956. Three methylated single-strand miRNA mimics of miR-4961a (5'-UCUUUGUGUGUAUGUAUGUAU-3'), miR-4961b (5'-UCUGUAUGUCUAUGUAUGUAU-3') and miR-4979 (5'-UACAUGUGUGUGUAUAUAUAU-3') and one negative control mimic (5'-UCACAACCUCCUAGAAAGAGUAGA-3') were synthesized by Shanghai GenePharma Co. Ltd., China.
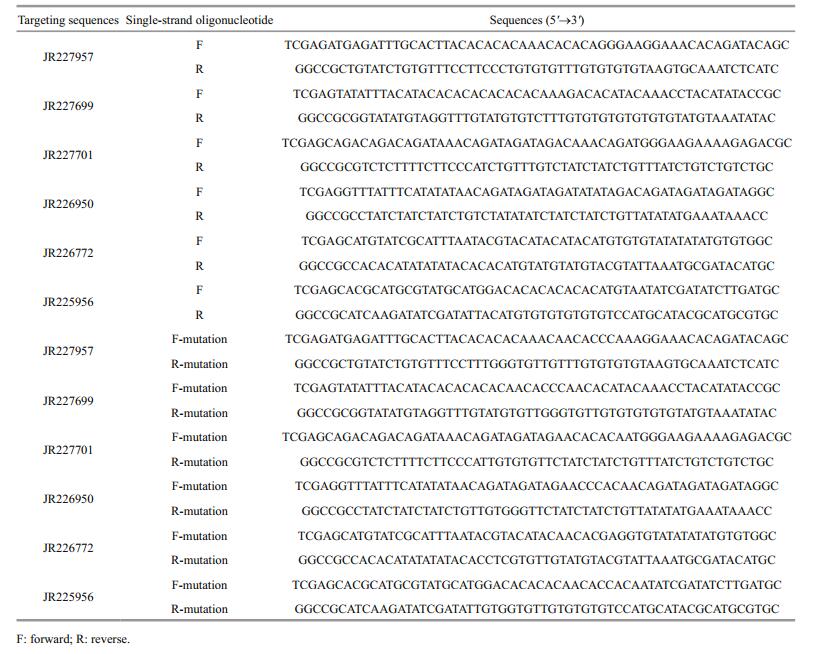
All sequences of the 6 selected target genes were extracted from EST-GSS database and the corresponding sequences of 52 bp fragment in length flanking the predicted targeting site was truncated, respectively. And then for each of the 6 truncated fragments, a pair of complementary single-strand oligonucleotides with an addition of XhoⅠ or NotⅠ sites at both ends, 59 bp in length each, were synthesized by BGI company (Shenzhen, China), respectively, and then subjected to 5'-end addition of phosphate and annealing reaction to form doublestrand insertion unit. The annealing reaction was performed in 20 μL total volume consisting of 8 μL 0.1 nmol/μL oligonucleotide for each strand, 0.5 μL 10 mg/mLATP-Na2, 0.4 μL 10 U/μL T4 polynucleotide kinase (PNK), 2 μL 10×T4 PNK buffer and 1.1 μL ddH2O and then incubated at 37℃ for 30 min, followed by 95℃ for 5 min and cooled to room temperature. After that, the obtained double-strand oligonucleotide was directionally inserted into a reporter plasmid of psiCHECK-2 (kindly provided by Jerame Hui, School of life science, Chinese University of Hong Kong, China). The ligation reaction mix in 15 μL total volume included 10 μL of the obtained double-strand oligonucleotide, 0.7 μL 0.5 μg/μL linear psiCheck-2 plasmid DNA, 1 μL 350 U/μL T4 ligase, 1.5 μL 10×T4 ligase buffer and 1.8 μL ddH2O. At the same time, for each of the 6 target genes tested, a mutant double-strand oligonucleotide with mutations introduced in the regions corresponding to the seed sequences of pmo-miRNAs was also prepared in the same way as mentioned above, respectively. All the synthesized wild-type and mutant target sequences were listed in Table 2.
One day prior to transfection, Neuro-2a cells were seeded into each well of a 24-well culture plate. Approximately 60%–70% confluence was anticipated at the time of transfection. All the stock solutions of 12 psiCHECK-2 recombinant plasmids containing wild-type (WT) or mutant target gene regions, 3 synthesized single-strand miRNA mimics and 1 synthesized negative control mimic were prepared in RNase-free water to a concentration of 25 μmol/L. Then to validate the targeting between pmo-miRNAs and their target genes tested, varied psiCHECK-2 recombinant plasmid DNAs with the same concentration of 250 ng/μL wild-type or mutant target gene regions were co-transformed with their corresponding miRNA mimic or negative control mimic (25 μmol/L) into the Neuro-2a cells using Lipofactamine 2000 transfection reagent (Invitrogen) following the product manual. The following groups were tested: 1: negative control mimic (1.6 μL) + psiCHECK2-WT (4 μL); 2: miRNA mimic (1.6 μL) + psiCHECK2-WT (4 μL); 3: negative control mimic (1.6 μL) + psiCHECK2-mutant (4 μL); 4: miRNA mimic (1.6 μL) + psiCHECK2-mutant (4 μL). For each group, all volumes were multiplied by 3.5 to account for the triplicate samples and liquid loss during pipetting.
At 48 h post-transfection, the old medium in each well was removed and the cells were rinsed with PBS twice. Then 150 μL per well of cell lysis buffer was added and the culture plate was shaken gently at room temperature for 15 min to ensure complete lysis. After that, all the cell lysate in each well was transferred into a 1.5-mL centrifuge tube and centrifuged at 9 000×g for 5 min. Then 20 μL of cell lysate supernatant was taken out and added into another 1.5 mL centrifuge tube containing 100 μL of luciferase assay reagent and mixed quickly by pipetting two or three times. Then the tube was immediately placed in the luminometer (GloMax, Promega) and the luminescence (relative light units, RLU) due to firefly luciferase activity was measured. After that, the sample tube was removed from the luminometer and 100 μL of stop reagent was added and mixed briefly, then the sample tube was put in the luminometer again, and the luminescence due to Renilla luciferase activity was recorded. As the Renilla luciferase gene was set as reporter gene and firefly luciferase gene was set as inner reference in the plasmid map of psiCHECK2, the ratios of Renilla luciferase activity to firefly luciferase activity was calculated for each sample and used to detect the targeting activity between pmo-miRNAs and target genes. The SPSS software was used for data statistical analysis.
3 RESULT AND DISCUSSION 3.1 Eight miRNA candidates were identified from P. monodonA total of 2 669 known miRNAs were collected from 17 different species of phylum arthropoda, which were in close relationship with P. monodon. Multiple sequence alignment removed 485 repeat miRNAs from this data set. The remaining 2 184 nonredundant miRNAs were blasted individually as query sequence against ESTs (39 908 sequences) and GSS (21 124 sequences) of P. monodon using local BLAST-2.2.28+. The results obtained showed that, 155 sequences (70 from EST and 85 from GSS) had less than 3 nt mismatches with the known query miRNAs and selected for subsequent miRNAs identification. From these selected sequences, 37 protein-encoding sequences were further removed by BLASTX program. After that, the secondary structures of the remaining 118 sequences were predicted and generated by RNAfold web server, and then they were screened for potential miRNAs by evaluating the secondary structures and sequences based on the criteria described in Methods. Finally, a total of 8 potential miRNA sequences (4 from EST and 4 from GSS) were obtained from P. monodon.
The sequences and hairpin structures of the identified eight miRNAs were shown in Fig. 2. They belong to 7 different miRNA families, named as pmomiRNA4961a, pmo-miRNA4961b, pmo-miRNA4979, pmo-miRNA6489, pmo-miRNA6491, pmo-miRNA6492, pmo-miRNA6493 and pmo-miRNA3819. These miRNA families have been reported previously in other animals, indicating the functional conservation of miRNA members in these families.

