Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Cuicui(王翠翠), ZHANG Kai(张凯), XIE Jun(谢骏), LIU Qigen(刘其根), YU Deguang(余德光), WANG Guangjun(王广军), YU Ermeng(郁二蒙), GONG Wangbao(龚望宝), LI Zhifei(李志斐)
- Denitrification potential evaluation of a newly indigenous aerobic denitrifier isolated from largemouth bass Micropterus salmoides culture pond
- Chinese Journal of Oceanology and Limnology, 36(3): 913-925
- http://dx.doi.org/10.1007/s00343-018-7029-5
Article History
- Received Mar. 30, 2017
- accepted in principle May. 4, 2017
- accepted for publication May. 27, 2017
2 College of Fisheries and Life Sciences, Shanghai Ocean University, Shanghai 201306, China
Contamination with nitrogenous compounds, especially ammonia, nitrite and nitrate, is a serious concern in fish culture systems. If widely distributed, ammonium along with its oxidation products (nitrate and nitrite) cause water-quality problems in systems for largemouth bass, a species which feeds mainly on live baitfish. Xu et al. (2007) reported an increase in 1 g of fish that were fed trash-fish and mass produced 34.4–67.2 mg nitrogen in urine, uneaten feed, feces, and caduceus scales. Moreover, the increased presence of aqueous ammonium is harmful for aquatic life, especially fish (Šiljeg et al., 2010), and nitrate can damage immune ability (Grguric et al., 2000). Thus, a stringent research topic has long been to determine how to efficiently remove the overburden of nitrogen in aquaculture systems.
Previous studies on water quality have noted improvements by the addition of probiotics, especially Bacillus spp. (Verschuere et al., 2000; Kolndadacha et al., 2011); however, large-scale application of this still presents numerous problems. Probiotic strains intended for colonization performance in water for fish culture are difficult to attain (Skjermo et al., 2015). The addition of some microbial products has shown limited ability to improve production performance and water quality in freshwater polyculture (Tang et al., 2016). As yet, it is difficult to produce strains in commercial quantities and apply them to good effect on a large scale (Michael et al., 2014).
Aerobic denitrification, as first described by Robertson and Kuenen (1984), was a potential way to remove nitrogen in aquaculture, with efficient removal of nitrogen achieved even under high DO levels. To date, studies of aerobic denitrification have focused on ammonium screened mainly from domestic wastewater and lakes, and research on the treatment of ammonium in aquatic culture was rare (Kumar et al., 2010). Previous researchers have described a few types of aerobic denitrifying bacteria isolated from activated sludge or wastewater treatment facilities, but these have not been truly suitable for the aquaculture industry due to poor tolerance of DO, C/N ratio or organic carbon, and because of low denitrification efficiency (Lukow and Diekmann, 1997; Pai et al., 1999; Joo et al., 2005; Obaja et al., 2005; Chen et al., 2012). Furthermore, those denitrifiers might acclimate poorly in eutrophic freshwaters, which could limit the denitrification process (Obaja et al., 2005; Guo et al., 2013). Therefore, to overcome this problem, an autochthonous strain able to perform aerobic denitrification might be helpful in attaining eco-friendly aquaculture systems.
The aim of this research was to evaluate nitrogenremoving potential of a novel autochthonous aerobic denitrification bacteria that was isolated from a largemouth-bass culture pond.
2 METHOD 2.1 MediumThe media used in our study included screening medium (SM), solid bromothymol blue medium (BTB), and liquid denitrification medium (DM).
The SM (pH 7.2) included the following reagents per liter: sodium citrate, 1.0 g; KNO3, 10 mmol/L; NH4Cl, 10 mmol/L; KH2PO4, 1.36 g; (NH4)2SO4, 0.27 g; yeast extract, 1 g; MgSO4·7H2O, 0.19 g; TE (trace element) solution, 1 mL.
The Tris-EDTA buffer (TE) solution contained the following components (per liter): 57.1 g of ethylenediamine-N, N, N', N'-tetraacetic acid, disodium salt, dihydrate (EDTA·2Na), 7 g of CaCl2·2H2O, 5.0 g of FeSO4·7H2O, 3.9 g of ZnSO4·7H2O, 1.6 g of CuSO4·5H2O, 1.1 g of (NH4)6Mo7O24·4H2O, 5.1 g of MnCl2·4H2O, and 1.6 g of CoCl2·6H2O (pH=7.0).
The ingredients of the solid BTB (pH 7.0–7.3) were as follows per liter: sodium citrate, 1.31 g; KNO3, 0.181 g; NH4Cl, 0.096 g; KH2PO4, 1 g; K 2HPO4, 5.0 g; MgSO4·7H2O, 0.2 g; BTB regent (1% in alcohol), 1 mL; TE solution, 1 mL; agar, 20 g. The components of TE solution were as described by Pai et al. (1999).
The ingredients of DM were identical with the solid BTB except for the addition of agar and BTB regent. The DM used involved three types: to detect the transformation of NO3ˉ-N, using KNO3 as the sole nitrogen resource; to detect the transformation of NH4+-N, using NH4Cl as the sole nitrogen resource; to detect the combined transformation of NH4+-N and NO3ˉ-N, using KNO3 and NH4Cl as the sole nitrogen resource.
A Luria-Bertani medium (LB) used for culture preservation contained 10 g/L of peptone, 10 g/L of NaCl, and 5 g/L of yeast extract.
2.2 Source of the isolated aerobic denitrifying bacteriaThe original source of the isolated aerobic denitrifier was bottom-water samples from the Nansha Aquaculture Base for largemouth bass (Nansha, Guangzhou, China). Water samples (at a depth of 1.5 m) were collected with 1-L sterile bottles from enclosures (5 m×5 m×1.5 m) in August 2015. The samples were immediately brought back to the laboratory; 100-mL samples were transferred to sterilize 100-mL SM to enrich the denitrifying bacteria. The enrichment samples were cultured on BTB plates at 30℃ for 2 days to verify whether the blue cloudy colonies could grow; next, any colonies were purified four times, and then selected for further assay.
2.3 Assays of the nitrogen removal rate of the isolatesPurified strains of the bacteria were second screened on DM culture containing 25×10-6 NH4+-N and 25×10-6 NO3ˉ-N. Each isolate (A600=0.610) was centrifuged and then transferred to 99-mL liquid DM, and finally cultured at 180 r/min at 30℃ for 24 h. Liquid samples of 10 mL were taken before and after the culturing procedure and centrifuged at 8 000×g for 10 min; the supernatant was used to measure the nitrogen (N) levels (i.e., ammonia-N, nitrate-N and total inorganic nitrogen). The concentration of ammonia-N, nitrate-N, nitrite-N, and TN concentration was determined according to the GB 7493-87 (GBT, 1987) method. The formula used to determine the rate of N removal was as follows:
N (ammonia-N, nitrate-N, nitrite-N, and TN) removal rate (%)=(Cend-Coriginal)/Coriginal×100%, where, Coriginal is N (ammonia-N, nitrate-N, nitrite-N and TN) concentration before the culture, and Cend is N (ammonia-N, nitrate-N, nitrite-N and TN) concentration at the end of the culture period.
After these procedures, the most promising bacteria identified, named strain CW-2, was stored in tubes at -80℃ after purification.
2.4 Bacterial identification and denitrificationgene amplificationThe genomic DNA of isolate CW-2 was extracted using a Bacteria DNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA) for bacterial identification. Bacterial common primers 27F/1492R were used for 16S rRNA amplification by a PTC-100TM Programmable Thermal Controller (MJ Research, Inc., Watertown, MA, USA), under the following conditions: 5 min at 94℃, 30 cycles of 94℃ for 1 min, 55℃ for 50S, 72℃ for 1.5 min, and a final step of 10 min at 72℃. The PCR products were sequenced by Shanghai Sangon Biological Technology Co. Ltd. Finally, the 16S rRNA partial sequence of the isolate was conducted with BLAST (http://www.ncbi.nlm.nih.gov/BLAST/bast.cgi). MEGA version 4.0 (MEGA, Tempe, AZ, USA) software was used to check alignment and construct the phylogenetic tree.
