Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Xiaolin(陈晓琳), SUN Yuhao(孙雨豪), HU Linfeng(胡林峰), LIU Song(刘松), YU Huahua(于华华), XING Rong'e(邢荣娥), LI Rongfeng(李荣锋), WANG Xueqin(王雪芹), LI Pengcheng(李鹏程)
- In vitro prebiotic effects of seaweed polysaccharides
- Chinese Journal of Oceanology and Limnology, 36(3): 926-932
- http://dx.doi.org/10.1007/s00343-018-6330-7
Article History
- Received Dec. 22, 2016
- accepted in principle Apr. 6, 2017
- accepted for publication May. 3, 2017
2 School of Chemistry & Chemical Engineering, Henan Institute of Science and Technology, Xinxiang 453003, China
The human intestinal microbial ecosystem is responsible for various processes affecting host health, such as energy allocation of indigestible compounds, stimulation of the gut immune system, and synthesis of essential vitamins (Turnbaugh et al., 2007; Leser and Mølbak, 2009; Possemier et al., 2009; Marzorati et al., 2010). Some studies have focused on what is referred to as gastrointestinal resource management, which is the management of complex gut microbiota and metabolism to improve host health (Cummings and Macfarlane, 1991; Macfarlane and Macfarlane, 1997; Manning and Gibson, 2004). To this end, a common practice is to introduce beneficial exogenous materials such as probiotics, bifidobacteria, and lactobacilli, or prebiotics. Probiotics are often used as live microbial dietary additives. They are believed to confer health advantages and have a long history of use in humans and animals (Tzortzis et al., 2005; Wang et al., 2006). Prebiotics are non-digestible food ingredients that benefit the host by selectively stimulating the growth and activity of one or a limited number of bacterial species, usually bifidobacteria and lactobacilli in the colon, thereby improving host health (Gibson and Roberfroid, 1995; Hu et al., 2006). Prebiotics have attracted attention owing to their high bioactivity and low toxicity. Prebiotics that are currently used in the functional food industry include oligosaccharides e.g., lactulose, fructo-oligosaccharides, inulin, galactooligosaccharides, and arabinoxylan-oligosaccharides (Manning and Gibson, 2004; Sanchez et al., 2009).
Oligosaccharides extracted from seaweeds, including alginate and agar, have been assessed for their potential as prebiotics. Studies have shown that oligosaccharides isolated from seaweed exhibited greater prebiotic activity than that of fructooligosaccharides (Wang et al., 2006; Sanchez et al., 2009). In addition, some studies have indicated that seaweed polysaccharides have various structure-dependent functional properties; they can act as anticoagulants (Qi et al., 2012), antioxidants (Cho et al., 2011), and immunomodulators (Wang et al., 2009) and have antitumor (Jiao et al., 2009) and antiviral (Mori et al., 2012) activities. However, few reports are available on the prebiotic effects of seaweed polysaccharides and their mechanism in vitro. On the other hand, seaweed has prominent popular uses in various places worldwide. Marine algae are a traditional part of the oriental diet in the Asian Pacific region, while applications in western countries include pharmaceutical, cosmetic, and the food industries for their extracts (Gómez-Ordóñez et al., 2010; Wijesinghe and Jeon, 2012). Polysaccharides in seaweeds are economically important and potentially global industries. Therefore, in this study, we selected four representative seaweed polysaccharides, systematically studied their prebiotic properties in vitro, and assessed the effect of polysaccharides obtained from marine algae on both beneficial and harmful bacteria. In addition, we inferred the functional mechanism of the four polysaccharides on both beneficial and harmful bacteria.
2 MATERIAL AND METHOD 2.1 Materials and bacteriaGrateloupia filicina and Ulva pertusa were sampled from Jiaozhou Bay, Qingdao, China. Eucheuma spinosum and Ascophyllum nodosum were supplied by Qingdao Jiashidi Seaweed Co., Ltd. (Shandong, China). The algae samples were washed, air-dried, and stored in plastic bags at room temperature before use. Dialysis membranes (3 600 Da) were produced by Spectrum Co. and were used as the cutoff. Beneficial bacteria were purchased from Ran Biological Technology Co. Ltd. (Hebei, China) and primarily included bifidobacteria and lactobacilli. Man-Rogosa-Sharp (MRS) medium was purchased from Qingdao Nussui Biotechnology Co. Ltd. (Shandong, China) Mueller-Hinton broth (MHB) medium was purchased from Qingdao Hope BiolTechnology Co. Ltd. (Shandong, China). All reagents were of analytical grade.
2.2 Preparation of polysaccharides from seaweedDried U. pertusa and A. nodosum (100 g each) were cut and autoclaved in 4-L distilled water at 125℃ for 2 h. Dried E. spinosum (100 g each) was cut into pieces and reacted for 4 h in 4-L distilled water at 90℃ (Du et al., 2012). Dried G. filicina (100 g each) was cut and reacted for 4 h in 5-L distilled water at 100℃ (Yu et al., 2012). Then, these hot aqueous solutions were separated by successive filtration with gauze and siliceous earth and dialyzed with tap water and distilled water for 48 h separately using 3 600 Da Mw cutoff dialysis membranes. At last, the solutions were concentrated to approximately 500 mL under reduced pressure. A 1 500-mL aliquot of 95% (v/v) ethanol was added to precipitate the polysaccharides. The resulting precipitate was washed three times with 100% ethanol and lyophilized for further chemical analysis.
