Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SIRIWAN Suksri, BOONSATIEN Boonsoong
- The post-larval and juvenile fish assemblage in the Sukhothai Floodplain, Thailand
- Chinese Journal of Oceanology and Limnology, 36(3): 1013-1024
- http://dx.doi.org/10.1007/s00343-018-6258-y
Article History
- Received Nov. 1, 2016
- accepted in principle Dec. 22, 2016
- accepted for publication Mar. 7, 2017
A floodplains is an ecotone that forms a transition between aquatic and terrestrial environments. Floodplains are very important because they provide a variety of habitats and areas of high biodiversity. All this depends on the natural water flow and the predictable, seasonal pattern of rising and falling floodwaters (Moss, 2010). Floodplains are one of the most important fishery resources, providing spawning and rearing grounds. Large river floodplains are considered key nursery habitats for many species of riverine fish (Górski et al., 2011), which allows aquatic organisms to migrate into inundated floodplains. One of the particular characteristics of river floodplains is that they provide dependable sources of food. Fish returning to the main channels as the floodwater recedes are fat from this source. In addition, floodplains provide some of the most productive and diverse freshwater fisheries, especially in tropical regions (Moss, 2010).
Asia is the continent most frequently affected by floods, especially tropical parts of the continent. During the past 30 year, more flood disasters have occurred in Asia (40% of the total) than on any other continent, including the Americas (25%), Africa (17%), Europe (14%), and Oceania (4%) (Dutta and Herath, 2004). Asia contains many floodplains, the largest three of which are in China, India and Bangladesh, respectively (Hussain, 2010). The largest floodplain in Southeast Asia is the Great Lake, located in the Mekong River basin of Cambodia. The water level of this area increases standing water bodies by up to 9 m in flood season. Most floodplains are covered by several meters of floodwater for 3‒4 months annually. In flood season, land and water together in the major flood zones account for 58 000 km2, much of which is covered with rice fields. During floods, adult fish move onto the floodplain to feed and spawn (Hortle, 2009). The Sukhothai floodplain is the second large floodplain of Thailand, and covers area about 320 km2. This floodplain is an importance area for fish productivity, spawning and rearing ground.
Unfortunately, the basic taxonomic and ecological status of fish larvae assemblages on the floodplains in Thailand has received little study, and most previous studies have focused on adult fish communities in various parts of Thailand (Choi et al., 2005; Kottelat, 2013; Phomikong et al., 2015). Tanaka et al. (2015) surveyed fish assemblages and investigated environmental and landscape parameters in a total of 135 floodplain waterbodies in the Chao Phraya River Basin. The study showed the population of juvenile fishes increasing in temporarily connected floodplain waterbodies to main rivers compared with isolated waterbodies (Tanaka et al., 2015). The hypotheses of this study were 1) fish larval assemblage are variable by the annual and seasonal patterns and 2) fish larvae and environmental factors are relate to those patterns. The objective of the present study was to evaluate the spatiotemporal variation in the abundance and diversity of fish larvae in the Sukhothai floodplain.
2 MATERIAL AND METHOD 2.1 Study areaThe Sukhothai floodplain is part of the Yom River Basin in northern Thailand. The floodplain is located in the Yom River Valley in southernmost northern Thailand (16°76′‒17°04′N, 99°82′‒100°13′E), at an altitude of 40‒52 m above mean sea level. The Yom River Basin has a total catchment area of 23 616 km2, and its main channel runs down a steep valley at about 1:700. There are narrow plains along the river itself, which flows southwest through a large plain (Thailand Water Partnership, 2014).
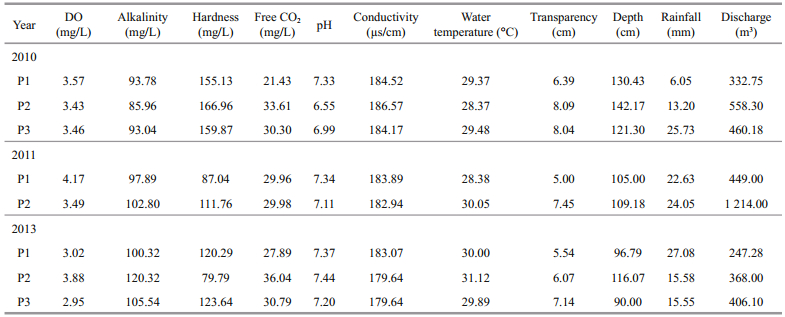
2.2 Sample collection and processingSampling was undertaken at 33 stations in the Sukhothai floodplain. The stations were divided into 4 habitat types: (1) river (R), 5 stations; (2) main canal (M), 8 stations; (3) branch canal (B), 3 stations; and (4) flood areas (F), 16 stations (Fig. 1). To sample flow within the floodplain, 3 distinct types of flood periods were chosen, each defined by flow orientation: (1) river flow runs into the floodplain (P1, August); (2) constant water level in floodplain (P2, September); and (3) river flow runs off of the floodplain (P3, October). Samples were obtained during all 3 flooding periods in all 3 collection years: 2010, 2011 and 2013. Samples were taken at the water’s surface. The pH, water temperature and electrical conductivity were measured in the field using the pH meter (YSI, pH100A), thermometer and conductivity meter (YSI, EC 300), respectively. Using titration methods, the water samples were analyzed in the field for dissolved oxygen (mg/L), alkalinity (mg/L), hardness (mg/L) and free CO2 (mg/L). The transparency and depth were measured (m) for physical parameters. The daily periodicity of the discharge (m3) and rainfall (mm) data were obtained from Hydrology Irrigation for the Lower Northern Region, the Royal Irrigation Department of Thailand (RID) (Royal Irrigation Department, 2014).