Fragments of the nitrous oxide reductase (nos) gene, nitrite reductase (nir) gene, and nitric oxide reductase (nor) gene of pseudomonas strain CW-2 were amplified using primer pairs nosLb/nosRb (Throbäck et al., 2004), nirSFcd3a/nirSR3cd (Throbäck et al., 2004), nirKFlaCu/nirKR3Cu (Garbeva et al., 2007), and norBF/norBR (Garbeva et al., 2007). The PCR conditions were the same as described in Wan et al. (2011).
2.5 Nitrogen resources consumed by strain CW-2To evaluate the nitrogen removal ability of strain CW-2, we used nitrate-N, ammonium-N, and the combination of the two nitrogens as the sole nitrogen source in the separate mediums. The three types of DM medium (i.e., 50 mg/L nitrate, 50 mg/L ammonium, and the combination of 25 mg/L nitrate and 25 mg/L ammonium) are described in Section 2.1, above. The strain was first activated in the LB medium and then added to three types of DM, in equal doses, at 30℃ with a shaking speed of 180 r/min for 40 h. During the experiments, the cultures were periodically sampled (every 5 h) to determine the extent of cell growth and the nitrogen residual.
2.6 Effects of different carbon sources, C/N ratio, DO, and pH on the ammonia-N removal rate by Pseudomonas strain CW-2The rate of ammonium-N removal was determined under different conditions, including carbon source, C/N ratio, DO and pH. Each 1-mL sample of activated Pseudomonas strain CW-2 was incubated in 99-mL of sterile DM, with 50 mg/L initial ammonia-N, and cultured for 24 h in the shakers (200 rotations per minute). After the liquid culture was centrifuged at 8 000×g for 10 min, the supernatant was used to measure the ammonia-N concentration. The carbon sources were sodium citrate, sodium acetate, sucrose and glucose; the C/N ratios were set at 2, 4, 6, 8 and 10; the speeds, representing different DO concentrations, were set at 0, 100, 150, 200, 250 and 300 rotations per minute. DO was determined according to the methods of Carpenter (1965). To observe the effects of pH on nitrogen removal, the pH was adjusted to 3, 5, 7, 9 and 11. All experiments were done in triplicate.
2.7 Ammonium removal performance in largemouth-bass pond waterTo determine the potential nitrogen removal capability of strain CW-2, we designated four treatments, labelled: -C–S (without additional carbon, and without CW-2), -C+S (without additional carbon, and with CW-2), +C–S (with additional carbon, and without CW-2), and +C+S (with additional carbon, and with CW-2). Given the initial concentration of ammonium (150 mg/L) in the water collected from the largemouth-bass ponds, the densities of nitrate-N, ammonium-N, and nitrite-N were periodically measured over 7 days. A 5-mL suspension of strain CW-2 at the late exponential phase of growth was also added to 100-mL samples of synthetic pond water (-C+S and +C+S); and, sodium citrate was added to two treatments (+C–S and +C+S) at the level C/N=8. The systems were incubated for one week at 30℃, 200 r/min, and pH 7. All experiments were done in triplicate.
2.8 Sequencing of the microbial community in the synthetic water 2.8.1 PCR amplification, and quantitation of the amplicon sequencingTotal genome DNA from the samples was extracted using the CTAB/SDS method. DNA was diluted to 1 ng/μL using sterile water and stored at -20℃ for later use.
All PCR reactions were carried out in 30-μL volumes per final reaction: 15 μL Master Mix (New England Biolabs); 0.2 μmol/L of forward and reverse primers (515F [5'-GTGCCAGCMGCCGCGGTAA- 3'] and 806R [5'-GGACTACHVGGGTWTCTAAT- 3']), and about 10 ng template DNA. The procedure involved 98℃ for 1 min, followed by 30 cycles of 98℃ for 10 s, annealing at 50℃ for 30 s, elongation at 72℃ for 60 s, and 72℃ for 5 min in the end.
The PCR products were detected by electrophoresis on 2% agarose gel. Samples with a bright main strip between 400–450 bp were used for further experiments. The PCR products were mixed in equidensity ratios, then the PCR product mixtures were purified with GeneJET Gel Extraction Kit (Thermo Scientific). Sequencing libraries were generated using NEB Next® Ultra™ DNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer's recommendations, and finally index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. At last, the library was sequenced on an Illumina MiSeq platform, and 250 bp/300 bp paired-end reads were generated.
2.8.2 Phylogenetic distances and community distributionQIIME calculates both the weighted and unweighted unifrac distances, which are phylogenetic measures of beta diversity. We used the unweighted pair-group method with arithmetic mean (UPGMA). UPGMA clustering is a hierarchical clustering method using average linkage and can be used to interpret the distance matrix.
2.9 Statistical analysisThe data generated in this study were analyzed with SPSS 13.0 software. Means were compared by one-way ANOVA, and the significance level was P < 0.05.
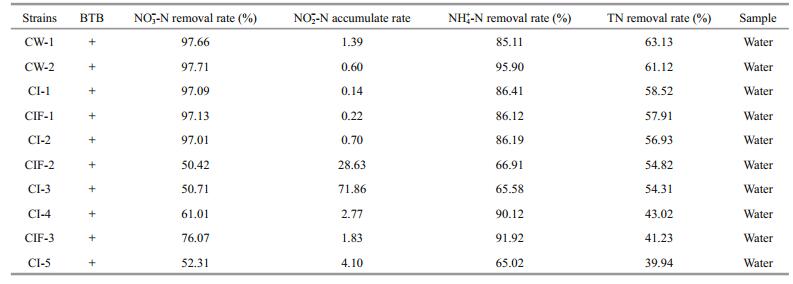
3 RESULT 3.1 Isolation and screening of the aerobic denitrifying bacteria, strain Pseudomonas CW-2The strain CW-2 displayed a high efficiency to remove nitrite and ammonium, and thus was selected as the most promising bacterium (Table 1). Moreover, the strain presented even better denitrification performance after subculture to the tenth generation, as illustrated in Fig. 1, with stable ability for removal of ammonium-N, nitrate-N, nitrite-N, and total inorganic nitrogen.

|
| Figure 1 Nitrogen removal of strain CW-2 of subculture generations |
Following the 16S rRNA gene sequence analysis, the strain CW-2 was identified as Pseudomonas. The sequence obtained in this study is available in GenBank under accession number KP747656.
The key enzyme nir, nor, and nos denitrification genes are shown in Fig. 2. Of the two nir reductase genes, cytochrome cd1 was expressed by nirS and copper nitrite reductase was expressed by nirK. The nirK gene and nosZ gene were positive in the CW-2 isolate, and the norB gene was detected with low specificity, while nirS amplification was negative.
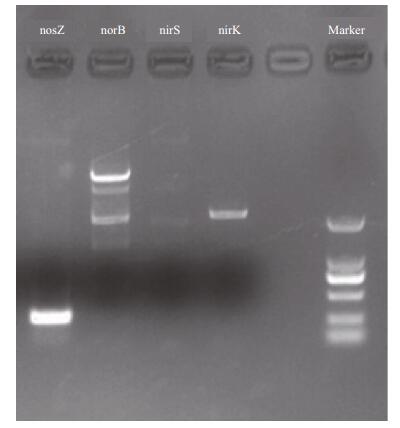
|
| Figure 2 Result of denitrification genes amplication of strain CW-2 |
During incubation of strain CW-2 in the nitrate-N, joint NO3ˉ/NH4+ and ammonia-N medium, the nitrogen concentration was measured and the cell growth of the organism (indicated by OD600) was observed (Fig. 3).