|
| Figure 2 Sequences and harpin sturctures of the identified miRNAs from P. monodon The mature miRNA sequences are underlined and in red colour. The length of the actual miRNAs precursor might be longer than what is presented here. |
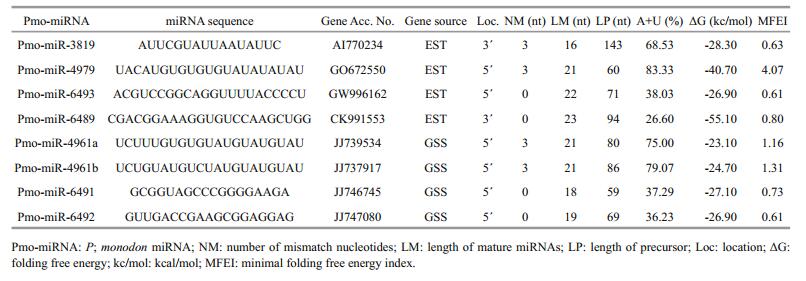
The identified pmo-miRNAs were diverse not only in the variety of miRNA families they belong to, but also in the length of pre-miRNAs, (A+U) contents and location of mature miRNA sequence within their pre-miRNAs. For all the identified pmo-miRNAs, the length of pre-miRNAs varied from 59 nt to 143 nt, but the precursors containing 60–95 nt accounted for 87.50%. In contrast, the lengths of the majority of the plant pre-miRNAs were more consistent, typically 70–80 nt (Yin et al., 2008). The (A+U) contents in each pre-pmo-miRNAs ranged from 26.60% to 83.33%, which conforms to the standard that miRNA precursors should have 30%–70% (A+U) contents. MFEI (minimal folding free energy index) is an important parameter to distinguish miRNAs from other types of RNAs. As shown in Table 3, the MEFI values for all the identified pmo-miRNAs ranged from 0.60 to 4.07, which was in agreement with previous studies (Zhang et al., 2006a). Of the identified eight pmo-miRNAs, six pmo-miRNAs have mature sequences located in the 5' end of their secondary hairpin structures, and the other two in the 3' end instead. And also, three mature sequences started with 5' uracil, indicating an important role of uracil in the regulatory function of miRNAs mediated by RNA-induced silencing complex (RISC) (Bartel, 2004; Gregory et al., 2005; Kim, 2005).
As shown in Table 3, about 0–3 nt base substitutions exist in the mature miRNA sequences between P. monodon and other known miRNAs, showing both conservation and divergence of miRNAs in evolution (Zhang et al., 2005). Although animals have more non-conserved miRNAs than plants (Axtell et al., 2011), animal miRNAs are still highly conserved among different species and a large amount of miRNA candidates can be found using homological search methods. But Zhang et al. (2006b) reported a low prediction frequency of miRNAs from EST using comprehensive bioinformatics approach as 0.01%. Similar result was obtained in this study. Only 8 miRNA candidates were predicted from 61 132 EST sequences, the miRNA prediction frequency was about 0.013%. Two reasons may account for the above low prediction frequency: (1) miRNAs were transcribed from either sense or antisense strand of DNA at the miRNA genomic loci (Stark et al., 2008). miRNAs located in the complementary strands of EST sequences have been missed; (2) in order to improve the reliability of prediction, only known miRNAs from 17 species of the phylum arthropoda, close to shrimps in relationship, were used as reference query for blast in this study. This markedly decreased the miRNA prediction frequency, too. Despite all that, the reliability of the predicted pmo-miRNAs could be high because they are screened by combination of stringent criteria and secondary structure prediction.
Works on the whole genome sequencing of shrimp are going on. When more genomic information of shrimp is publicly available, more shrimp miRNAs will be discovered using both classic experiment methods and bioinformatics approaches.
3.3 PCR validation of the predicted pmo-miRNAsPCR was performed to experimentally validate the 8 pmo-miRNAs predicted using specific primers flanking the pre-pmo-miRNAs sequences and the obtained PCR products were separated on a 1.5% agarose gel. As shown in Fig. 3, five computationally predicted pmo-miRNAs including pmo-miR-4961a, pmo-miR-4961b, pmo-miR-4979, pmo-miR-6492 and pmo-miR-6493 were successfully amplified from the cDNA templates of muscle tissues of P. monodon with the expected product sizes (Table 1). All the amplified fragments were also successfully verified by sequencing, indicating a 100% positive confirmation. However, other three pmo-microRNAs precursors were not detected using PCR. Limited tissues sampled and inappropriate PCR reaction condition possibly accounted for the failure.
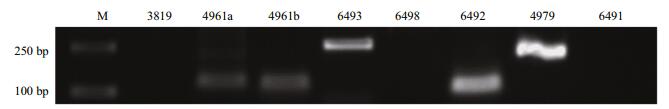
|
| Figure 3 PCR validation of the identified 8 pmo-miRNAs M: molecular weight marker 2 000. |
To show the evolutionary relationship of both intra- and inter-family of miRNAs, all the mature pmo-miRNAs or pre-pmo-miRNAs predicted were included in the construction of phylogenetic trees (Fig. 4). As shown in the phylogenetic tree for mature pmo-miRNAs (Fig. 4a), five mature pmo-miRNAs were found to have highly conserved counterpart miRNAs from different species and clustered in the same position, e.g. pmo-miR-3819 and tca-miR- 3819-5p, pmo-miR-6489 and mja-miR-6489-3p, pmo-miR-6493 and mja-miR-6493-5p, pmomiR-6491 and mja-miR-6491, pmo-miR-6492 and mja-miR-6492. However, pmo-miR-4979 and dmemiR-4979-3p were clustered in the same branches but different evolutionary distances. Of interest, pmomiR-4961a and pmo-miR-4961b were from the same species and same precursor but deposited in different branches, showing a divergent evolution rate in the tree. It can be concluded that different miRNAs might evolve at different rates in both inter- and intraspecies.
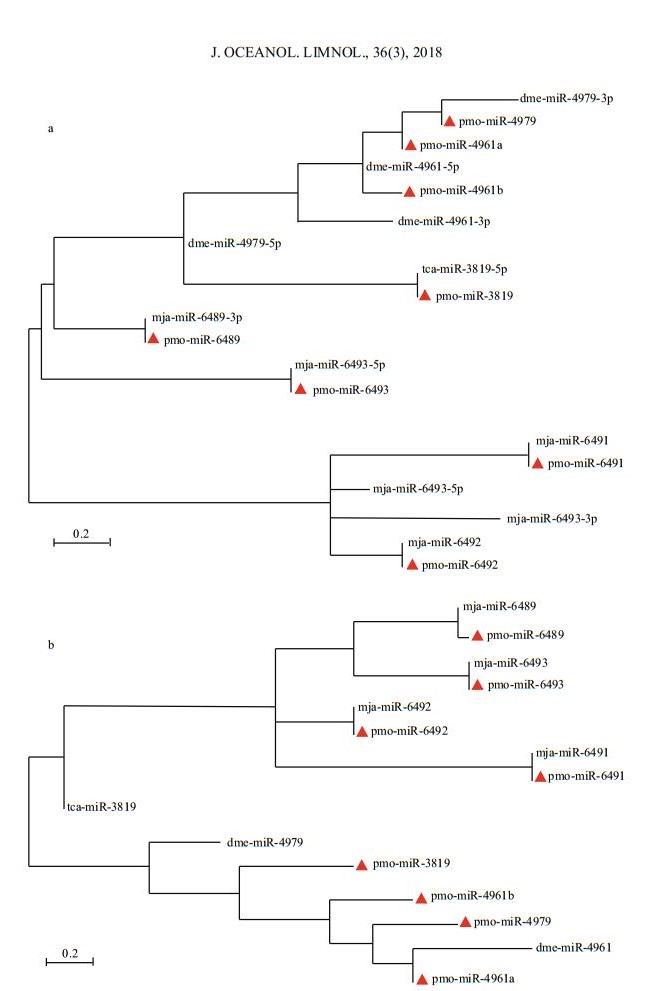
|
| Figure 4 Phylogenetic analysis of mature pmo-miRNAs (a) and pre- pmo-miRNAs (b) Identified pmo-miRNAs are labeled with red triangle. Other published miRNAs from the same families of the 8 pmo-miRNAs are from species of Tribolium castaneum (tca), Drosophila melanogaster (dme) and Marsupenaeus japonicus (mja), respectively. Precursors of dme-miR-4961, dme-miR-4979, tcamiR-3819, mja-miR-6489 and mja-miR 6493 can produce two mature miRNAs. They are dme-miR-4961-5p and dme-miR-4961-3p, dme-miR-4979-5p and dme-miR-4979-3p, tca-miR-3819-5p and tca-miR-3819-3p, mja-miR-6489-5p and mja-miR-6489-3p, mja-miR-6493-5p and mja-miR-6493-3p, respectively. |
Rodriguez et al. (2004) suggested that pre-miRNA sequences were conserved, too. Similar results were obtained in this study. Unlike the mature pmomiRNAs, in the phylogenetic tree for the pre-pmomiRNAs, pre-pmo-miR-6489 and pre-mja-miR-6489 were clustered in the same branch but with different evolutionary distances, pre-pmo-miR-3819 and pretca-miR-3819 were located in completely divergent branch, indicating that the flanking sequences outside the core mature sequence were relatively less conserved than the mature sequence in the miRNA.