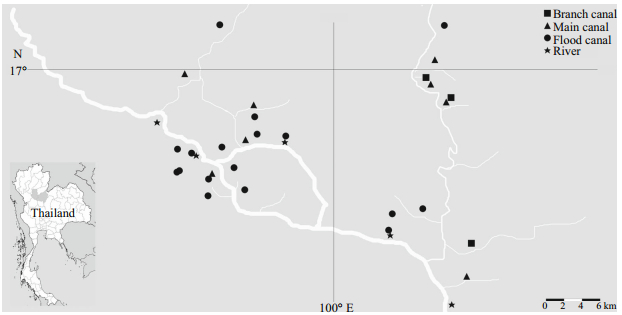
|
| Figure 1 Map showing the location of sampling stations in the Sukhothai floodplain |
In the margins, fish larvae were sampled using a micromesh seine net 30 m wide and 4 m deep, with 1-mm sized mesh. At each sample site, 3 sweeps of the net were made in different locations.
The samples were fixed in 10% neutral buffered formalin. Then, specimens were transferred to glass vials and preserved in 70% ethyl alcohol for identification. Post-larval and juvenile stages of fishes were identified to the lowest possible taxon, usually the species level, according to Termvidchakorn(2003, 2005), Termvidchakorn et al.(2005, 2007) and Termvidchakorn and Hortle (2013).
2.3 Data analysisFrom the sample data for each site, abundance of larvae was standardized using population density methods (Wootton, 1992). Fish larvae assemblage change in response to habitats and years were visualized by performing a non-metric, multidimensional scaling algorithm (nMDS) on abundance data matrices (habitats) and presence/ absence data (years). Temporal change of environmental variables among years was analyzed by a Principal Component Analysis (PCA). The vectors of all environmental variables to evidence the relationship between variables, axes and samples were shown in PCA ordination. It required an initial standardization of abundance and environmental data, which was performed by the general relativizations. A Sorensen distance matrix, using presence/absence data of taxa among years, was analysed by nMDS. The distance was computed, random staring configurations were used and 100 iterations were completed, by sample, with the sample and abundance of species collected in each sampling site, sampling period and year. The relationship between species distribution (abundance) and environmental factors was investigated using canonical correspondence analysis (CCA). All statistical analyses were performed using PC-ORD software version 6.08 (McCune and Mefford, 2011).
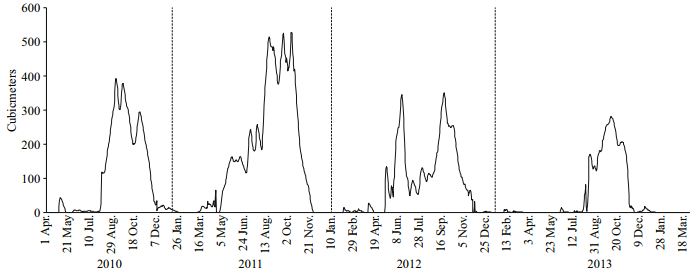
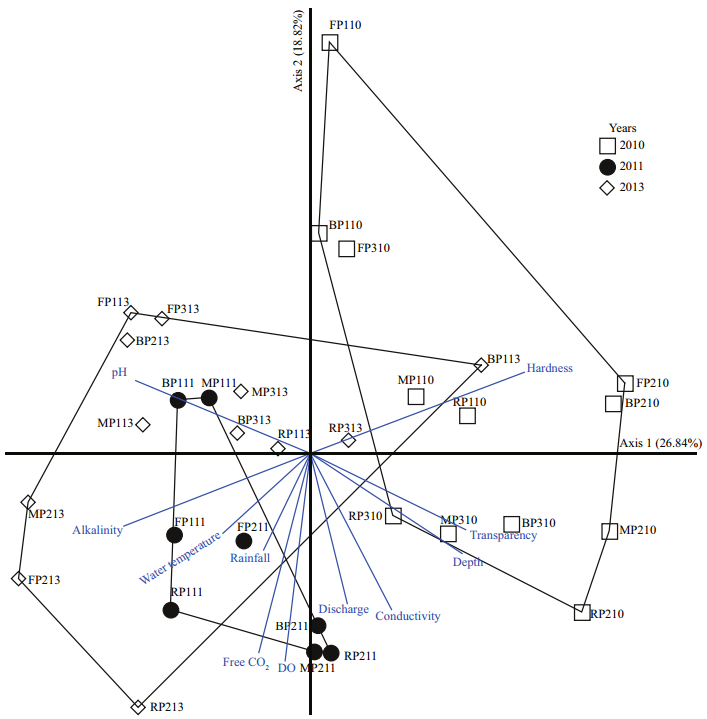
3 RESULT 3.1 Environmental variablesTable 1 contains the study’s physico-chemical parameters and hydrological data. Both physicochemical and hydrological parameters varied by period and year. Slight variations in environmental variables were found, including dissolved oxygen (DO) (range, 2.95‒4.17 mg/L), alkalinity (range, 85.96‒120.32 mg/L), free CO2 (range, 21.43‒ 36.04 