|
| Figure 3 Dynamics of Pseudomonas CW-2 cell growth and the nitrogen-removal curves in different incubation mediums a. nitrate-N medium; b. joint NO3ˉ/NH4+ medium; c. ammonia-N medium. |
The nitrate-N level decreased sharply during the first 15 h, as did the TN concentration (Fig. 3a). The nitrate-N concentration apparently did not decrease after 30 h. A declination trend in the TN concentration appeared until 25 h, and thereafter barely varied. Nitrite began to accumulate after 5 h due to nitrate reduction, and then the level did not decrease until 15 h. As of 40 h into the experiment, no ammonium-N was detected in the culture medium. The cell-biomass growth trend (determined by OD600) increased slightly at first, then rapidly, but the production was low (no more than 0.6). It had an initial lag phase at 10 h, then an exponential growth phase from 10 to 20 h; cell growth was not significant after 20 h.
Figure 3b depicts the variations in ammonium and nitrate concentrations when approximately 25 mg NO3ˉ-N/L and 25 mg NH4+-N/Lwere added to the DM. During an initial 10-h period, remarkable changes in the ammonia level were tracked, while the amount of nitrate was reduced only slightly. However, the ammonia-N and nitrate-N in the medium were nearly the same in the end, as correspondingly less nitrite accumulated in the medium as compared with nitrate as a sole nitrogen resource. Simultaneously, the OD600 values of the strain rose rapidly within 25 h, and the amounts of the various nitrogen forms began to plateau after 20 h.
When ammonium was used as the sole nitrogen source, the OD600 value of strain CW-2 increased significantly between 10 and 15 h (Fig. 3c). The growth characteristics of the strain were identical with what had occurred in the nitrate medium, but its ammoniumremoval ability was higher than what occurred in nitrate (Fig. 3a). The ammonium-N was almost consumed within 20 h, with only a trace amount left after 25 h. However, the TN-concentration trends differed significantly between the ammonium medium and the nitrate medium. The value of TN decreased during the first 5 h, followed by a slow rise, and then a slender declination from 10 to 20 h, when it finally achieved relative balance. Throughout the processes of the ammonium removal experiment, neither nitrate nor nitrite was detected. Strain CW-2 displayed high ammonium-removal potential, which was well correlated with its higher growth rates in the cultures.
3.4 Effects of carbon source, C/N ratio, and DO on denitrification by Pseudomonas CW-2The assays were also used to investigate the effects of several vital factors, namely carbon source, C/N ratio, pH and DO, on the aerobic denitrification process (Fig. 4).
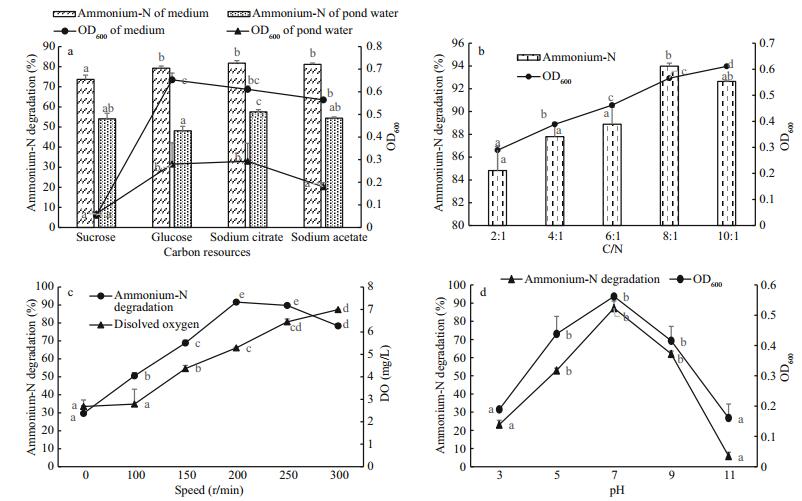
|
| Figure 4 Effects of several vital factors on ammonium removal by Pseudomonas CW-2 a. carbon sources; b. C/N ratio; c. DO; d. pH. |
The results depicted in Fig. 4a show the effect of carbon resources on ammonium-N removal and Pseudomonas CW-2 growth. Strain CW-2 absorbed sodium citrate, sodium acetate, and glucose better than did sucrose, with up to 80% removal when used in the DM medium. Citrate could well support either ammonium removal in the medium or pond water, with nitrogen removal rates over 80% and 58%, respectively. Sucrose and glucose were not beneficial to nitrogen removal in the medium and pond water, respectively, and resulted in relatively poor denitrification performance by strain CW-2.
Figure 4b shows the relationship between C/N and ammonium removal. The nitrogen-removal potential of strain CW-2 is affected not only by organic carbon species but also by their ratios to nitrogen (Guo et al., 2013). The removal rate rose with the rise of C/N initially, NH4+-N was completely consumed, and the removal rate peaked at C/N=8, amounting to 94%. But a slight decrease occurred thereafter, at C/N=10, and the conversion rate of ammonium-N appeared delayed before it leveled off. While the strain grew as the C/N ratio increased, the maximum absorbance of CW-2 was 6.2 when C/N=10.
The influences of two other factors, DO and pH, were also examined. Figure 4c describes the DO effect on the ammonium-N removal rate; the results showed that the removal rate increased with ascending values of dissolved oxygen until DO reached 5.29, and thereafter decreased gradually. The optimum DO concentration for ammonium removal was 5–6 mg/L; the otherwise high nitrogen removal rate would drop with increased DO after that point.
Figure 4d describes the relationship between pH and the ammonium-N removal rate; the results showed that denitrification by Pseudomonas CW-2 peaked at pH 7.0: the removal rate of ammonia increased with ascending pH values up to 7, and thereafter decreased gradually.
3.5 Ammonium-removal potential in largemouthbass pond waterThe aerobic denitrifier investigated was verified to have robust adaptability to remove nitrogen waste in synthetic pond water (Figs. 5 and 6). Given the ammonium-N concentration 150 mg/L, we recorded the transformation of ammonium-N and nitrite-N in the four treatments (-C–S, -C+S, +C–S and +C+S). Figure 5 reveals no definite change in the ammonium-N level in the control group. Comparatively, the -C+S group, NH4+-N decreased during the first 3 days and then accumulated slowly during the days thereafter. Whether looking at the +C–S or +C+S groups, the ammonium-removal phenomenon was detected immediately in the first day and lasted until the end of the trial. The trend in the nitrification intermediate NO2ˉ-N is shown in Fig. 6. NO2ˉ-N fluctuated unevenly in the control cultures; in the -C+S group, however, the concentration kept rising until the fourth day and then decreased during subsequent days. The nitrite-N accumulation rate was low and relatively stable both in the +C–S and +C+S culture solutions.

|
| Figure 5 Effects of strain CW-2 on ammonium concentration in synthetic pond water |
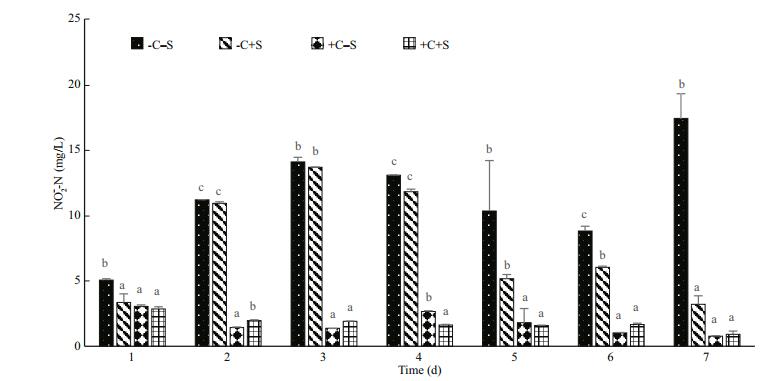
|
| Figure 6 Effects of strain CW-2 on nitrite concentration in synthetic pond water |
For a full understanding of the microbial community in a synthetic version of the largemouthbass pond water, one feasible and effective approach was to investigate the massive genetic information contained in the water. Ten different sequence groups were identified, and 29 libraries showed very dissimilar 16S rRNA profiles in the phylum-level distributions (Fig. 7). On the whole, SaCa (controlwater) libraries included the maximum number of phyla, where Proteobacteria, Bacteroidetes, Planctomycetes, and Actinobacteria were the most important groups and accounted for 87.21% of the reads. The SbCa (water with strain CW-2, and without carbon) library was numerically dominated by Proteobacteria and Bacteroidetes, and these phyla represented 82.04% of the reads. The SaCb (water with carbon, and without strain CW-2) and SbCb (water with carbon, and with strain CW-2) libraries showed relatively simple and similar diversity, and Proteobacteria and Bacteroidetes represented over 95% of the reads. Notably, SbCb4 and SbCb6 showed complex diversity and proportions which were analogous to the control group.