The phylogenetic trees for mature and precursor pmo-miRNAs also revealed that four of the identified pmo-miRNAs have already been discovered in M. japonicus (Huang et al., 2012), a closely related species to P. monodon, e.g. mja-miR-6489, mjamiR-6491 mja-miR-6492 and mja-miR-6493. This confirmed to some degree the reliability and accuracy of computational identification of miRNAs. Of course, there are some miRNA members found in M. japonicus but not predicted in this study, referring that they might not be deposited in the EST or GSS datasets yet. Thus, deep sequencing and genome-wide identification are needed to help to solve this problem in the future (Bar et al., 2008; Lu et al., 2008; Zhou et al., 2010).
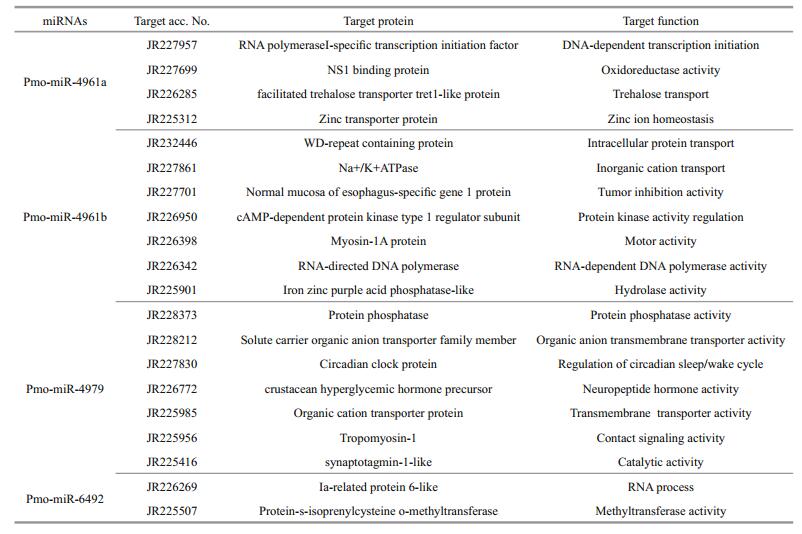
3.5 Prediction of the target genes of the 8 pmomiRNAsIdentification of the target genes of the 8 pmomiRNAs is an easy way to gain an insight into their roles in various cellular functions and gene regulation network. It has been reported that miRNAs always perfectly or near-perfectly match to their target mRNAs and regulate gene expression in posttranscriptional level by inhibiting the protein translation or facilitating mRNA degradation (Brown and Sanseau, 2005; Sethupathy et al., 2006), and during this process only the evolutionarily conserved mature miRNA sequences seem to be necessary and thus often used in the target gene identification. Based on the complementary between miRNAs and targets, putative target genes for the 8 pmo-miRNAs were identified using miRanda software under stringent criteria. As shown in Table 4 and Fig. 5, only 4 pmomiRNAs of pmo-miR-4961a, pmo-miR-4961b, pmomiR-4979 and pmo-miR-6492 successfully matched to 20 target genes after searching against the downloaded mRNA database of P. monodon from NCBI. This suggested that one pmo-miRNA could target multiple genes in P. monodon, too. These different genes targeted by one pmo-miRNA often had quite different biological functions and were even involved in different cellular process and metabolic pathways. The mechanism of action for this is still unclear and more works are needed. The remaining 4 pmo-miRNAs of pmo-miR-3819, pmo-miR-6489, pmo-miR-6491 and pmo-miR-6493 failed to match annotated target genes instead. The incompleteness of mRNA database of P. monodon may account for the failure.
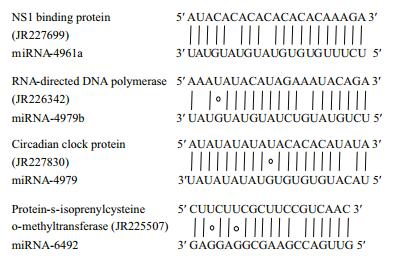
|
| Figure 5 Representative matches between pmo-miRNAs and their target genes Top strands show the pmo-miRNA binding sites in the target genes, and the bottom strands shows the pmo-miRNAs. Watson-Crick pairing (vertical dashes) and G:U wobble pairing (circles) are indicated. |
Many genes associated with transcription process were reported as the targets of miRNAs in both animals and plants (Guo et al., 2009). In this study, we found that pmo-miR-4961, a targeted RNA polymerase Ⅰ-specific transcription initiation factor of P. monodon (JR227957), indicating its role in the mRNA transcription.
The growth and development of animals are an intrinsic process in which many signal pathways and gene regulation networks were involved. Previous studies have demonstrated that miRNAs played an important role in the regulation of growth, development and apoptosis (Alvarez-Garcia and Miska, 2005; Cheng et al., 2005). Here, the pmomiR-4979 was found by both computational prediction and experimental validation to target the gene of crustacean hyperglycemic hormone (CHH) precursor (JR226772, Table 4 and Fig. 7). CHH protein, a member of neuropeptide hormone family present only in arthropods, plays a pivotal role in the modulation of hemolymph glucose levels, molting, reproduction and the stress response. It regulates the growth, reproduction and molting of crustacean by interacting with molt-inhibiting hormone (MIH), gonad-inhibiting hormone (GIH) and mandibular organ-inhibiting hormone (Chan et al., 2003; Fanjul-Moles, 2006). The target of pmo-miR-4979 to CHH gene inferred an involvement of this pmo-miRNA in the growth and molting of P. monodon. Circadian clock protein gene, which responds to light stimulus, was also identified as target of pmo-miR-4979, suggesting another function of this miRNA in the regulation of cellular response to light/dark. Up to date, several miRNAs have been previously reported in the sensory cells and organs (Ason et al., 2006; Li et al., 2006; Weston et al., 2006), for example, miR183 has been reported to be expressed conservatively in epithelial cells of S. kowalevskii and sensory organs of D. melanogaster and nematode (Pierce et al., 2008).