mg/L), conductivity (range, 179.64‒186.57 μs/ cm), water temperature (range, 28.37‒31.12℃) and transparency (range, 5.00‒8.09 cm). Water pH varied from 6.55‒7.44. Hardness (range, 79.79‒166.96 mg/L) and depth (range, 90‒142.17 cm) increased during 2010. During the study period, peak discharge occurred in 2011, and the lowest discharge in 2013 (Fig. 2). The rainfall (range, 6.05‒27.08 mm) and discharge (range, 247.28‒1 214.00 m3) increased during periods of a constant water level in the floodplain (P2) during 2010 and 2011. In addition, the PCA analysis revealed that the environmental parameters in 2010 were clearly different from those in 2011 and 2013. Among the environmental variables, discharge strongly correlated with axis 2 and increased in periods of a constant water level in the floodplain (P2) in 2011 (Fig. 3).
|
|
| Figure 2 Hydrograph showing the annual flow events (discharge, m3) in the Sukhothai during the study period (2010‒2013) |
|
| Figure 3 Biplot of sample coordinates on the first two axis of PCA with distance-based biplot scores, including all 32 samples for those an environmental variables |
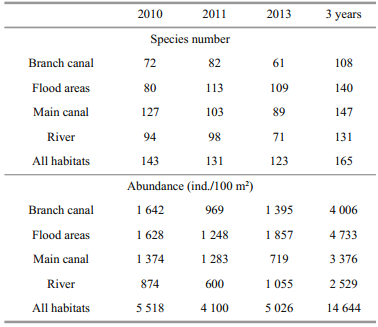
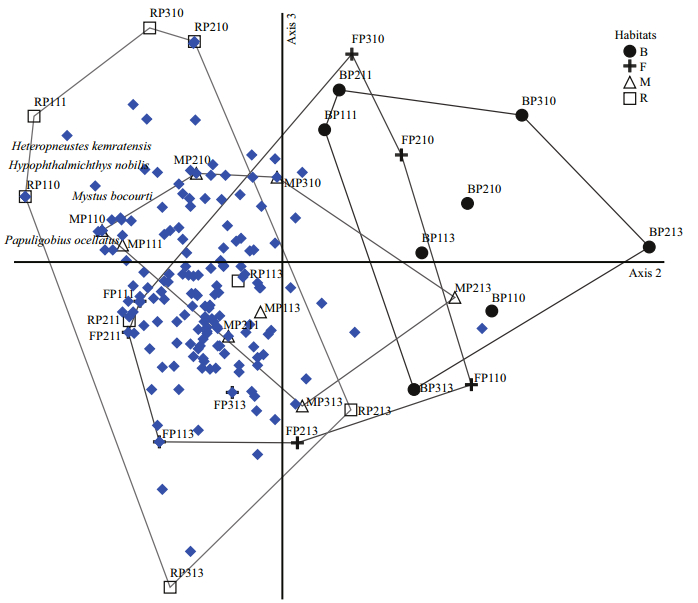
Table 2 contains the species number and abundance of fish larvae in each habitat. The main canal (M) and flood areas (F) habitats accounted for 147 species and 140 species, respectively, of the total catch, while the river (R) and branch canal (B) habitats accounted for 131 and 108 species, respectively. In terms of larval density, the catch was dominated by taxa associated with the flood areas and branch canal habitats, which accounted for 4 733 and 4 006 ind./100 m2, respectively. The remaining portion of the catch consisted of taxa associated with the main canal (3 376 ind./100 m2) and river (2 529 ind./100 m2) habitats. Figure 4 presents the results of ordination using nMDS analysis on transformed larval fish-abundance data. It is clear that the river habitat samples on the left side of the plot (axis 3) were separated from the other habitats on the right side of the plot. Four species were positively correlated with the river habitat, including Papuligobius ocellatus, Mystus bocourti, Hypophthalmichthys nobilis and Heteropneustes kemratensis, indicating that they were the mostabundant species in the river habitat. This, in turn, indicates that the spatial pattern of the larval fish abundance was better represented by the river habitat type.