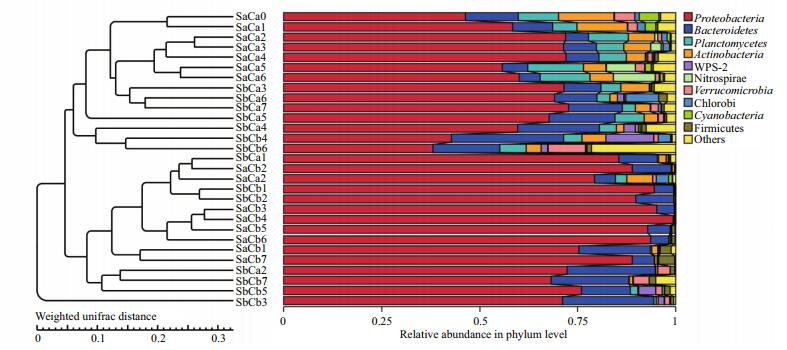
|
| Figure 7 The microbial community composition: relative reads of abundance of different bacterial phyla within the different communities Sequences that could not be classified into any known group were designated as "others". SaCa0 represents the initial control water; SaCa (1, 2, 3, 4, 5, 6 and 7) represents the control water at day 1, day 2, day 3, day 4, day 5, day 6 and day 7. SbCa (1, 2, 3, 4, 5, 6 and 7) represents the simulated pond water with strain CW-2 at day 1, day 2, day 3, day 4, day 5, day 6 and day 7; SaCb (1, 2, 3, 4, 5, 6 and 7) represents the simulated pond water with carbon at day 1, day 2, day 3, day 4, day 5, day 6 and day 7; SbCb (1, 2, 3, 4, 5, 6 and 7) represents the simulated pond water with strain CW-2 and carbon at day 1, day 2, day 3, day 4, day 5, day 6 and day 7. |
The biomass of some characteristic microorganisms were identified at the phylum level. The relative abundances of Proteobacteria, Bacteroidetes, Planctomycetes, and Nitrospirae continuously changed in relation to carbon and the addition of strain CW-2. Overall, carbon increased the relative abundance of Proteobacteria but decreased the relative abundance of Planctomycetes and Nitrospirae (at the phylum level). That is, the Proteobacteria phylum assumed a greater proportion in the +C–S and +C+S groups. Two other heterotrophic bacteria, Planctomycetes and Nitrospirae, were significantly more abundant in the control and -C+S groups than in the +C–S and +C+S treatment groups.
4 DISCUSSIONOur evaluation of the isolated Pseudomonas strain CW-2 applied to largemouth-bass culture ponds indicates its tremendous potential for ammonium-N removal, especially owing to its autochthonous characteristic, which allowed efficient nitrogen removal from synthetic pond water. The specific denitrification characteristics of strain CW-2, as follows, are likely to support its capacity for improving water quality under intensive culture conditions.
Several nitrogen reductase functional genes were closely related to the bacteria's denitrification properties. The nirK gene expression indicated that the strain could express copper nitrite reductase. The copper nitrite reductase expressed by the nirK gene can catalyze reduction reactions of NO2ˉ to NO (Robertson et al., 1988; van Niel et al., 1992). Another key denitrification gene of N2 generation was nosZ, and its existence in strain CW-2 indicates that it could effectively generate N2, making it applicable as an aerobic denitrifier to simultaneously achieve nitrification and denitrification via nitrite reduction processes.
Further analyses showed that bacterial-culture time had little effect on denitrification efficiency, as the demonstrated stability of the strain is considerably promising. According to the data on the transformation of nitrate and ammonium, the strain utilized both nitrate and ammonium, when nitrate was the sole nitrogen resource; the strain displayed a strong growth phenomenon, decreased the amounts of nitrate and total nitrogen, and increased the level of nitrite. Together these results indicate that strain CW-2 could play a role in denitrification, and part of the nitrate nitrogen was transformed into nitrogen gas. When ammonium was the only nitrogen resource, strain CW-2 mainly maintained its reproduction through the assimilation of ammonia nitrogen. Zhu et al. (2012a) reported similar phenomena in P. mendocina 3–7. The same as with the carbon source, ammonium was used as an alternative electron donor during denitrification, which might well explain this phenomenon. When nitrate and ammonia were the co-nitrogen resource, strain CW-2 prioritized ammonia assimilation over nitrate denitrification under aerobic conditions. Therefore, when the strain served as a solvation of reparation for nitrogen-rich water, the first action was to strengthen the nitrification process in the water (i.e., convert ammonia to nitrate as much as possible). Then the denitrification process occurred under low oxygen circumstances, or the strain mainly conducted ammonia assimilation rather than denitrification under the condition of sufficient oxygen, but which could ultimately weaken the effect of TN removability. For this reason, intermittent aeration could improve the denitrification effect.
Carbon usually serves as the energy and electron source for heterotrophic bacteria. In our study, ammonium removal and cell growth were shown to be significantly affected by carbon sources. Poorer growth and nitrification performance of the strain were observed in glucose and sucrose groups, implying that strain CW-2 has less inclination to the two carbons. When sodium acetate and sodium citrate were used as the sole carbon sources, strain CW-2 exhibited efficient ammonium removal ability and cell growth. Zhang et al. (2012) suggested that microorganisms perform aerobic denitrification via different mechanisms on different carbon sources. For example, the removal process with glucose included transformation into pyruvate first, which was then converted to ethanol under anaerobic conditions with the effect of acetyl coenzyme A, and stepped up by further oxidation to acetic acid, and finally entered into the tricarboxylic acid cycle to provide energy to bacteria afterwards (Xu, 1994). This complicated cascade reaction by glucose was less efficient than that of citrate, which could enter into the tricarboxylic acid cycle directly: consequently, sodium citrate was employed in the following research.
Different carbons correlate to different C/N ratios, and the optimal quantity of carbon (C/N) is a key parameter in the denitrification process (Patureau et al., 2000). Denitrification efficiency and the growth performance of denitrifying bacteria will decrease under extremely low or high carbon concentrations (Huang and Tseng, 2001). Therefore, it was important to optimize the C/N ratio for each denitrifier. In our study, we considered cost and ammonium removal performance, and found that C/N=8 would be appropriate for the strain's denitrification processes. The C/N ratio required by strain CW-2 was relatively close to that of other strains, as reported in previous studies (Joo et al., 2005; Taylor et al., 2009). The denitrification rate slowed down while the strain's growth was simultaneously elevated, at C/N=10, which might be due to the amounts of ammonium consumed, mainly for cell synthesis (intracellular N), at C/N=10. At higher C/N ratios, the nitrogen source was utilized by the denitrifier for both cell synthesis and the denitrification process to maintain a certain micro-ecosystem balance (Joo et al., 2005).