|
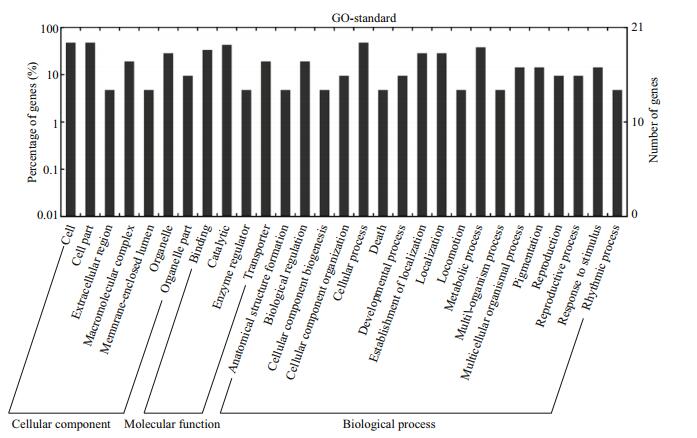
| Figure 6 Gene ontology categories and distribution of pmo-miRNAs target genes |
|
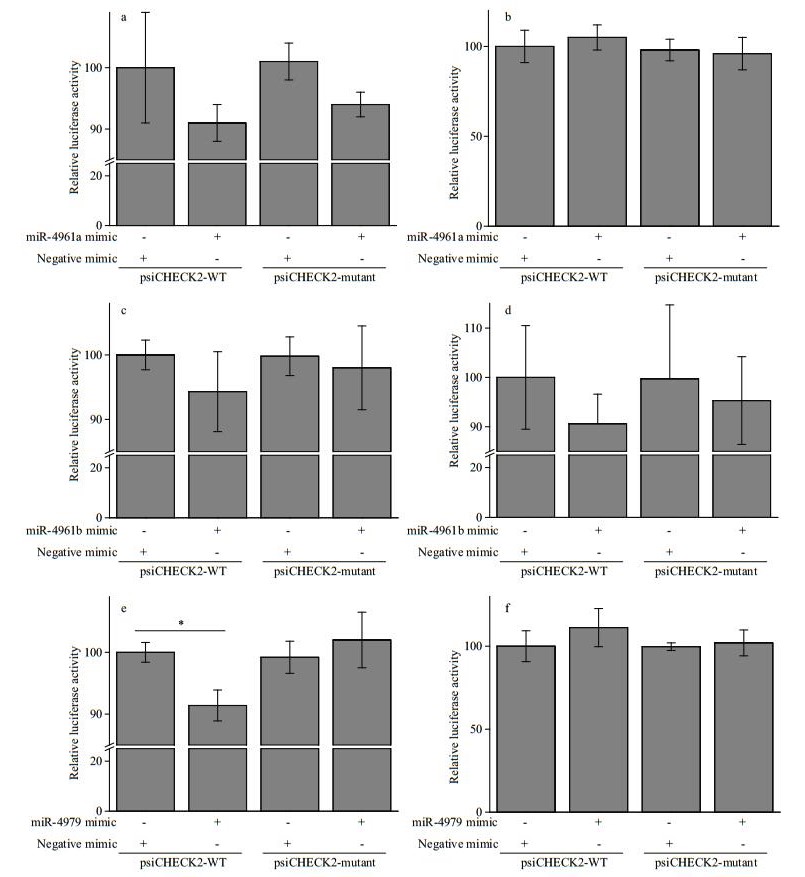
| Figure 7 Experimental validation of the targeting of 3 pmo-miRNAs to their corresponding target genes in Neuro-2a cells by dual luciferase reporter assay a. miRNA-4961a and its target gene (JR227957); b. miRNA-4961a and its target gene (JR227699); c. miRNA-4961b and its target gene (JR227701); d. miRNA-4961b and its target gene (JR226950); e. miR-4979 and its target gene (JR226772); f. miR-4979 and its target gene (JR225956); All data are expressed as mean±SE (n=3). One-way ANOVA in SPSS was used for statistical analysis. F02A indicates significant difference (P < 0.05). |
Of interest, cAMP-dependent protein kinase type 1 regulator subunit (JR226950) and protein phosphatase (JR228373) were also identified as target genes for pmo-miR-4961b and pmo-miR-4979, respectively. The above two enzymes act as opposite regulators for the activity of target proteins by phosphorylation and dephosphorylation (Fabbri et al., 2007; Kato et al., 2009; O'Connell et al., 2009). This means that pmomiR-4961b and pmo-miR-4979 may participate in the functional regulation of the same protein target in the signaling pathways via knocking down the activity of kinase and phosphatase, respectively. And also, RNA directed DNA polymerase (JR226342) was found as the target of pmo-miR-4961b. As we know, the infection and replication of RNA virus in host cells greatly depend on this enzyme. Shrimp RNA virus like Taura syndrome virus (TSV) is a big threat to shrimp industry, thus pmo-miR-4961b might provide us a useful tool to treat TSV infection.
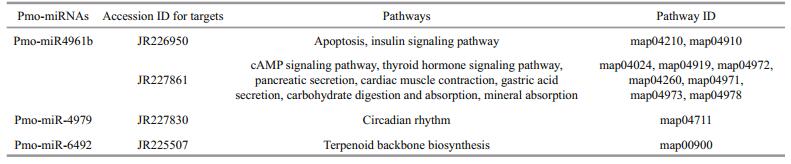
3.6 GO classification and KEGG pathway analysis of the predicted 20 target genesTo further understand the cellular functions and regulatory pathways involved by pmo-miRNAs, all the predicted target genes were subjected to GO and KEGG database for analysis. GO terms were categorized into three classes of cellular component, molecular function and biological process (Ashburner et al., 2000). As shown in Table 5, GO analysis showed that the 20 pmo-miRNA target genes were assigned to 7 categories at cellular component level, 4 categories at molecular functions level and 18 categories at biological process level. Although number of pmo-miRNA targets predicted in this study is limited, the distribution of assigned GO terms was also produced by Wego output to show gene function visually and deeply (Fig. 7). As shown in Fig. 6, more pmo-miRNA targets were located in cell and cell part, followed by those located in organelle and macromolecular complex. All putative target genes presented a narrow range of molecular function including binding, catalytic, enzyme regulator and transporter. The biological process involved by the pmo-miRNA targets includes cellular process, establishment of location and metabolic process, etc. Several target genes of pmo-miRNAs were identified in the process of development, reproduction and death. This enriched the known miRNA library (Carrington and Ambros, 2003; Kloosterman and Plasterk, 2006). The results of KEGG annotation have shown that pmo-miRNA targets participate in many pathways, such as terpenoid backbone biosynthesis, apoptosis, insulin signaling pathway and thyroid hormone signal pathway (Table 6). Information on the metabolic networks of pmo-miRNA targets will help us to understand the functions and target pathways of pmo-miRNAs in more detail and guide the future research of interest.
The targeting activity of 3 PCR-confirmed pmomiRNAs of pmo-miR-4961a, pmo-miR-4961b and pmo-miR-4979 to their predicted gene candidates were investigated in Neuro-2a cells by dual luciferase reporter gene assay. As shown in Fig. 7, obvious down-regulation of the expression of target genes was found in experimental groups of pmo-miR-4961a and JR227957, pmo-miR-4961b and JR227701, pmomiR-4961b and JR226950, pmo-miR-4979 and JR226772. However, significant targeting activity was found only between pmo-miR-4979 and JR226772 (P < 0.05). The target gene JR226772 encoded crustacean hyperglycemic hormone precursor, suggesting the involvement of pmomiR-4979 in the regulation of neuropeptide hormone activity. However, the remaining three experimental groups failed to detect obvious targeting activity, indicating again that prediction data based on computational algorithm need to be experimentally validated to avoid potential false-positive result. In this study, a mammalian cell line was used in the validation experiment of miRNA-mRNA interaction due to the absence of immortalized shrimp cell line. Difference in genetic background between these two cell lines might to some degree account for the failure mentioned above. The mechanism of miRNA-mediated gene silencing is very different between vertebrate and invertebrate. In vertebrate, a miRNA is loaded onto Argonaut 2, while a miRNA is loaded onto Argonaut 1 in invertebrate.
4 CONCLUSIONIn this study, a total of 8 miRNAs were computationally identified from EST or GSS database of P. monodon. Of these, pmo-miR-6489, pmomiR-6491, pmo-miR-6492 and pmo-miR-6493 have been reported previously in M. japonicus, but pmomiR-4961a, pmo-miR-4961b, pmo-miR-4979 and pmo-miR-3819 were first identified in shrimps. And also five out of the eight predicted pmo-miRNAs including pmo-miR-4961a, pmo-miR-4961b, pmomiR-4979, pmo-miR-6492 and pmo-miR-6493 were subsequently validated by PCR and sequencing. Experimental validation by dual luciferase reporter assay confirmed the targeting between 3 pmomiRNAs and one or two of their target genes, especially the pmo-miR-4979 which could significantly down-regulate the expression of target gene (JR226772). This study updates the miRNAs library and their targets in P. monodon and lays a solid foundation for future RNAi study.