2.3 Chemical analysisTotal sugar content was analyzed by the phenolsulfuric acid method using galactose, rhamnose, and fucose as standards for G. filicina (GFP), E. spinosum (ESP), U. pertusa (UPP), and A. nodosum (ANP) (Dubois et al., 1956). Sulfate content was measured by the barium chloride gelatin method (Kawai et al., 1969). The molar ratios of the monosaccharides were determined with reference to Zhang et al. (2009). Briefly, polysaccharides were hydrolyzed in trifluoroacetic acid, followed by neutralization with sodium hydroxide. Then, pre-column derivatization with 3-methyl-1-phenyl-2-pyrazolin-5-one (PMP; 99%) was carried out to neutralize the mixture, and the solutions were separated by high performance liquid chromatography on an YMC Pack ODS AQ column (4.6 mm×250 mm) (Yan et al., 2013). The standards for the monosaccharide composition analysis and PMP were obtained from Sigma Aldrich (St. Louis, MO, USA). A high performance gel permeation chromatography method was used to determine the molecular weights of the samples against a dextran standard (Hou et al., 2012).
2.4 Culture of bacteria and growth measurementsCarbohydrate-free MRS medium was used as the base growth medium to study the ability of beneficial bacteria (mainly bifidobacteria and lactobacilli) to utilize the four polysaccharides. The polysaccharides were sterilized and added to the sterilized medium to reach final concentrations of 0.1%, 0.2%, 0.3%, 0.4%, and 0.5% (w/v). Beneficial bacteria (100 μL, OD620 0.13) were cultured overnight and were used to inoculate 100 μL of the sterilized medium supplemented with polysaccharides on 96-well plates. The plates were filled with nitrogen, sealed with Parafilm, and cultured anaerobically at 37℃ for 72 h. The absorbance of the culture medium at 620 nm was determined to assess bacterial growth. A 100-μL overnight culture of each species of beneficial bacteria was used as a control to inoculate 100-μL of sterilized medium.
Similarly, carbohydrate-free MHB medium was used as the base growth medium to study the inhibitory ability of the seaweed polysaccharide extracts on Staphylococcus aureus subsp. aureus and Escherichia coli. The polysaccharides were sterilized and added to the sterilized medium to yield final concentrations of 0.031 25%, 0.062 5%, 0.125%, 0.25%, 0.5%, and 1% (w/v). A 100-μL overnight culture of each bacterial species was used to inoculate 100-μL sterilized medium supplemented with polysaccharides on 96- well plates. The bacteria were cultured anaerobically at 37℃ for 20 h, and absorbance of the culture medium was determined at 630 nm to study bacterial growth. Bacteria (100 μL) cultured overnight in 100 μL of sterilized MHB medium served as the control. All measurements were performed in triplicate.
2.5 Statistical analysisData are presented as mean±SD. The statistical analysis was performed using SPSS ver. 16.0 software (SPSS Inc., Chicago, IL, USA). Differences between means were considered statistically significant if P < 0.05.
3 RESULT 3.1 Chemical analysis of the four sulfated polysaccharidesThe polysaccharides extracted from the four seaweeds were examined for total sugar content, sulfate content, monosaccharide composition, and molecular weight, as shown in Table 1.
Total sugar and sulfate contents differed among the four seaweeds. ESP had the highest total sugar (62.11%) and sulfate contents (21.35%). Furthermore, individual variations were observed in monosaccharide composition. The most abundant components of GFP, ESP, UPP, and ANP were galactose, galactose, rhamnose, and fucose, respectively, with large variations in chemical composition of the four polysaccharides, from which different functional properties may have resulted. In addition, mean molecular weights of the polysaccharides from GFP, ESP, UPP, and ANP were 216, 272, 159, and 57.5 kDa, respectively.
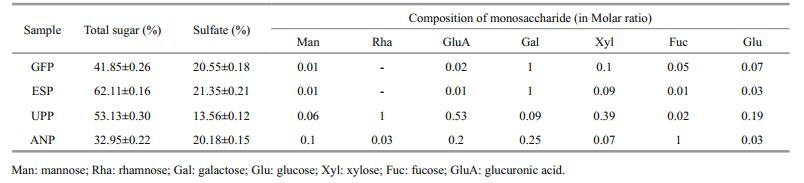
3.2 Effects of the four seaweed polysaccharide extracts on growth of beneficial bacteria in vitroFigure 1 shows the effect of GFP concentration on growth of beneficial bacteria. GFP promoted the growth of beneficial bacteria at all concentrations (0.1%–0.5%) compared with the control. The beneficial bacteria grew well in all polysaccharide groups 6 h after inoculation. As the polysaccharide concentration increased, growth improved. However, growth did not differ markedly between the 0.4% and 0.5% groups. A growth plateau appeared in all concentration groups after 48 h. Overall, the growth of beneficial bacteria in the 0.4% GFP concentration group was better than the other groups.