Dissolved oxygen (DO) concentration is another key factor in aerobic denitrification. In the present study, the denitrification rate by strain CW-2 increased with DO level: that is, it first climbed and then dropped; this tendency differed to the results reported by Patureau et al. (2000), yet was nearly identical with a report by Song et al. (2011). Hence, we supposed that strain CW-2 might tolerate high levels of DO, which was adapted to the aquaculture system. The DO level indicated that it would be suitable for polyculture with Ctenopharyngodon idellus, Hypophthalmichthys molitrix, Hypophthalmichthys nobilis, and Pelteobagrus fulvidraco in largemouth bass Micropterus salmoides ponds, since the DO content would not affect the activity of the aerobic denitrifier.
In our experiments, strain CW-2 showed a strong ability to adapt to variations in pH, between 5.0 and 9.0, indicating it is adapted to a slightly alkaline range. The ammonia nitrogen removal rate was approximately 90% at pH 7.0.
Denitrification of strain CW-2 in synthetic water from largemouth bass ponds would have more reference value for evaluation of its denitrification ability. The average removal rates of NH4+-N were high in the treatment groups, for the first 7 days (except for the control group), reaching 46.52%, 59.66% and 64.5%, in groups -C+S, +C–S and +C+S, respectively. This finding implied that either strain CW-2 or the exogenous carbon was effective for removing ammonium. But since carbon was obviously able to improve the efficiency of the strain, we recommend that the nitrogen removal rate is definitely improved if combining the strain with carbon.
More specifically, a significant and rapid consumption of NH4+-N caused a temporary accumulation of NO2ˉ-N on days 2 and 3, which might be the result of the strain entering a logarithmic growth phase. The entire denitrification process largely occurred in the log growth phase of the bacterium (Shao and Yu, 2008). As NH4+-N was being consumed, NO2ˉ-N was utilized as a preliminary intermediate, and then as the next electron acceptor, and finally further reduced to other intermediates.
NH4+-N was consumed completely, and the accumulation of NO2ˉ-N was low in the +C–S and +C+S solutions after incubation for 4 days, from day 4 to day 7. Zheng et al. (2012) demonstrated previously that during the entire growth phase, NH4+-N was mainly converted to biomass nitrogen and gaseous nitrogen.
Moreover, in current study, the carbon resource acted as a more positive force of longer duration than strain CW-2 exclusively used to remove ammonium-N. For strain CW-2, sodium citrate was more favorable; if induced it could easily provide energy and act as a rapid reduction force (Zheng et al., 2012). The other assumption of the phenomenon could be due to indigenous microorganisms that might be activated and propagated by additional carbon and a proper C/N ratio. Endogenous denitrification was reportedly highly efficient when appropriate carbon was supplied for inherent metabolism, in cases when an autochthonal bacterium is better adapted to the ambient circumstances than allochthonous microorganisms (Abufayed and Schroeder, 1986; Zheng et al., 2012).
The microbial community in the synthetic water was profiled in detail; the microbe diversities differed among the four water substrates, which implied that microorganisms in the synthetic pond water were affected by carbon or the bacterial strain during the observation period. The effective proliferation of some bacteria relevant to nitrogen removal was meaningful for nitrogen waste elimination. The relative abundance of Proteobacteria proliferated intensively, indicating that carbon or exogenous bacteria was most likely the dominant factor influencing these properties. Another explanation of elevated Proteobacteria in water with carbon or added strain is that proliferation was indirectly driven by increased abundance of the dominant Pseudomonas which belongs to the Proteobacteria phylum (Yao et al., 2014).
Propagation of the nitrite-oxidizing bacteria (NOB) Nitrospirae and ammonium-oxidizing bacteria (AOB) Planctomycetes were more abundant in the control group; the conventional nitrification reaction might also exist in natural largemouth-bass pond water. Traditional nitrification entails the oxidation of ammonia to nitrite and further to nitrate, by AOB and NOB, respectively; nitrate was thereafter reduced to molecular nitrogen by denitrifying bacteria (Zhao et al., 2015). In this study, these functional microorganisms typically involved in nitrogen denitrification were relatively predominant in the control pond water. Previous studies had indicated that many species of Planctomycetes possess anaerobic ammonium oxidizing (anammox) activity, which is responsible for the simultaneous removal of nitrite and ammonia under anaerobic conditions (Ye and Zhang, 2010; Ye et al., 2011). In the current study, the appearance of Planctomycetes in the dominant phyla demonstrated that the nitrogen removal pathway of anammox probably existed and was responsible for the removal of nitrogen in the control pond water. Less distribution of Planctomycetes and Nitrospirae in water containing carbon or strain CW-2 indicated that the traditional denitrification process might have been weakened in the +C–S and +C+S treatments. This means that carbon or the added CW-2 strain favored and modified the denitrification process, with pond production no longer requiring two separate systems for anaerobic denitrification and aerobic nitrification, which otherwise add cost and timeconsuming work.
In the current study, strain CW-2 was identified as Pseudomonas sp. according to 16S rRNA gene sequence analysis. Previous reports on Pseudomonas denitrifying bacteria mainly included three types: one was for a conventional mode of aerobic denitrification using NO3ˉ-N as the nitrogen resource (Takaya et al., 2003; Xiang et al., 2006; Kim et al., 2008; Miyahara et al., 2010; Zhu et al., 2012a; Wang et al., 2013); another method stated that NO2ˉ-N was directly removed through simultaneous processes of nitrification and denitrification (SND) (Song et al., 2011; Wan et al., 2011; Liang et al., 2014); and, the third reported method was heterotrophic nitrification and aerobic denitrification (HNAD), as mostly found in sewage treatment systems (Su et al., 2006; Zhang et al., 2011; Qiu et al., 2012; Zhu et al., 2012b; Guo et al., 2013). A broad view of the characteristics of aerobic denitrification by Pseudomonas CW-2 in the present study highlights its combined effect on NO3ˉ-N and NH4+-N, together with low levels of NO2ˉ-N accumulation and a high rate of NH4+-N removal, indicating that strain CW-2 might be a HNAD bacterium. The combination of the two activities (heterotrophic nitrification and aerobic denitrification) in one strain mean that direct transformation of ammonia nitrogen to gaseous nitrogen could be achieved; this conclusion merits deeper research to support it.
The present research results indicate that strain CW-2 has outstanding application potential for nitrogen removal in intensive aquaculture systems. First, due to its high specific growth rate and aerobic denitrification characteristics it could simultaneously remove both nitrate and ammonium efficiently. Second, high nitrogen content (50 mg/L) in the DM solution or in synthetic pond water could be well remediated by the isolated strain. Furthermore, the strain tolerated high levels dissolved oxygen, and equipped with the nosZ gene could transfer nitrous oxide to nitrogen gas, all characteristics which make it very adaptable to intensive fish culture environments.
5 CONCLUSIONThe current study evaluated the potential of a newly isolated indigenous aerobic denitrifier, identified as Pseudomonas strain CW-2, for removing ammonium under laboratory conditions and in synthetic water from largemouth bass culture ponds. The isolated strain exhibited heterotrophic nitrification/aerobic denitrification characteristics and showed strong cell growth and substantial nitrogenremoval capability. Meanwhile, as a denitrifier, CW-2 was verified to have robust adaptability to remove nitrogen waste in simulated pond water as its addition effectively improved the structure and abundance of denitrifying bacteria in ambient water. We believe that the excellent ability for nitrogen removal exhibited by strain CW-2 warrants its application as a favorable aerobic denitrifier for intensive aquaculture.