5 ACKNOWLEDGEMENTWe thank Dr. Jerame Hui (School of Life Science, Chinese University of Hong Kong, China) for kindly providing the reporter plasmid of psiCHECK-2.
Adai A, Johnson C, Mlotshwa S, Archer-Evans S, Manocha V, Vance V, Sundaresan V. 2005. Computational prediction of miRNAs in Arabidopsis thaliana. Genome Research, 15(1): 78-91. DOI:10.1101/gr.2908205 |
Altschul S F, Madden T L, Schäffer A A, Zhang J H, Zhang Z, Miller W, Lipman D J. 1997. Gapped BLAST and PSIBLAST:a new generation of protein database search programs. Nucleic Acids Res., 25(17): 3 389-3 402. DOI:10.1093/nar/25.17.3389 |
Alvarez-Garcia I, Miska E A. 2005. MicroRNA functions in animal development and human disease. Development, 132(21): 4 653-4 662. DOI:10.1242/dev.02073 |
Ashburner M, Ball C A, Blake J A, et al. 2000. Gene ontology:tool for the unification of biology. Nature Genetics, 25(1): 25-29. DOI:10.1038/75556 |
Ason B, Darnell D K, Wittbrodt B, Berezikov E, Kloosterman W P, Wittbrodt J, Antin P B, Plasterk R H. 2006. Differences in vertebrate microRNA expression. Proceedings of the National Academy of Sciences of the United States of America, 103(39): 14 385-14 389. DOI:10.1073/pnas.0603529103 |
Axtell M J, Westholm J O, Lai E C. 2011. Vive la différence:biogenesis and evolution of microRNAs in plants and animals. Genome Biol., 12(4): 221. DOI:10.1186/gb-2011-12-4-221 |
Bar M, Wyman S K, Fritz B R, et al. 2008. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells, 26(10): 2 496-2 505. DOI:10.1634/stemcells.2008-0356 |
Bartel D P. 2004. MicroRNAs:genomics, biogenesis, mechanism, and function. Cell, 116(2): 281-297. DOI:10.1016/S0092-8674(04)00045-5 |
Baskerville S, Bartel D P. 2005. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA, 11(3): 241-247. DOI:10.1261/rna.7240905 |
Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk R H A, Cuppen E. 2005. Phylogenetic shadowing and computational identification of human microRNA genes. Cell, 120(1): 21-24. DOI:10.1016/j.cell.2004.12.031 |
Brown J R, Sanseau P. 2005. A computational view of microRNAs and their targets. Drug Discovery Today, 10(8): 595-601. DOI:10.1016/S1359-6446(05)03399-4 |
Carrington J C, Ambros V. 2003. Role of microRNAs in plant and animal development. Science, 301(5631): 336-338. DOI:10.1126/science.1085242 |
Carthew R W, Sontheimer E J. 2009. Origins and mechanisms of miRNAs and siRNAs. Cell, 136(4): 642-655. DOI:10.1016/j.cell.2009.01.035 |
Chan S M, Gu P L, Chu K H, Tobe S S. 2003. Crustacean neuropeptide genes of the CHH/MIH/GIH family:implications from molecular studies. General and Comparative Endocrinology, 134(3): 214-219. DOI:10.1016/S0016-6480(03)00263-6 |
Chen X M. 2005. MicroRNA biogenesis and function in plants. FEBS Letters, 579(26): 5 923-5 931. DOI:10.1016/j.febslet.2005.07.071 |
Cheng A M, Byrom M W, Shelton J, Ford L P. 2005. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res., 33(4): 1 290-1 297. DOI:10.1093/nar/gki200 |
Chi W, Tong C B, Gan X N, He S P. 2011. Characterization and comparative profiling of miRNA transcriptomes in bighead carp and silver carp. PLoS One, 6(8): e23549. DOI:10.1371/journal.pone.0023549 |
Conesa A, Götz S, García-Gómez J M, Terol J, Talón M, Robles M. 2005. Blast2GO:a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18): 3 674-3 676. DOI:10.1093/bioinformatics/bti610 |
Cui Y L, Huang T Z, Zhang X B. 2015. RNA editing of microRNA prevents RNA-induced silencing complex recognition of target mRNA. Open Biology, 5(12): 150 126. DOI:10.1098/rsob.150126 |
Davis B N, Hata A. 2009. Regulation of MicroRNA biogenesis:a miRiad of mechanisms. Cell Communication and Signaling, 7(1): 18. DOI:10.1186/1478-811X-7-18 |
Fabbri M, Garzon R, Cimmino A, et al. 2007. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proceedings of the National Academy of Sciences of the United States of America, 104(40): 15 805-15 810. DOI:10.1073/pnas.0707628104 |
Fanjul-Moles M L. 2006. Biochemical and functional aspects of crustacean hyperglycemic hormone in decapod crustaceans:review and update. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 142(3-4): 390-400. |
Gomes C P C, Cho J H, Hood L, Franco O L, Pereira R W, Wang K. 2013. A review of computational tools in microRNA discovery. Front. Genet., 4: 81. |
Götz S, García-Gómez J M, Terol J, Williams T D, Nagaraj S H, Nueda M J, Robles M, Talón M, Dopazo J, Conesa A. 2008. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res., 36(10): 3420-3 435. DOI:10.1093/nar/gkn176 |
Gregory R I, Chendrimada T P, Cooch N, Shiekhattar R. 2005. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell, 123(4): 631-640. DOI:10.1016/j.cell.2005.10.022 |
Griffiths-Jones S, Grocock R J, Van Dongen S, Bateman A, Enright A J. 2006. miRBase:microRNA sequences, targets and gene nomenclature. Nucleic Acids Res., 34(S1): D140-D144. |
Guo H S, Xie Q, Fei J F, Chua N H. 2005. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. The Plant Cell, 17(5): 1 376-1 386. DOI:10.1105/tpc.105.030841 |
Hofacker I L. 2003. Vienna RNA secondary structure server. Nucleic Acids Res., 31(13): 3 429-3 431. DOI:10.1093/nar/gkg599 |
Huang T Z, Xu D D, Zhang X B. 2012. Characterization of host microRNAs that respond to DNA virus infection in a crustacean. BMC Genomics, 13(1): 159. DOI:10.1186/1471-2164-13-159 |
Huang T Z, Zhang X B. 2012. Functional analysis of a crustacean microRNA in host-virus interactions. Journal of Virology, 86(23): 12 997-13 004. DOI:10.1128/JVI.01702-12 |
John B, Enright A J, Aravin A, Tuschl T, Sander C, Marks D S. 2004. Human microRNA targets. PLoS Biology, 2(11): e363. DOI:10.1371/journal.pbio.0020363 |
Kanehisa M, Goto S. 2000. KEGG:kyoto encyclopedia of genes and genomes. Nucleic Acids Res., 28(1): 27-30. DOI:10.1093/nar/28.1.27 |
Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi J J, Natarajan R. 2009. TGF-β activates Akt kinase through a microRNAdependent amplifying circuit targeting PTEN. Nature Cell Biology, 11(7): 881-889. DOI:10.1038/ncb1897 |
Kim V N. 2005. MicroRNA biogenesis:coordinated cropping and dicing. Nature Reviews Molecular Cell Biology, 6(5): 376-385. DOI:10.1038/nrm1644 |
Kloosterman W P, Plasterk R H A. 2006. The diverse functions of microRNAs in animal development and disease. Developmental Cell, 11(4): 441-450. DOI:10.1016/j.devcel.2006.09.009 |
Kloosterman W P, Steiner F A, Berezikov E, de Bruijn E, van de Belt J, Verheul M, Cuppen E, Plasterk R H A. 2006. Cloning and expression of new microRNAs from zebrafish. Nucleic Acids Res., 34(9): 2 558-2 569. DOI:10.1093/nar/gkl278 |
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. 2002. Identification of tissue-specific microRNAs from mouse. Current Biology, 12(9): 735-739. DOI:10.1016/S0960-9822(02)00809-6 |
Lai E C, Tomancak P, Williams R W, Rubin G M. 2003. Computational identification of Drosophila microRNA genes. Genome Biol., 4(7): R42. DOI:10.1186/gb-2003-4-7-r42 |