|
| Figure 1 Effect of GFP concentration on growth of beneficial bacteria |
Similarly, ESP concentration in the culture medium also affected growth of the beneficial bacteria (Fig. 2). Among all groups, the 0.10% polysaccharide concentration resulted in the largest increase in growth of beneficial bacteria, although the growth plateau appeared to be behind the other groups. Beneficial bacteria proliferated substantially in all ESP groups 6 h after inoculation, and grew faster than that of the control, which indicated that it had the prebiotic activity of the polysaccharides. In addition, ESP exhibited stronger prebiotic activity than that of GFP, which was probably due to the higher total sugar and sulfate content in ESP.

|
| Figure 2 Effect of ESP concentration on growth of beneficial bacteria |
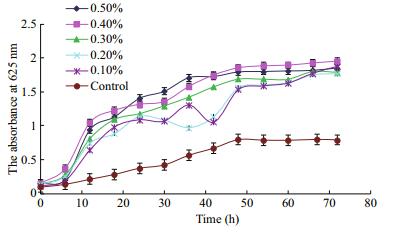
Figure 3 depicts the opposite trend to those shown in Figs. 1 and 2. Unlike GFP and ESP, UPP did not promote growth but inhibited beneficial bacteria in all UPP groups. Beneficial bacteria in the control began to grow rapidly 6 h after inoculation and reached a growth plateau 48 h after inoculation (Fig. 3). In contrast, growth in the UPP groups was inhibited during a 72-h culture.

|
| Figure 3 Effect of UPP concentration on growth of beneficial bacteria |
As shown in Fig. 4, the 0.4% polysaccharide concentration exhibited similar trends to that of the control; however, all other concentration groups inhibited the growth of beneficial bacteria, which differed from results reported by Dierick et al. (2009) who postulated that polysaccharide extracts from brown algae improve gut health of pigs. This difference could also be explained by the in vitro vs. in vivo nature of the experiments. When whole brown algae or extracted polysaccharides are added to feed, they boost the animal's immune response to improve the gut health; however, this effect may not work in vitro.
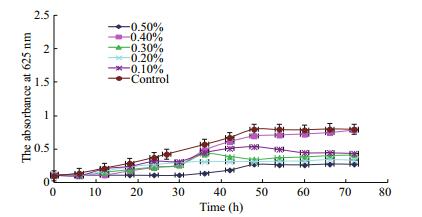
|
| Figure 4 Effect of ANP concentration on growth of beneficial bacteria |
Figure 5 showed the antibacterial activity of penicillin, GFP, ESP, UPP, and ANP on S. aureus subsp. aureus after a 20-h culture. Penicillin at a concentration of 0.031 25% significantly inhibited growth of S. aureus subsp. aureus and was chosen as the positive control. However, GFP, ESP, UPP, and ANP did not exhibit any antibacterial activity. Instead, they significantly promoted the growth of S. aureus subsp. aureus, particularly UPP at a low concentration of 0.031 5%. In addition, no significant increase in absorbance was observed regardless of UPP concentration ranging from 0.031 25% to 1.000 00%. However, absorbance at 625 nm increased significantly in the GFP, ESP, and ANP groups as concentrations increased from 0.031 25% to 1.000 00%.
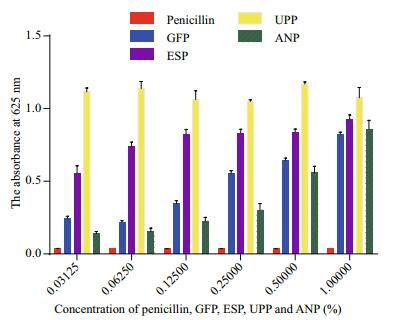
|
| Figure 5 Antibacterial activity of penicillin, Grateloupia filicina (GFP), Eucheuma spinosum (ESP), Ulva pertusa (UPP), and Ascophyllum nodosum (ANP) on Staphylococcus aureus subsp. aureus growth during a 20-h culture |
It is well known that penicillin inhibits growth of E. coli; thus, we used it as the positive control (Fig. 6). GFP, ESP, UPP, and ANP markedly promoted growth of E. coli 20 h after inoculation, and the effect of UPP was the strongest. Absorbance at 625 nm was weaker when UPP concentration was > 0.500 00%, than that at 1.000 0%. Absorbance increased with increasing concentration for the others.