6 DATA AVAILABILITY STATEMENTAll data generated or analyzed during this study are included in the manuscript.
Abufayed A A, Schroeder E D. 1986. Kinetics and stoichiometry of SBR/denitrification with a primary sludge carbon source. J. Water Pollut. Control Fed., 58: 398-405. |
Carpenter J H. 1965. The Chesapeake Bay institute technique for the Winkler dissolved oxygen method. Limnology and Oceanography, 10(1): 141-143. DOI:10.4319/lo.1965.10.1.0141 |
Chen P Z, Li J, Li Q X, Wang Y C, Li S P, Ren T Z, Wang L G. 2012. Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24.Bioresource Technology, 116: 266-270. DOI:10.1016/j.biortech.2012.02.050 |
Garbeva P, Baggs E M, Prosser J I. 2007. Phylogeny of nitrite reductase (nirK) and nitric oxide reductase (norB) genes from Nitrosospira species isolated from soil. FEMS Microbiology Letters, 266(1): 83-89. DOI:10.1111/fml.2007.266.issue-1 |
GBT 7493-1987 Water quality-determination of nitrogen(nitrite)-spectrophotometric method. 1987. The State Environmental Protection Administration of China, p. 144-148.
|
Grguric G, Wetmore S S, Fournier R W. 2000. Biological denitrification in a closed seawater system. Chemosphere, 40(5): 549-555. |
Guo L Y, Chen Q K, Fang F, Hu Z X, Wu J, Miao A J, Xiao L, Chen X F, Yang L Y. 2013. Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. Bioresource Technology, 142: 45-51. DOI:10.1016/j.biortech.2013.05.021 |
Huang H K, Tseng S K. 2001. Nitrate reduction by Citrobacter diversusunder aerobic environment. AppliedMicrobiology& Biotechnology, 55(1): 90-94. |
Joo H S, Hirai M, Shoda M. 2005. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. Journal of Bioscience and Bioengineering, 100(2): 184-191. |
Kim M, Jeong S Y, Yoon S J, Cho S J, Kim Y H, Kim M J, Ryu E Y, Lee S J. 2008. Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. Journal of Bioscience and Bioengineering, 106(5): 498-502. |
Kolndadacha O D, Adikwu I A, Okaeme A N, Atiribom R Y, Mohammed A, Musa Y M. 2011. The role of probiotics in aquaculture in Nigeria-a review. Continental Journal of Fisheries and Aquatic Sciences, 5(1): 8-15. |
Kumar G S, Ramakrishnan A, Hung Y T. 2010. Simultaneous removal of carbon and nitrogen from domestic wastewater in an aerobic RBC. In: Wang L K, Tay J H, Tay S T L, Hung Y T eds. Environmental Bioengineering: Volume 11. Humana Press, Totowa, NJ, USA. p. 403-443.
|
Liang Y H, Li D, Zhang X J, Zeng H P, Yang Z, Zhang J. 2014. Microbial characteristics and nitrogen removal of simultaneous partial nitrification, anammox and denitrification (SNAD) process treating low C/N ratio sewage. Bioresource Technology, 169: 103-109. DOI:10.1016/j.biortech.2014.06.064 |
Lukow T, Diekmann H. 1997. Aerobic denitrification by a newly isolated heterotrophic bacterium strain TLl. Biotechnology Letters, 19(11): 1 157-1 159. DOI:10.1023/A:1018465232392 |
Michael E T, Amos S O, Hussaini L T. 2014. A review on probiotics application in aquaculture. Fisheries and Aquaculture Journal, 5(4): 111. |
Miyahara M, Kim S W, Fushinobu S, Takaki K, Yamada T, Watanabe A, Miyauchi K, Endo G, Wakagi T, Shoun H. 2010. Potential of aerobic denitrification by Pseudomonas stutzeri TR2 to reduce nitrous oxide emissions from wastewater treatment plants. Applied and Environmental Microbiology, 76(14): 4 619-4 625. DOI:10.1128/AEM.01983-09 |
Obaja D, Macé S, Mata-Alvarez J. 2005. Biological nutrient removal by a sequencing batch reactor (SBR) using an internal organic carbon source in digested piggery wastewater. Bioresource Technology, 96(1): 7-14. DOI:10.1016/j.biortech.2004.03.002 |
Pai S L, Chong N M, Chen C H. 1999. Potential applications of aerobic denitrifying bacteria as bioagents in wastewater treatment. Bioresource Technology, 68(2): 179-185. DOI:10.1016/S0960-8524(98)00140-0 |
Patureau D, Bernet N, Delgenès J P, Moletta R. 2000. Effect of dissolved oxygen and carbon-nitrogen loads on denitrification by an aerobic consortium. Applied Microbiology and Biotechnology, 54(4): 535-542. |
Qiu X F, Wang T W, Zhong X M, Du G C, Chen J. 2012. Screening and characterization of an aerobic nitrifying-denitrifying bacterium from activated sludge. Biotechnology and Bioprocess Engineering, 17(2): 353-360. DOI:10.1007/s12257-011-0467-y |
Robertson L A, van Niel E W J, Torremans R A M, Kuenen J G. 1988. Simultaneous nitrification and denitrification in aerobic chemostat cultures of Thiosphaera pantotropha. Applied and Environmental Microbiology, 54(11): 2 812-2 818. |
Robertson L A, Kuenen J G. 1984. Aerobic denitrification:a controversy revived. Archives of Microbiology, 139(4): 351-354. DOI:10.1007/BF00408378 |
Shao Q, Yu X B. 2008. Isolation and characterization of a strain denitrobacteria. Biotechnology, 18(3): 63-65. |
Šiljeg M, Foglar L, Kukučka M. 2010. The ground water ammonium sorption onto Croatian and Serbian clinoptilolite. Journal of Hazardous Materials, 178(1-3): 572-577. DOI:10.1016/j.jhazmat.2010.01.123 |
Skjermo J, Bakke I, Dahle S W, Vadstein O. 2015. Probiotic strains introduced through live feed and rearing water have low colonizing success in developing Atlantic cod larvae. Aquaculture, 438: 17-23. DOI:10.1016/j.aquaculture.2014.12.027 |
Song Z F, An J, Fu G H, Yang X L. 2011. Isolation and characterization of an aerobic denitrifying Bacillus sp. YX-6 from shrimp culture ponds. Aquaculture, 319(1-2): 188-193. |
Su J J, Yeh K S, Tseng P W. 2006. A strain of Pseudomonas sp. isolated from piggery wastewater treatment systems with heterotrophic nitrification capability in Taiwan. Current Microbiology, 53(1): 77-81. |
Takaya N, Catalan-Sakairi M A B, Sakaguchi Y, Kato I, Zhou Z M, Shoun H. 2003. Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Applied and Environmental Microbiology, 69(6): 3 152-3 157. DOI:10.1128/AEM.69.6.3152-3157.2003 |
Tang J Y, Dai Y X, Li Y M, Qin J G, Wang Y. 2016. Can application of commercial microbial products improve fish growth and water quality in freshwater polyculture?. North American Journal of Aquaculture, 78(2): 154-160. DOI:10.1080/15222055.2015.1116474 |
Taylor S M, He Y L, Zhao B, Huang J. 2009. Heterotrophic ammonium removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Providencia rettgeri YL. Journal of Environmental Sciences, 21(10): 1 336-1 341. DOI:10.1016/S1001-0742(08)62423-7 |
Throbäck I N, Enwall K, Jarvis A, Hallin S. 2004. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiology Ecology, 49(3): 401-417. DOI:10.1016/j.femsec.2004.04.011 |
van Niel E W J, Braber K J, Robertson L A, Kuene J G. 1992. Heterotrophic nitrification and aerobic denitrification in Alcaligenes faecalis strain TUD. Antonie van Leeuwenhoek, 62(3): 231-237. DOI:10.1007/BF00582584 |