Li L, Xu J Z, Yang D Y, Tan X R, Wang H F. 2010. Computational approaches for microRNA studies:a review. Mammalian Genome, 21(1-2): 1-12. DOI:10.1007/s00335-009-9241-2 |
Li Y, Wang F, Lee J A, Gao F B. 2006. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes & Development, 20(20): 2 793-2 805. |
Lim L P, Lau N C, Weinstein E G, Abdelhakim A, Yekta S, Rhoades M W, Burge C B, Bartel D P. 2003. The microRNAs of Caenorhabditis elegans. Genes & Development, 17(8): 991-1 008. |
Liu C G, Calin G A, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru C D, Shimizu M, Zupo S, Dono M, Alder H, Bullrich F, Negrini M, Croce C M. 2004. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proceedings of the National Academy of Sciences of the United States of America, 101(26): 9 740-9 744. DOI:10.1073/pnas.0403293101 |
Lu C, Jeong D H, Kulkarni K, et al. 2008. Genome-wide analysis for discovery of rice microRNAs reveals natural antisense microRNAs (nat-miRNAs). Proceedings of the National Academy of Sciences of the United States of America, 105(12): 4 951-4 956. DOI:10.1073/pnas.0708743105 |
Meemak P, Phongdara A, Chotigeat W, Tammi M T. 2013. Computational identification of Penaeus monodon microRNA genesan d their targets. Songklanakarin Journal of Science & Technology, 35(2): 143-148. |
O'Connell R M, Chaudhuri A A, Rao D S, Baltimore D. 2009. Inositol phosphatase SHIP1 is a primary target of miR-155. Proceedings of the National Academy of Sciences of the United States of America, 106(17): 7 113-7 118. DOI:10.1073/pnas.0902636106 |
Pierce M L, Weston M D, Fritzsch B, Gabel H W, Ruvkun G, Soukup G A. 2008. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evolution & Development, 10(1): 106-113. |
Ramachandra R K, Salem M, Gahr S, Rexroad C E, Yao J B. 2008. Cloning and characterization of microRNAs from rainbow trout (Oncorhynchus mykiss):their expression during early embryonic development. BMC Developmental Biology, 8(1): 41. DOI:10.1186/1471-213X-8-41 |
Re A, Corá D, Taverna D, Caselle M. 2009. Genome-wide survey of microRNA-transcription factor feed-forward regulatory circuits in human. Molecular BioSystems, 5(8): 854-867. DOI:10.1039/b900177h |
Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P. 1998. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture, 167(3-4): 301-313. DOI:10.1016/S0044-8486(98)00305-6 |
Rodriguez A, Griffiths-Jones S, Ashurst J L, Bradley A. 2004. Identification of mammalian microRNA host genes and transcription units. Genome Research, 14(10a): 1 902-1 910. DOI:10.1101/gr.2722704 |
Saini H K, Griffiths-Jones S, Enright A J. 2007. Genomic analysis of human microRNA transcripts. Proceedings of the National Academy of Sciences of the United States of America, 104(45): 17 719-17 724. DOI:10.1073/pnas.0703890104 |
Sethupathy P, Megraw M, Hatzigeorgiou A G. 2006. A guide through present computational approaches for the identification of mammalian microRNA targets. Nature Methods, 3(11): 881-886. DOI:10.1038/nmeth954 |
Soares A R, Pereira P M, Santos B, Egas C, Gomes A C, Arrais J, Oliveira J L, Moura G R, Santos M A. 2009. Parallel DNA pyrosequencing unveils new zebrafish microRNAs. BMC Genomics, 10(1): 195. DOI:10.1186/1471-2164-10-195 |
Stark A, Bushati N, Jan C H, Kheradpour P, Hodges E, Brennecke J, Bartel D P, Cohen S M, Kellis M. 2008. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands. Genes & Development, 22(1): 8-13. |
Strimmer K, von Haeseler A. 1996. Quartet puzzling:a quartet maximum-likelihood method for reconstructing tree topologies. Molecular Biology and Evolution, 13(7): 964-969. DOI:10.1093/oxfordjournals.molbev.a025664 |
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2 731-2 739. DOI:10.1093/molbev/msr121 |
Wang J Y, Yang X D, Xu H B, Chi X Y, Zhang M, Hou X L. 2012. Identification and characterization of microRNAs and their target genes in Brassica oleracea. Gene, 505(2): 300-308. DOI:10.1016/j.gene.2012.06.002 |
Weston M D, Pierce M L, Rocha-Sanchez S R, Beisel K W, Soukup G A. 2006. MicroRNA gene expression in the mouse inner ear. Brain Research, 1111(1): 95-104. DOI:10.1016/j.brainres.2006.07.006 |
Wienholds E, Kloosterman W P, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz H R, Kauppinen S, Plasterk R H A. 2005. MicroRNA expression in zebrafish embryonic development. Science, 309(5732): 310-311. DOI:10.1126/science.1114519 |
Xi Q Y, Xiong Y Y, Wang Y M, Cheng X, Qi Q E, Shu G, Wang S B, Wang L N, Gao P, Zhu X T, Jiang Q Y, Zhang Y L, Liu L. 2015. Genome-wide discovery of novel and conserved microRNAs in white shrimp (Litopenaeus vannamei). Molecular Biology Reports, 42(1): 61-69. DOI:10.1007/s11033-014-3740-2 |
Xie C X, Xu S L, Yang L L, Ke Z H, Xing J B, Gai J W, Gong X L, Xu L X, Bao B L. 2011. mRNA/microRNA profile at the metamorphic stage of olive flounder (Paralichthys olivaceus). Comparative And functional Genomics, 2011: 256 038. |
Xu Z Q, Qin Q, Ge J C, Pan J L, Xu X F. 2012. Bioinformatic identification and validation of conservative microRNAs in Ictalurus punctatus. Molecular Biology Reports, 39(12): 10 395-10 405. DOI:10.1007/s11033-012-1918-z |
Yang G, Yang L, Zhao Z, Wang J J, Zhang X B. 2012. Signature miRNAs involved in the innate immunity of invertebrates. PLoS One, 7(6): e39015. DOI:10.1371/journal.pone.0039015 |
Yang L D, He S P. 2014. A bioinformatics-based update on microRNAs and their targets in rainbow trout(Oncorhynchus mykiss). Gene, 533(1): 261-269. DOI:10.1016/j.gene.2013.09.060 |
Yin Z J, Li C H, Han X L, Shen F F. 2008. Identification of conserved microRNAs and their target genes in tomato(Lycopersicon esculentum). Gene, 414(1-2): 60-66. DOI:10.1016/j.gene.2008.02.007 |
Zhang B H, Pan X P, Cannon C H, Cobb G P, Anderson T A. 2006b. Conservation and divergence of plant microRNA genes. The Plant Journal, 46(2): 243-259. DOI:10.1111/tpj.2006.46.issue-2 |
Zhang B H, Pan X P, Cox S B, Cobb G P, Anderson T A. 2006a. Evidence that miRNAs are different from other RNAs. Cellular and Molecular Life Sciences, 63(2): 246-254. DOI:10.1007/s00018-005-5467-7 |
Zhang B H, Pan X P, Wang Q L, Cobb G P, Anderson T A. 2005. Identification and characterization of new plant microRNAs using EST analysis. Cell Research, 15(5): 336-360. DOI:10.1038/sj.cr.7290302 |
Zhang B H, Wang Q L, Wang K B, Pan X P, Liu F, Guo T L, Cobb G P, Anderson T A. 2007. Identification of cotton microRNAs and their targets. Gene, 397(1-2): 26-37. DOI:10.1016/j.gene.2007.03.020 |
Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology, 7(1-2): 203-214. DOI:10.1089/10665270050081478 |
Zhou L G, Liu Y H, Liu Z C, Kong D Y, Duan M, Luo L J. 2010. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. Journal of Experimental Botany, 61(15): 4 157-4 168. DOI:10.1093/jxb/erq237 |