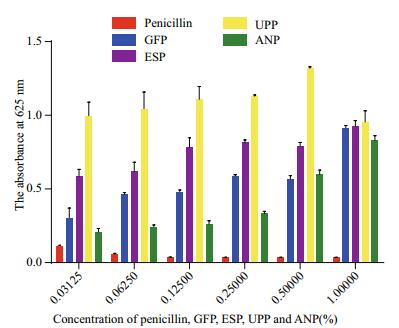
|
| Figure 6 Antibacterial activity of penicillin, Grateloupia filicina (GFP), Eucheumas spinosum (ESP), Ulva pertusa (UPP), and Ascophyllum nodosum (ANP) on Escherichia coli 20 h after inoculation |
Non-digestible oligosaccharides, such as lactulose, fructo-oligosaccharide, inulin, galactooligosaccharide, and arabinoxylan-oligosaccharide can benefit host health by selectively stimulating the growth and activity of health-promoting bacteria (Zaporozhets et al., 2014). Most oligosaccharides currently used as prebiotics include raffinose and soybean oligosaccharides, and they are extracted from natural sources through enzymatic hydrolysis of polysaccharides (Depeint et al., 2008). Because of the high price of enzymes used in the synthesis, oligosaccharide prebiotics are often expensive. If polysaccharides could be directly utilized as prebiotics, the price would be dramatically reduced.
In recent years, seaweeds have attracted attention as a source of bioactive compounds for the development of new drugs and health foods (Jiao et al., 2011). Sulfated polysaccharides from seaweeds are known to exhibit many biological and physiological activities, including anticoagulant, antiviral, antitumor, antiinflammatory, and antioxidant effects (Cumashi et al., 2007; Almeida-Lima et al., 2010; Costa et al., 2010). However, few studies have correlated seaweed polysaccharides to a prebiotic effect. One study (Silva et al., 2012) reported that a sulfated-polysaccharide fraction from the seaweed Gracilaria birdiae prevents naproxen-induced gastrointestinal damage in rats after determining glutathione and malondialdehyde concentrations as well as myeloperoxidase activity, but the study did not refer to variations in intestinal flora. In this study, we determined the effect of four seaweed polysaccharides from GFP, ESP, ANP, and UPP on proliferation of beneficial bacteria. The results demonstrated that polysaccharides extracted from GFP and ESP exhibited the strongest prebiotic effects in vitro. The effect of ESP was better than that of GFP because 0.1% ESP achieved the same result as that of 0.4% GFP after a 72-h culture of beneficial bacteria. In addition, the proliferation of beneficial bacteria was significantly affected by the concentration of polysaccharides, particularly during the first 30 h. Higher concentrations led to rapid proliferation of beneficial bacteria in response to ESP but no significant difference was observed with GFP. After the 72 h culture, 0.4% GFP and 0.1% ESP obtained the best results. Generally speaking, these two polysaccharides are potential sources of prebiotics. However, polysaccharides obtained from UPP and ANP did not exhibit a prebiotic effect; instead, they inhibited the growth of beneficial bacteria. This may have been due to the differences in monosaccharide composition of these polysaccharides. Some enzymes produced by a particular species of bacteria may have hydrolyzed the polysaccharides to oligosaccharides, which, in turn, may act as prebiotics. Indeed, the primary monosaccharide constituents of GFP and ESP was galactose, which forms galacto-oligosaccharides with prebiotic effects demonstrated previously (Kim et al., 2010; Vulevic et al., 2013, 2015). However, the main monosaccharide constituents of UPP and ANP were rhamnose and fucose, respectively but the oligosaccharides formed by rhamnose and fucose cannot be easily utilized by beneficial bacteria. At present, no prebiotic effects have been reported for them.
Prebiotics promote the growth of beneficial bacteria. On the other hand, they can inhibit the growth of harmful bacteria such as S. aureus subsp. aureus and E. coli. In this study, we examined the effect of polysaccharides from four seaweeds on the growth of these two harmful bacteria. Contrary to our expectation, the four polysaccharides did not exhibit strong antibacterial activity but somehow promoted the growth of harmful bacteria. This may have been due to the D-galactose, rhamnose, and fucose which may have been utilized as carbon sources by the harmful bacteria (Boronat and Aguilar, 1981; Qi et al., 2012). Sweeney et al. (2011) reported that a diet containing fucoidan increases the number of lactobacilli in the caecum of weaned pigs, and also increases shedding of fecal Salmonella Typhimurium at selected sampling periods in an experimental study. However, the mechanism was not clear. Therefore, future in vivo studies are required to determine the prebiotic effects of the four polysaccharides and understand the mechanisms of the different prebiotic behavior in these polysaccharides, as is the case for alginate oligosaccharides and neoagarooligosaccharides (Hu et al., 2006; Wang et al., 2006).
5 CONCLUSIONWe found that GFP and ESP significantly promoted proliferation of bifidobacterium at a polysaccharide concentration of 0.10%, whereas UPP and ANP inhibited the growth of beneficial bacteria at concentrations from 0.031 25% to 1.000 00%, which may have resulted from differences in composition, particularly the monosaccharides, and the structures of the seaweed polysaccharides. The main monosaccharide found in GFP and ESP was galactose, which forms galacto-oligosaccharides with known prebiotic effects. In addition, these polysaccharides showed no antibacterial activity at any of the tested concentrations.
In conclusion, polysaccharides isolated from G. filicina and E. spinosum might be putative prebiotics. Moreover, these polysaccharides could be utilized without enzyme hydrolysis after extraction from seaweed, an abundant raw material. However, further work is needed to investigate their effects in vivo.