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reviews, 64(4): 655-671. DOI:10.1128/MMBR.64.4.655-671.2000 |
Wan C L, Yang X, Lee D J, Du M A, Wan F, Chen C. 2011. Aerobic denitrification by novel isolated strain using NO2- -N as nitrogen source. Bioresource Technology, 102(15): 7 244-7 248. DOI:10.1016/j.biortech.2011.04.101 |
Wang P, Yuan Y Z, Li Q, Yang J Z, Zheng Y L, He M Q, Geng H, Xiong L, Liu D L. 2013. Isolation and immobilization of new aerobic denitrifying bacteria. International Biodeterioration & Biodegradation, 76: 12-17. |
Xiang M F, Wang P, Liu X T, Zhai Q. 2006. Selection and identification of aerobic denitrifiers in denitrification process of wastewater. Food Science and Technology, 31(7): 153-156. |
Xu Y T. 1994. The effect of different carbon sources on biological denitrification. Chinese Journal of Environmental Science, 15(2): 28-32, 44. |
Xu Z N, Lin X T, Lin Q, Yang Y F, Wang Y X. 2007. Nitrogen, phosphorus, and energy waste outputs of four marine cage-cultured fish fed with trash fish. Aquaculture, 263(1-4): 130-141. DOI:10.1016/j.aquaculture.2006.10.020 |
Yao M J, Rui J P, Li J B, Dai Y M, Bai Y F, Heděnec P, Wang J M, Zhang S H, Pei K Q, Liu C, Wang Y F, He Z L, Frouz J, Li X Z. 2014. Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe. Soil Biology and Biochemistry, 79: 81-90. DOI:10.1016/j.soilbio.2014.09.009 |
Ye L, Shao M F, Zhang T, Tong A H Y, Lok S. 2011. Analysis of the bacterial community in a laboratory-scale nitrification reactor and a wastewater treatment plant by 454-pyrosequencing. Water Research, 45(15): 4 390-4 398. DOI:10.1016/j.watres.2011.05.028 |
Ye L, Zhang T. 2010. Estimation of nitrifier abundances in a partial nitrification reactor treating ammonium-rich saline wastewater using DGGE, T-RFLP and mathematical modeling. Applied Microbiology and Biotechnology, 88(6): 1 403-1 412. DOI:10.1007/s00253-010-2837-3 |
Zhang J B, Wu P X, Hao B, Yu Z N. 2011. Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresource Technology, 102(21): 9 866-9 869. DOI:10.1016/j.biortech.2011.07.118 |
Zhang Q L, Liu Y, Ai G M, Miao L L, Zheng H Y, Liu Z P. 2012. The characteristics of a novel heterotrophic nitrification-aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresource Technology, 108: 35-44. DOI:10.1016/j.biortech.2011.12.139 |
Zhao Y G, Fang Y, Jin Y L, Huang J, Ma X R, He K Z, He Z M, Wang F, Zhao H. 2015. Microbial community and removal of nitrogen via the addition of a carrier in a pilotscale duckweed-based wastewater treatment system. Bioresource Technology, 179: 549-558. DOI:10.1016/j.biortech.2014.12.037 |
Zheng H Y, Liu Y, Gao X Y, Ai G M, Miao L L, Liu Z P. 2012. Characterization of a marine origin aerobic nitrifyingdenitrifying bacterium. Journal of Bioscience and Bioengineering, 114(1): 33-37. DOI:10.1016/j.jbiosc.2012.02.025 |
Zhu L, Ding W, Feng L J, Dai X, Xu X Y. 2012a. Characteristics of an aerobic denitrifier that utilizes ammonium and nitrate simultaneously under the oligotrophic niche. Environmental Science and Pollution Research, 19(8): 3 185-3 191. DOI:10.1007/s11356-012-0822-3 |
Zhu L, Ding W, Feng L J, Kong Y, Xu J, Xu X Y. 2012b. Isolation of aerobic denitrifiers and characterization for their potential application in the bioremediation of oligotrophic ecosystem. Bioresource Technology, 108: 1-7. DOI:10.1016/j.biortech.2011.12.033 |
| Abufayed A A, Schroeder E D, 1986. Kinetics and stoichiometry of SBR/denitrification with a primary sludge carbon source. J. Water Pollut. Control Fed., 58: 398–405. |
| Carpenter J H, 1965. The Chesapeake Bay institute technique for the Winkler dissolved oxygen method. Limnology and Oceanography, 10(1): 141–143. Doi: 10.4319/lo.1965.10.1.0141 |
| Chen P Z, Li J, Li Q X, Wang Y C, Li S P, Ren T Z, Wang L G, 2012. Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24.Bioresource Technology, 116: 266–270. Doi: 10.1016/j.biortech.2012.02.050 |
| Garbeva P, Baggs E M, Prosser J I, 2007. Phylogeny of nitrite reductase (nirK) and nitric oxide reductase (norB) genes from Nitrosospira species isolated from soil. FEMS Microbiology Letters, 266(1): 83–89. Doi: 10.1111/fml.2007.266.issue-1 |
| GBT 7493-1987 Water quality-determination of nitrogen(nitrite)-spectrophotometric method. 1987. The State Environmental Protection Administration of China, p. 144-148. |
| Grguric G, Wetmore S S, Fournier R W, 2000. Biological denitrification in a closed seawater system. Chemosphere, 40(5): 549–555. |
| Guo L Y, Chen Q K, Fang F, Hu Z X, Wu J, Miao A J, Xiao L, Chen X F, Yang L Y, 2013. Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. Bioresource Technology, 142: 45–51. Doi: 10.1016/j.biortech.2013.05.021 |
| Huang H K, Tseng S K, 2001. Nitrate reduction by Citrobacter diversusunder aerobic environment. AppliedMicrobiology& Biotechnology, 55(1): 90–94. |
| Joo H S, Hirai M, Shoda M, 2005. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. Journal of Bioscience and Bioengineering, 100(2): 184–191. |
| Kim M, Jeong S Y, Yoon S J, Cho S J, Kim Y H, Kim M J, Ryu E Y, Lee S J, 2008. Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. Journal of Bioscience and Bioengineering, 106(5): 498–502. |
| Kolndadacha O D, Adikwu I A, Okaeme A N, Atiribom R Y, Mohammed A, Musa Y M, 2011. The role of probiotics in aquaculture in Nigeria-a review. Continental Journal of Fisheries and Aquatic Sciences, 5(1): 8–15. |
| Kumar G S, Ramakrishnan A, Hung Y T. 2010. Simultaneous removal of carbon and nitrogen from domestic wastewater in an aerobic RBC. In: Wang L K, Tay J H, Tay S T L, Hung Y T eds. Environmental Bioengineering: Volume 11. Humana Press, Totowa, NJ, USA. p. 403-443. |
| Liang Y H, Li D, Zhang X J, Zeng H P, Yang Z, Zhang J, 2014. Microbial characteristics and nitrogen removal of simultaneous partial nitrification, anammox and denitrification (SNAD) process treating low C/N ratio sewage. Bioresource Technology, 169: 103–109. Doi: 10.1016/j.biortech.2014.06.064 |
| Lukow T, Diekmann H, 1997. Aerobic denitrification by a newly isolated heterotrophic bacterium strain TLl. Biotechnology Letters, 19(11): 1 157–1 159. Doi: 10.1023/A:1018465232392 |
| Michael E T, Amos S O, Hussaini L T, 2014. A review on probiotics application in aquaculture. Fisheries and Aquaculture Journal, 5(4): 111. |
| Miyahara M, Kim S W, Fushinobu S, Takaki K, Yamada T, Watanabe A, Miyauchi K, Endo G, Wakagi T, Shoun H, 2010. Potential of aerobic denitrification by Pseudomonas stutzeri TR2 to reduce nitrous oxide emissions from wastewater treatment plants. Applied and Environmental Microbiology, 76(14): 4 619–4 625. Doi: 10.1128/AEM.01983-09 |
| Obaja D, Macé S, Mata-Alvarez J, 2005. Biological nutrient removal by a sequencing batch reactor (SBR) using an internal organic carbon source in digested piggery wastewater. Bioresource Technology, 96(1): 7–14. Doi: 10.1016/j.biortech.2004.03.002 |