| Adai A, Johnson C, Mlotshwa S, Archer-Evans S, Manocha V, Vance V, Sundaresan V, 2005. Computational prediction of miRNAs in Arabidopsis thaliana. Genome Research, 15(1): 78–91. Doi: 10.1101/gr.2908205 |
| Altschul S F, Madden T L, Schäffer A A, Zhang J H, Zhang Z, Miller W, Lipman D J, 1997. Gapped BLAST and PSIBLAST:a new generation of protein database search programs. Nucleic Acids Res., 25(17): 3 389–3 402. Doi: 10.1093/nar/25.17.3389 |
| Alvarez-Garcia I, Miska E A, 2005. MicroRNA functions in animal development and human disease. Development, 132(21): 4 653–4 662. Doi: 10.1242/dev.02073 |
| Ashburner M, Ball C A, Blake J A, et al, 2000. Gene ontology:tool for the unification of biology. Nature Genetics, 25(1): 25–29. Doi: 10.1038/75556 |
| Ason B, Darnell D K, Wittbrodt B, Berezikov E, Kloosterman W P, Wittbrodt J, Antin P B, Plasterk R H, 2006. Differences in vertebrate microRNA expression. Proceedings of the National Academy of Sciences of the United States of America, 103(39): 14 385–14 389. Doi: 10.1073/pnas.0603529103 |
| Axtell M J, Westholm J O, Lai E C, 2011. Vive la différence:biogenesis and evolution of microRNAs in plants and animals. Genome Biol., 12(4): 221. Doi: 10.1186/gb-2011-12-4-221 |
| Bar M, Wyman S K, Fritz B R, et al, 2008. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells, 26(10): 2 496–2 505. Doi: 10.1634/stemcells.2008-0356 |
| Bartel D P, 2004. MicroRNAs:genomics, biogenesis, mechanism, and function. Cell, 116(2): 281–297. Doi: 10.1016/S0092-8674(04)00045-5 |
| Baskerville S, Bartel D P, 2005. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA, 11(3): 241–247. Doi: 10.1261/rna.7240905 |
| Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk R H A, Cuppen E, 2005. Phylogenetic shadowing and computational identification of human microRNA genes. Cell, 120(1): 21–24. Doi: 10.1016/j.cell.2004.12.031 |
| Brown J R, Sanseau P, 2005. A computational view of microRNAs and their targets. Drug Discovery Today, 10(8): 595–601. Doi: 10.1016/S1359-6446(05)03399-4 |
| Carrington J C, Ambros V, 2003. Role of microRNAs in plant and animal development. Science, 301(5631): 336–338. Doi: 10.1126/science.1085242 |
| Carthew R W, Sontheimer E J, 2009. Origins and mechanisms of miRNAs and siRNAs. Cell, 136(4): 642–655. Doi: 10.1016/j.cell.2009.01.035 |
| Chan S M, Gu P L, Chu K H, Tobe S S, 2003. Crustacean neuropeptide genes of the CHH/MIH/GIH family:implications from molecular studies. General and Comparative Endocrinology, 134(3): 214–219. Doi: 10.1016/S0016-6480(03)00263-6 |
| Chen X M, 2005. MicroRNA biogenesis and function in plants. FEBS Letters, 579(26): 5 923–5 931. Doi: 10.1016/j.febslet.2005.07.071 |
| Cheng A M, Byrom M W, Shelton J, Ford L P, 2005. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res., 33(4): 1 290–1 297. Doi: 10.1093/nar/gki200 |
| Chi W, Tong C B, Gan X N, He S P, 2011. Characterization and comparative profiling of miRNA transcriptomes in bighead carp and silver carp. PLoS One, 6(8): e23549. Doi: 10.1371/journal.pone.0023549 |
| Conesa A, Götz S, García-Gómez J M, Terol J, Talón M, Robles M, 2005. Blast2GO:a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18): 3 674–3 676. Doi: 10.1093/bioinformatics/bti610 |
| Cui Y L, Huang T Z, Zhang X B, 2015. RNA editing of microRNA prevents RNA-induced silencing complex recognition of target mRNA. Open Biology, 5(12): 150 126. Doi: 10.1098/rsob.150126 |
| Davis B N, Hata A, 2009. Regulation of MicroRNA biogenesis:a miRiad of mechanisms. Cell Communication and Signaling, 7(1): 18. Doi: 10.1186/1478-811X-7-18 |
| Fabbri M, Garzon R, Cimmino A, et al, 2007. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proceedings of the National Academy of Sciences of the United States of America, 104(40): 15 805–15 810. Doi: 10.1073/pnas.0707628104 |
| Fanjul-Moles M L, 2006. Biochemical and functional aspects of crustacean hyperglycemic hormone in decapod crustaceans:review and update. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 142(3-4): 390–400. |
| Gomes C P C, Cho J H, Hood L, Franco O L, Pereira R W, Wang K, 2013. A review of computational tools in microRNA discovery. Front. Genet., 4: 81. |
| Götz S, García-Gómez J M, Terol J, Williams T D, Nagaraj S H, Nueda M J, Robles M, Talón M, Dopazo J, Conesa A, 2008. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res., 36(10): 3420–3 435. Doi: 10.1093/nar/gkn176 |
| Gregory R I, Chendrimada T P, Cooch N, Shiekhattar R, 2005. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell, 123(4): 631–640. Doi: 10.1016/j.cell.2005.10.022 |
| Griffiths-Jones S, Grocock R J, Van Dongen S, Bateman A, Enright A J, 2006. miRBase:microRNA sequences, targets and gene nomenclature. Nucleic Acids Res., 34(S1): D140–D144. |
| Guo H S, Xie Q, Fei J F, Chua N H, 2005. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. The Plant Cell, 17(5): 1 376–1 386. Doi: 10.1105/tpc.105.030841 |
| Hofacker I L, 2003. Vienna RNA secondary structure server. Nucleic Acids Res., 31(13): 3 429–3 431. Doi: 10.1093/nar/gkg599 |
| Huang T Z, Xu D D, Zhang X B, 2012. Characterization of host microRNAs that respond to DNA virus infection in a crustacean. BMC Genomics, 13(1): 159. Doi: 10.1186/1471-2164-13-159 |
| Huang T Z, Zhang X B, 2012. Functional analysis of a crustacean microRNA in host-virus interactions. Journal of Virology, 86(23): 12 997–13 004. Doi: 10.1128/JVI.01702-12 |
| John B, Enright A J, Aravin A, Tuschl T, Sander C, Marks D S, 2004. Human microRNA targets. PLoS Biology, 2(11): e363. Doi: 10.1371/journal.pbio.0020363 |
| Kanehisa M, Goto S, 2000. KEGG:kyoto encyclopedia of genes and genomes. Nucleic Acids Res., 28(1): 27–30. Doi: 10.1093/nar/28.1.27 |
| Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi J J, Natarajan R, 2009. TGF-β activates Akt kinase through a microRNAdependent amplifying circuit targeting PTEN. Nature Cell Biology, 11(7): 881–889. Doi: 10.1038/ncb1897 |
| Kim V N, 2005. MicroRNA biogenesis:coordinated cropping and dicing. Nature Reviews Molecular Cell Biology, 6(5): 376–385. Doi: 10.1038/nrm1644 |
| Kloosterman W P, Plasterk R H A, 2006. The diverse functions of microRNAs in animal development and disease. Developmental Cell, 11(4): 441–450. Doi: 10.1016/j.devcel.2006.09.009 |
| Kloosterman W P, Steiner F A, Berezikov E, de Bruijn E, van de Belt J, Verheul M, Cuppen E, Plasterk R H A, 2006. Cloning and expression of new microRNAs from zebrafish. Nucleic Acids Res., 34(9): 2 558–2 569. Doi: 10.1093/nar/gkl278 |
| Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T, 2002. Identification of tissue-specific microRNAs from mouse. Current Biology, 12(9): 735–739. Doi: 10.1016/S0960-9822(02)00809-6 |
| Lai E C, Tomancak P, Williams R W, Rubin G M, 2003. Computational identification of Drosophila microRNA genes. Genome Biol., 4(7): R42. Doi: 10.1186/gb-2003-4-7-r42 |
| Li L, Xu J Z, Yang D Y, Tan X R, Wang H F, 2010. Computational approaches for microRNA studies:a review. Mammalian Genome, 21(1-2): 1–12. Doi: 10.1007/s00335-009-9241-2 |