Almeida-Lima J, Costa L S, Silva N B, Melo-Silveira R F, Silva F V, Felipe M B M C, Medeiros S R B, Leite E L, Rocha H A O. 2010. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. Journal of Applied Toxicology, 30(7): 708-715. DOI:10.1002/jat.1547 |
Boronat A, Aguilar J. 1981. Metabolism of L-fucose and L-rhamnose in Escherichia coli:differences in induction of propanediol oxidoreductase. Journal of Bacteriology, 147(1): 181-185. |
Cho M, Lee H S, Kang I J, Won M H, You S G. 2011. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chemistry, 127(3): 999-1 006. DOI:10.1016/j.foodchem.2011.01.072 |
Costa L S, Fidelis G P, Cordeiro S L, Oliveira R M, Sabry D A, Câmara R B G, Nobre L T D B, Costa M S S P, AlmeidaLima J, Farias E H C, Leite E L, Rocha H A O. 2010. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomedicine & Pharmacotherapy, 64(1): 21-28. |
Cumashi A, Ushakova N A, Preobrazhenskaya M E, D'incecco A, Piccoli A, Totani L, Tinari N, Morozevich G E, Berman A E, Bilan M I, Usov A I, Ustyuzhanina N E, Grachev A A, Sanderson C J, Kelly M, Rabinovich G A, Iacobelli S, Nifantiev N E. 2007. A comparative study of the antiinflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology, 17(5): 541-552. DOI:10.1093/glycob/cwm014 |
Cummings J H, Macfarlane G T. 1991. The control and consequences of bacterial fermentation in the human colon. Journal of Applied Bacteriology, 70(6): 443-459. DOI:10.1111/jam.1991.70.issue-6 |
Depeint F, Tzortzis G, Vulevic J, I'Anson K, Gibson G R. 2008. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans:a randomized, double-blind, crossover, placebocontrolled intervention study. The American Journal of Clinical Nutrition, 87(3): 785-791. DOI:10.1093/ajcn/87.3.785 |
Dierick N, Ovyn A, De Smet S. 2009. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. Journal of the Science of Food and Agriculture, 89(4): 584-594. DOI:10.1002/jsfa.v89:4 |
Du Y J, Zhao Y Q, Huang G J. 2012. Study on extraction of polysaccharides from Eucheuma. The Food Industry, 33(12): 75-78. |
Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3): 350-356. DOI:10.1021/ac60111a017 |
Gibson G R, Roberfroid M B. 1995. Dietary modulation of the human colonic microbiota:introducing the concept of prebiotics. The Journal of Nutrition, 125(6): 1 401-1 412. |
Gómez-Ordóñez E, Jiménez-Escrig A, Rupérez P. 2010. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Research International, 43(9): 2 289-2 294. DOI:10.1016/j.foodres.2010.08.005 |
Hou Y, Wang J, Jin W H, Zhang H, Zhang Q B. 2012. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydrate Polymers, 87(1): 153-159. DOI:10.1016/j.carbpol.2011.07.031 |
Hu B, Gong Q G, Wang Y, Ma Y M, Li J B, Yu W G. 2006. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe, 12(5-6): 260-266. DOI:10.1016/j.anaerobe.2006.07.005 |
Jiao G L, Yu G L, Zhang J Z, Ewart H S. 2011. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Marine Drugs, 9(12): 196-223. |
Jiao L L, Li X, Li T B, Jiang P, Zhang L X, Wu M J, Zhang L P. 2009. Characterization and anti-tumor activity of alkaliextracted polysaccharide from Enteromorpha intestinalis. International Immunopharmacology, 9(3): 324-329. DOI:10.1016/j.intimp.2008.12.010 |
Kawai Y, Seno N, Anno K A. 1969. A modified method for chondrosulfatase assay. Analytical Biochemistry, 32(2): 314-321. DOI:10.1016/0003-2697(69)90091-8 |
Kim J, Kong M K, Lee S Y, Lee P C. 2010. Carbon sourcesdependent carotenoid production in metabolically engineered Escherichia coli. World Journal of Microbiology and Biotechnology, 26(12): 2 231-2 239. DOI:10.1007/s11274-010-0408-5 |
Leser T D, Mølbak L. 2009. Better living through microbial action:the benefits of the mammalian gastrointestinal microbiota on the host. Environmental Microbiology, 11(9): 2 194-2 206. DOI:10.1111/emi.2009.11.issue-9 |
Macfarlane G T, Macfarlane S. 1997. Human colonic microbiota:ecology, physiology and metabolic potential of intestinal bacteria. Scandinavian Journal of Gastroenterology, 32(S222): 3-9. |
Manning T S, Gibson G R. 2004. Microbial-gut interactions in health and disease. Prebiotics. Best Practice & Research Clinical Gastroenterology, 18(2): 287-298. |
Marzorati M, Verhelst A, Luta G, Sinnott R, Verstraete W, Van de Wiele T, Possemiers S. 2010. In vitro modulation of the human gastrointestinal microbial community by plantderived polysaccharide-rich dietary supplements. International Journal of Food Microbiology, 139(3): 168-176. DOI:10.1016/j.ijfoodmicro.2010.02.030 |