| Pai S L, Chong N M, Chen C H, 1999. Potential applications of aerobic denitrifying bacteria as bioagents in wastewater treatment. Bioresource Technology, 68(2): 179–185. Doi: 10.1016/S0960-8524(98)00140-0 |
| Patureau D, Bernet N, Delgenès J P, Moletta R, 2000. Effect of dissolved oxygen and carbon-nitrogen loads on denitrification by an aerobic consortium. Applied Microbiology and Biotechnology, 54(4): 535–542. |
| Qiu X F, Wang T W, Zhong X M, Du G C, Chen J, 2012. Screening and characterization of an aerobic nitrifying-denitrifying bacterium from activated sludge. Biotechnology and Bioprocess Engineering, 17(2): 353–360. Doi: 10.1007/s12257-011-0467-y |
| Robertson L A, van Niel E W J, Torremans R A M, Kuenen J G, 1988. Simultaneous nitrification and denitrification in aerobic chemostat cultures of Thiosphaera pantotropha. Applied and Environmental Microbiology, 54(11): 2 812–2 818. |
| Robertson L A, Kuenen J G, 1984. Aerobic denitrification:a controversy revived. Archives of Microbiology, 139(4): 351–354. Doi: 10.1007/BF00408378 |
| Shao Q, Yu X B, 2008. Isolation and characterization of a strain denitrobacteria. Biotechnology, 18(3): 63–65. |
| Šiljeg M, Foglar L, Kukučka M, 2010. The ground water ammonium sorption onto Croatian and Serbian clinoptilolite. Journal of Hazardous Materials, 178(1-3): 572–577. Doi: 10.1016/j.jhazmat.2010.01.123 |
| Skjermo J, Bakke I, Dahle S W, Vadstein O, 2015. Probiotic strains introduced through live feed and rearing water have low colonizing success in developing Atlantic cod larvae. Aquaculture, 438: 17–23. Doi: 10.1016/j.aquaculture.2014.12.027 |
| Song Z F, An J, Fu G H, Yang X L, 2011. Isolation and characterization of an aerobic denitrifying Bacillus sp. YX-6 from shrimp culture ponds. Aquaculture, 319(1-2): 188–193. |
| Su J J, Yeh K S, Tseng P W, 2006. A strain of Pseudomonas sp. isolated from piggery wastewater treatment systems with heterotrophic nitrification capability in Taiwan. Current Microbiology, 53(1): 77–81. |
| Takaya N, Catalan-Sakairi M A B, Sakaguchi Y, Kato I, Zhou Z M, Shoun H, 2003. Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Applied and Environmental Microbiology, 69(6): 3 152–3 157. Doi: 10.1128/AEM.69.6.3152-3157.2003 |
| Tang J Y, Dai Y X, Li Y M, Qin J G, Wang Y, 2016. Can application of commercial microbial products improve fish growth and water quality in freshwater polyculture?. North American Journal of Aquaculture, 78(2): 154–160. Doi: 10.1080/15222055.2015.1116474 |
| Taylor S M, He Y L, Zhao B, Huang J, 2009. Heterotrophic ammonium removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Providencia rettgeri YL. Journal of Environmental Sciences, 21(10): 1 336–1 341. Doi: 10.1016/S1001-0742(08)62423-7 |
| Throbäck I N, Enwall K, Jarvis A, Hallin S, 2004. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiology Ecology, 49(3): 401–417. Doi: 10.1016/j.femsec.2004.04.011 |
| van Niel E W J, Braber K J, Robertson L A, Kuene J G, 1992. Heterotrophic nitrification and aerobic denitrification in Alcaligenes faecalis strain TUD. Antonie van Leeuwenhoek, 62(3): 231–237. Doi: 10.1007/BF00582584 |
| Verschuere L, Rombaut G, Sorgeloos P, Verstraete W, 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reviews, 64(4): 655–671. Doi: 10.1128/MMBR.64.4.655-671.2000 |
| Wan C L, Yang X, Lee D J, Du M A, Wan F, Chen C, 2011. Aerobic denitrification by novel isolated strain using NO2- -N as nitrogen source. Bioresource Technology, 102(15): 7 244–7 248. Doi: 10.1016/j.biortech.2011.04.101 |
| Wang P, Yuan Y Z, Li Q, Yang J Z, Zheng Y L, He M Q, Geng H, Xiong L, Liu D L, 2013. Isolation and immobilization of new aerobic denitrifying bacteria. International Biodeterioration & Biodegradation, 76: 12–17. |
| Xiang M F, Wang P, Liu X T, Zhai Q, 2006. Selection and identification of aerobic denitrifiers in denitrification process of wastewater. Food Science and Technology, 31(7): 153–156. |
| Xu Y T, 1994. The effect of different carbon sources on biological denitrification. Chinese Journal of Environmental Science, 15(2): 28–32, 44. |
| Xu Z N, Lin X T, Lin Q, Yang Y F, Wang Y X, 2007. Nitrogen, phosphorus, and energy waste outputs of four marine cage-cultured fish fed with trash fish. Aquaculture, 263(1-4): 130–141. Doi: 10.1016/j.aquaculture.2006.10.020 |
| Yao M J, Rui J P, Li J B, Dai Y M, Bai Y F, Heděnec P, Wang J M, Zhang S H, Pei K Q, Liu C, Wang Y F, He Z L, Frouz J, Li X Z, 2014. Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe. Soil Biology and Biochemistry, 79: 81–90. Doi: 10.1016/j.soilbio.2014.09.009 |
| Ye L, Shao M F, Zhang T, Tong A H Y, Lok S, 2011. Analysis of the bacterial community in a laboratory-scale nitrification reactor and a wastewater treatment plant by 454-pyrosequencing. Water Research, 45(15): 4 390–4 398. Doi: 10.1016/j.watres.2011.05.028 |
| Ye L, Zhang T, 2010. Estimation of nitrifier abundances in a partial nitrification reactor treating ammonium-rich saline wastewater using DGGE, T-RFLP and mathematical modeling. Applied Microbiology and Biotechnology, 88(6): 1 403–1 412. Doi: 10.1007/s00253-010-2837-3 |
| Zhang J B, Wu P X, Hao B, Yu Z N, 2011. Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresource Technology, 102(21): 9 866–9 869. Doi: 10.1016/j.biortech.2011.07.118 |
| Zhang Q L, Liu Y, Ai G M, Miao L L, Zheng H Y, Liu Z P, 2012. The characteristics of a novel heterotrophic nitrification-aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresource Technology, 108: 35–44. Doi: 10.1016/j.biortech.2011.12.139 |
| Zhao Y G, Fang Y, Jin Y L, Huang J, Ma X R, He K Z, He Z M, Wang F, Zhao H, 2015. Microbial community and removal of nitrogen via the addition of a carrier in a pilotscale duckweed-based wastewater treatment system. Bioresource Technology, 179: 549–558. Doi: 10.1016/j.biortech.2014.12.037 |
| Zheng H Y, Liu Y, Gao X Y, Ai G M, Miao L L, Liu Z P, 2012. Characterization of a marine origin aerobic nitrifyingdenitrifying bacterium. Journal of Bioscience and Bioengineering, 114(1): 33–37. Doi: 10.1016/j.jbiosc.2012.02.025 |
| Zhu L, Ding W, Feng L J, Dai X, Xu X Y, 2012a. Characteristics of an aerobic denitrifier that utilizes ammonium and nitrate simultaneously under the oligotrophic niche. Environmental Science and Pollution Research, 19(8): 3 185–3 191. Doi: 10.1007/s11356-012-0822-3 |
| Zhu L, Ding W, Feng L J, Kong Y, Xu J, Xu X Y, 2012b. Isolation of aerobic denitrifiers and characterization for their potential application in the bioremediation of oligotrophic ecosystem. Bioresource Technology, 108: 1–7. Doi: 10.1016/j.biortech.2011.12.033 |
 2018, Vol. 36
2018, Vol. 36