| Li Y, Wang F, Lee J A, Gao F B, 2006. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes & Development, 20(20): 2 793–2 805. |
| Lim L P, Lau N C, Weinstein E G, Abdelhakim A, Yekta S, Rhoades M W, Burge C B, Bartel D P, 2003. The microRNAs of Caenorhabditis elegans. Genes & Development, 17(8): 991–1 008. |
| Liu C G, Calin G A, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru C D, Shimizu M, Zupo S, Dono M, Alder H, Bullrich F, Negrini M, Croce C M, 2004. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proceedings of the National Academy of Sciences of the United States of America, 101(26): 9 740–9 744. Doi: 10.1073/pnas.0403293101 |
| Lu C, Jeong D H, Kulkarni K, et al, 2008. Genome-wide analysis for discovery of rice microRNAs reveals natural antisense microRNAs (nat-miRNAs). Proceedings of the National Academy of Sciences of the United States of America, 105(12): 4 951–4 956. Doi: 10.1073/pnas.0708743105 |
| Meemak P, Phongdara A, Chotigeat W, Tammi M T, 2013. Computational identification of Penaeus monodon microRNA genesan d their targets. Songklanakarin Journal of Science & Technology, 35(2): 143–148. |
| O'Connell R M, Chaudhuri A A, Rao D S, Baltimore D, 2009. Inositol phosphatase SHIP1 is a primary target of miR-155. Proceedings of the National Academy of Sciences of the United States of America, 106(17): 7 113–7 118. Doi: 10.1073/pnas.0902636106 |
| Pierce M L, Weston M D, Fritzsch B, Gabel H W, Ruvkun G, Soukup G A, 2008. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evolution & Development, 10(1): 106–113. |
| Ramachandra R K, Salem M, Gahr S, Rexroad C E, Yao J B, 2008. Cloning and characterization of microRNAs from rainbow trout (Oncorhynchus mykiss):their expression during early embryonic development. BMC Developmental Biology, 8(1): 41. Doi: 10.1186/1471-213X-8-41 |
| Re A, Corá D, Taverna D, Caselle M, 2009. Genome-wide survey of microRNA-transcription factor feed-forward regulatory circuits in human. Molecular BioSystems, 5(8): 854–867. Doi: 10.1039/b900177h |
| Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P, 1998. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture, 167(3-4): 301–313. Doi: 10.1016/S0044-8486(98)00305-6 |
| Rodriguez A, Griffiths-Jones S, Ashurst J L, Bradley A, 2004. Identification of mammalian microRNA host genes and transcription units. Genome Research, 14(10a): 1 902–1 910. Doi: 10.1101/gr.2722704 |
| Saini H K, Griffiths-Jones S, Enright A J, 2007. Genomic analysis of human microRNA transcripts. Proceedings of the National Academy of Sciences of the United States of America, 104(45): 17 719–17 724. Doi: 10.1073/pnas.0703890104 |
| Sethupathy P, Megraw M, Hatzigeorgiou A G, 2006. A guide through present computational approaches for the identification of mammalian microRNA targets. Nature Methods, 3(11): 881–886. Doi: 10.1038/nmeth954 |
| Soares A R, Pereira P M, Santos B, Egas C, Gomes A C, Arrais J, Oliveira J L, Moura G R, Santos M A, 2009. Parallel DNA pyrosequencing unveils new zebrafish microRNAs. BMC Genomics, 10(1): 195. Doi: 10.1186/1471-2164-10-195 |
| Stark A, Bushati N, Jan C H, Kheradpour P, Hodges E, Brennecke J, Bartel D P, Cohen S M, Kellis M, 2008. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands. Genes & Development, 22(1): 8–13. |
| Strimmer K, von Haeseler A, 1996. Quartet puzzling:a quartet maximum-likelihood method for reconstructing tree topologies. Molecular Biology and Evolution, 13(7): 964–969. Doi: 10.1093/oxfordjournals.molbev.a025664 |
| Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S, 2011. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2 731–2 739. Doi: 10.1093/molbev/msr121 |
| Wang J Y, Yang X D, Xu H B, Chi X Y, Zhang M, Hou X L, 2012. Identification and characterization of microRNAs and their target genes in Brassica oleracea. Gene, 505(2): 300–308. Doi: 10.1016/j.gene.2012.06.002 |
| Weston M D, Pierce M L, Rocha-Sanchez S R, Beisel K W, Soukup G A, 2006. MicroRNA gene expression in the mouse inner ear. Brain Research, 1111(1): 95–104. Doi: 10.1016/j.brainres.2006.07.006 |
| Wienholds E, Kloosterman W P, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz H R, Kauppinen S, Plasterk R H A, 2005. MicroRNA expression in zebrafish embryonic development. Science, 309(5732): 310–311. Doi: 10.1126/science.1114519 |
| Xi Q Y, Xiong Y Y, Wang Y M, Cheng X, Qi Q E, Shu G, Wang S B, Wang L N, Gao P, Zhu X T, Jiang Q Y, Zhang Y L, Liu L, 2015. Genome-wide discovery of novel and conserved microRNAs in white shrimp (Litopenaeus vannamei). Molecular Biology Reports, 42(1): 61–69. Doi: 10.1007/s11033-014-3740-2 |
| Xie C X, Xu S L, Yang L L, Ke Z H, Xing J B, Gai J W, Gong X L, Xu L X, Bao B L, 2011. mRNA/microRNA profile at the metamorphic stage of olive flounder (Paralichthys olivaceus). Comparative And functional Genomics, 2011: 256 038. |
| Xu Z Q, Qin Q, Ge J C, Pan J L, Xu X F, 2012. Bioinformatic identification and validation of conservative microRNAs in Ictalurus punctatus. Molecular Biology Reports, 39(12): 10 395–10 405. Doi: 10.1007/s11033-012-1918-z |
| Yang G, Yang L, Zhao Z, Wang J J, Zhang X B, 2012. Signature miRNAs involved in the innate immunity of invertebrates. PLoS One, 7(6): e39015. Doi: 10.1371/journal.pone.0039015 |
| Yang L D, He S P, 2014. A bioinformatics-based update on microRNAs and their targets in rainbow trout(Oncorhynchus mykiss). Gene, 533(1): 261–269. Doi: 10.1016/j.gene.2013.09.060 |
| Yin Z J, Li C H, Han X L, Shen F F, 2008. Identification of conserved microRNAs and their target genes in tomato(Lycopersicon esculentum). Gene, 414(1-2): 60–66. Doi: 10.1016/j.gene.2008.02.007 |
| Zhang B H, Pan X P, Cannon C H, Cobb G P, Anderson T A, 2006b. Conservation and divergence of plant microRNA genes. The Plant Journal, 46(2): 243–259. Doi: 10.1111/tpj.2006.46.issue-2 |
| Zhang B H, Pan X P, Cox S B, Cobb G P, Anderson T A, 2006a. Evidence that miRNAs are different from other RNAs. Cellular and Molecular Life Sciences, 63(2): 246–254. Doi: 10.1007/s00018-005-5467-7 |
| Zhang B H, Pan X P, Wang Q L, Cobb G P, Anderson T A, 2005. Identification and characterization of new plant microRNAs using EST analysis. Cell Research, 15(5): 336–360. Doi: 10.1038/sj.cr.7290302 |
| Zhang B H, Wang Q L, Wang K B, Pan X P, Liu F, Guo T L, Cobb G P, Anderson T A, 2007. Identification of cotton microRNAs and their targets. Gene, 397(1-2): 26–37. Doi: 10.1016/j.gene.2007.03.020 |
| Zhang Z, Schwartz S, Wagner L, Miller W, 2000. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology, 7(1-2): 203–214. Doi: 10.1089/10665270050081478 |
| Zhou L G, Liu Y H, Liu Z C, Kong D Y, Duan M, Luo L J, 2010. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. Journal of Experimental Botany, 61(15): 4 157–4 168. Doi: 10.1093/jxb/erq237 |
 2018, Vol. 36
2018, Vol. 36