Mori N, Nakasone K, Tomimori K, Ishikawa C. 2012. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World Journal of Gastroenterology, 18(18): 2 225-2 230. DOI:10.3748/wjg.v18.i18.2225 |
Possemiers S, Grootaert C, Vermeiren J, Gross G, Marzorati M, Verstraete W, Van de Wiele T. 2009. The intestinal environment in health and disease-recent insights on the potential of intestinal bacteria to influence human health. Current Pharmaceutical Design, 15(18): 2 051-2 065. DOI:10.2174/138161209788489159 |
Qi X H, Mao W J, Gao Y, Chen Y, Chen Y L, Zhao C Q, Li N, Wang C Y, Yan M X, Lin C, Shan J M. 2012. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydrate Polymers, 90(4): 1 804-1 810. DOI:10.1016/j.carbpol.2012.07.077 |
Sanchez J I, Marzorati M, Grootaert C, Baran M, Van Craeyveld V, Courtin C M, Broekaert W F, Delcour J A, Verstraete W, van de Wiele T. 2009. Arabinoxylanoligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the simulator of human intestinal microbial ecosystem. Microbial Biotechnology, 2(1): 101-113. DOI:10.1111/mbt.2009.2.issue-1 |
Silva R O, Santana A P M, Carvalho N S, Bezerra T S, Oliveira C B, Damasceno S R B, Chaves L S, Freitas A L P, Soares P M G, Souza M H L P, Barbosa A L R, Medeiros J V R. 2012. A sulfated-polysaccharide fraction from seaweed Gracilaria birdiae prevents naproxen-induced gastrointestinal damage in rats. Marine Drugs, 10(12): 2 618-2 633. DOI:10.3390/md10122618 |
Sweeney T, Dillon S, Fanning J, Egan J, O'Shea C J, Figat S, Gutierrez J J M., Mannion C, Leonard F, O'Doherty J V. 2011. Evaluation of seaweed-derived polysaccharides on indices of gastrointestinal fermentation and selected populations of microbiota in newly weaned pigs challenged with Salmonella Typhimurium. Animal Feed Science and Technology, 165(1-2): 85-94. DOI:10.1016/j.anifeedsci.2011.02.010 |
Turnbaugh P J, Ley R E, Hamady M, Fraser-Liggett C M, Knight R, Gordon J I. 2007. The human microbiome project. Nature, 449(7164): 804-810. DOI:10.1038/nature06244 |
Tzortzis G, Goulas A K, Gee J M, Gibson G R. 2005. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. The Journal of Nutrition, 135(7): 1 726-1 731. DOI:10.1093/jn/135.7.1726 |
Vulevic J, Juric A, Tzortzis G, Gibson G R. 2013. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of Nutrition, 143(3): 324-331. DOI:10.3945/jn.112.166132 |
Vulevic J, Juric A, Walton G E, Claus S P, Tzortzis G, Toward R E, Gibson G R. 2015. Influence of galactooligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. British Journal of Nutrition, 114(4): 586-595. DOI:10.1017/S0007114515001889 |
Wang T T, Sun Y X, Jin L J, Xu Y P, Wang L, Ren T J, Wang K L. 2009. Enhancement of non-specific immune response in sea cucumber (Apostichopus japonicus) by Astragalus membranaceus and its polysaccharides. Fish & Shellfish Immunology, 27(6): 757-762. |
Wang Y, Han F, Hu B, Li J B, Yu W G. 2006. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate. Nutrition Research, 26(11): 597-603. DOI:10.1016/j.nutres.2006.09.015 |
Wijesinghe W A J P, Jeon Y J. 2012. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds:a review. Carbohydrate Polymers, 88(1): 13-20. DOI:10.1016/j.carbpol.2011.12.029 |
Yan W, Niu Y G, Lv J L, Xie Z H, Jin L, Yao W B, Gao X D, Yu L L. 2013. Characterization of a heteropolysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydrate Polymers, 92(2): 2 111-2 117. DOI:10.1016/j.carbpol.2012.11.074 |
Yu Q M, Yan J, Wang S C, Ji L L, Ding K, Vella C, Wang Z T, Hu Z B. 2012. Antiangiogenic effects of GFP08, an agaran-type polysaccharide isolated from Grateloupia filicina. Glycobiology, 22(10): 1 343-1 352. DOI:10.1093/glycob/cws096 |
Zaporozhets T S, Besednova N, Kuznetsova T A, Zvyagintseva T N, Makarenkova I D, Kryzhanovsky S P, Melnikov V G. 2014. The prebiotic potential of polysaccharides and extracts of seaweeds. Russian Journal of Marine Biology, 40(1): 1-9. DOI:10.1134/S1063074014010106 |
Zhang J J, Zhang Q B, Wang J, Shi X L, Zhang Z S. 2009. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chinese Journal of Oceanology and Limnology, 27(3): 578-582. DOI:10.1007/s00343-009-9205-0 |
| Almeida-Lima J, Costa L S, Silva N B, Melo-Silveira R F, Silva F V, Felipe M B M C, Medeiros S R B, Leite E L, Rocha H A O, 2010. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. Journal of Applied Toxicology, 30(7): 708–715. Doi: 10.1002/jat.1547 |
| Boronat A, Aguilar J, 1981. Metabolism of L-fucose and L-rhamnose in Escherichia coli:differences in induction of propanediol oxidoreductase. Journal of Bacteriology, 147(1): 181–185. |
| Cho M, Lee H S, Kang I J, Won M H, You S G, 2011. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chemistry, 127(3): 999–1 006. Doi: 10.1016/j.foodchem.2011.01.072 |
| Costa L S, Fidelis G P, Cordeiro S L, Oliveira R M, Sabry D A, Câmara R B G, Nobre L T D B, Costa M S S P, AlmeidaLima J, Farias E H C, Leite E L, Rocha H A O, 2010. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomedicine & Pharmacotherapy, 64(1): 21–28. |
| Cumashi A, Ushakova N A, Preobrazhenskaya M E, D'incecco A, Piccoli A, Totani L, Tinari N, Morozevich G E, Berman A E, Bilan M I, Usov A I, Ustyuzhanina N E, Grachev A A, Sanderson C J, Kelly M, Rabinovich G A, Iacobelli S, Nifantiev N E, 2007. A comparative study of the antiinflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology, 17(5): 541–552. Doi: 10.1093/glycob/cwm014 |
| Cummings J H, Macfarlane G T, 1991. The control and consequences of bacterial fermentation in the human colon. Journal of Applied Bacteriology, 70(6): 443–459. Doi: 10.1111/jam.1991.70.issue-6 |
| Depeint F, Tzortzis G, Vulevic J, I'Anson K, Gibson G R, 2008. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans:a randomized, double-blind, crossover, placebocontrolled intervention study. The American Journal of Clinical Nutrition, 87(3): 785–791. Doi: 10.1093/ajcn/87.3.785 |
| Dierick N, Ovyn A, De Smet S, 2009. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. Journal of the Science of Food and Agriculture, 89(4): 584–594. Doi: 10.1002/jsfa.v89:4 |
| Du Y J, Zhao Y Q, Huang G J, 2012. Study on extraction of polysaccharides from Eucheuma. The Food Industry, 33(12): 75–78. |
| Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F, 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3): 350–356. Doi: 10.1021/ac60111a017 |
| Gibson G R, Roberfroid M B, 1995. Dietary modulation of the human colonic microbiota:introducing the concept of prebiotics. The Journal of Nutrition, 125(6): 1 401–1 412. |
| Gómez-Ordóñez E, Jiménez-Escrig A, Rupérez P, 2010. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Research International, 43(9): 2 289–2 294. Doi: 10.1016/j.foodres.2010.08.005 |
| Hou Y, Wang J, Jin W H, Zhang H, Zhang Q B, 2012. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydrate Polymers, 87(1): 153–159. Doi: 10.1016/j.carbpol.2011.07.031 |
| Hu B, Gong Q G, Wang Y, Ma Y M, Li J B, Yu W G, 2006. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe, 12(5-6): 260–266. Doi: 10.1016/j.anaerobe.2006.07.005 |
| Jiao G L, Yu G L, Zhang J Z, Ewart H S, 2011. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Marine Drugs, 9(12): 196–223. |
| Jiao L L, Li X, Li T B, Jiang P, Zhang L X, Wu M J, Zhang L P, 2009. Characterization and anti-tumor activity of alkaliextracted polysaccharide from Enteromorpha intestinalis. International Immunopharmacology, 9(3): 324–329. Doi: 10.1016/j.intimp.2008.12.010 |
| Kawai Y, Seno N, Anno K A, 1969. A modified method for chondrosulfatase assay. Analytical Biochemistry, 32(2): 314–321. Doi: 10.1016/0003-2697(69)90091-8 |
| Kim J, Kong M K, Lee S Y, Lee P C, 2010. Carbon sourcesdependent carotenoid production in metabolically engineered Escherichia coli. World Journal of Microbiology and Biotechnology, 26(12): 2 231–2 239. Doi: 10.1007/s11274-010-0408-5 |
| Leser T D, Mølbak L, 2009. Better living through microbial action:the benefits of the mammalian gastrointestinal microbiota on the host. Environmental Microbiology, 11(9): 2 194–2 206. Doi: 10.1111/emi.2009.11.issue-9 |
| Macfarlane G T, Macfarlane S, 1997. Human colonic microbiota:ecology, physiology and metabolic potential of intestinal bacteria. Scandinavian Journal of Gastroenterology, 32(S222): 3–9. |
| Manning T S, Gibson G R, 2004. Microbial-gut interactions in health and disease. Prebiotics. Best Practice & Research Clinical Gastroenterology, 18(2): 287–298. |
| Marzorati M, Verhelst A, Luta G, Sinnott R, Verstraete W, Van de Wiele T, Possemiers S, 2010. In vitro modulation of the human gastrointestinal microbial community by plantderived polysaccharide-rich dietary supplements. International Journal of Food Microbiology, 139(3): 168–176. Doi: 10.1016/j.ijfoodmicro.2010.02.030 |
| Mori N, Nakasone K, Tomimori K, Ishikawa C, 2012. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World Journal of Gastroenterology, 18(18): 2 225–2 230. Doi: 10.3748/wjg.v18.i18.2225 |
| Possemiers S, Grootaert C, Vermeiren J, Gross G, Marzorati M, Verstraete W, Van de Wiele T, 2009. The intestinal environment in health and disease-recent insights on the potential of intestinal bacteria to influence human health. Current Pharmaceutical Design, 15(18): 2 051–2 065. Doi: 10.2174/138161209788489159 |
| Qi X H, Mao W J, Gao Y, Chen Y, Chen Y L, Zhao C Q, Li N, Wang C Y, Yan M X, Lin C, Shan J M, 2012. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydrate Polymers, 90(4): 1 804–1 810. Doi: 10.1016/j.carbpol.2012.07.077 |
| Sanchez J I, Marzorati M, Grootaert C, Baran M, Van Craeyveld V, Courtin C M, Broekaert W F, Delcour J A, Verstraete W, van de Wiele T, 2009. Arabinoxylanoligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the simulator of human intestinal microbial ecosystem. Microbial Biotechnology, 2(1): 101–113. Doi: 10.1111/mbt.2009.2.issue-1 |
| Silva R O, Santana A P M, Carvalho N S, Bezerra T S, Oliveira C B, Damasceno S R B, Chaves L S, Freitas A L P, Soares P M G, Souza M H L P, Barbosa A L R, Medeiros J V R, 2012. A sulfated-polysaccharide fraction from seaweed Gracilaria birdiae prevents naproxen-induced gastrointestinal damage in rats. Marine Drugs, 10(12): 2 618–2 633. Doi: 10.3390/md10122618 |
| Sweeney T, Dillon S, Fanning J, Egan J, O'Shea C J, Figat S, Gutierrez J J M., Mannion C, Leonard F, O'Doherty J V, 2011. Evaluation of seaweed-derived polysaccharides on indices of gastrointestinal fermentation and selected populations of microbiota in newly weaned pigs challenged with Salmonella Typhimurium. Animal Feed Science and Technology, 165(1-2): 85–94. Doi: 10.1016/j.anifeedsci.2011.02.010 |
| Turnbaugh P J, Ley R E, Hamady M, Fraser-Liggett C M, Knight R, Gordon J I, 2007. The human microbiome project. Nature, 449(7164): 804–810. Doi: 10.1038/nature06244 |
| Tzortzis G, Goulas A K, Gee J M, Gibson G R, 2005. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. The Journal of Nutrition, 135(7): 1 726–1 731. Doi: 10.1093/jn/135.7.1726 |
| Vulevic J, Juric A, Tzortzis G, Gibson G R, 2013. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of Nutrition, 143(3): 324–331. Doi: 10.3945/jn.112.166132 |
| Vulevic J, Juric A, Walton G E, Claus S P, Tzortzis G, Toward R E, Gibson G R, 2015. Influence of galactooligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. British Journal of Nutrition, 114(4): 586–595. Doi: 10.1017/S0007114515001889 |
| Wang T T, Sun Y X, Jin L J, Xu Y P, Wang L, Ren T J, Wang K L, 2009. Enhancement of non-specific immune response in sea cucumber (Apostichopus japonicus) by Astragalus membranaceus and its polysaccharides. Fish & Shellfish Immunology, 27(6): 757–762. |
| Wang Y, Han F, Hu B, Li J B, Yu W G, 2006. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate. Nutrition Research, 26(11): 597–603. Doi: 10.1016/j.nutres.2006.09.015 |
| Wijesinghe W A J P, Jeon Y J, 2012. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds:a review. Carbohydrate Polymers, 88(1): 13–20. Doi: 10.1016/j.carbpol.2011.12.029 |
| Yan W, Niu Y G, Lv J L, Xie Z H, Jin L, Yao W B, Gao X D, Yu L L, 2013. Characterization of a heteropolysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydrate Polymers, 92(2): 2 111–2 117. Doi: 10.1016/j.carbpol.2012.11.074 |
| Yu Q M, Yan J, Wang S C, Ji L L, Ding K, Vella C, Wang Z T, Hu Z B, 2012. Antiangiogenic effects of GFP08, an agaran-type polysaccharide isolated from Grateloupia filicina. Glycobiology, 22(10): 1 343–1 352. Doi: 10.1093/glycob/cws096 |
| Zaporozhets T S, Besednova N, Kuznetsova T A, Zvyagintseva T N, Makarenkova I D, Kryzhanovsky S P, Melnikov V G, 2014. The prebiotic potential of polysaccharides and extracts of seaweeds. Russian Journal of Marine Biology, 40(1): 1–9. Doi: 10.1134/S1063074014010106 |
| Zhang J J, Zhang Q B, Wang J, Shi X L, Zhang Z S, 2009. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chinese Journal of Oceanology and Limnology, 27(3): 578–582. Doi: 10.1007/s00343-009-9205-0 |
 2018, Vol. 36
2018, Vol. 36