|
|
| Figure 4 nMDS ordination plot of samples by taxa dissimilarities (abundance data), with habitat identified (stress=16.82%), including all 32 samples for those a faunal list was established (solid dots=fish species) |
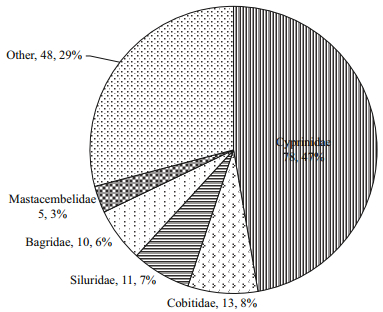
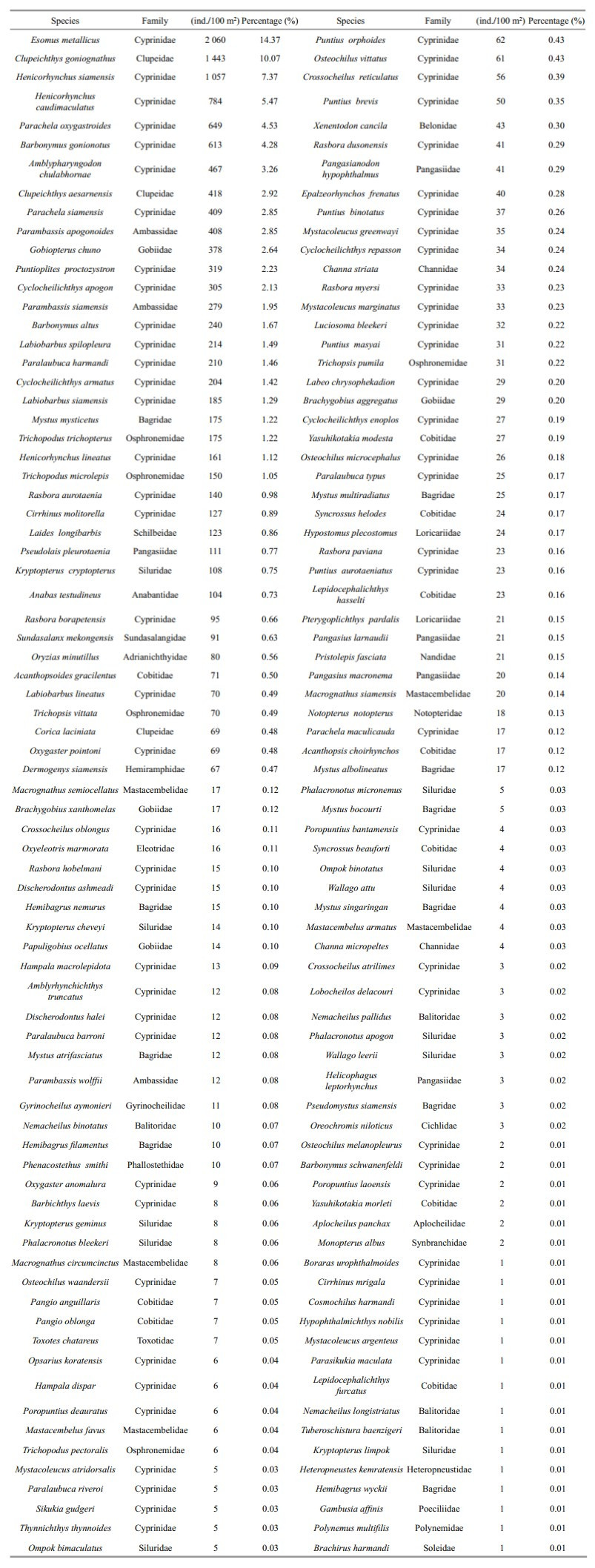
The study collected 149 296 fish larvae representing 11 orders, 32 families, 84 genera and 165 species from 33 sampling stations along the Sukhothai floodplain. Figure 5 shows each of the dominant families of fish larvae as a percentage of total catch, including the number of species within each family. The catch was dominated by five families: Cyprinidae (78 species, 47.27%), Cobitidae (13 species, 7.88%), Siluridae (11 species, 6.67%), Bagridae (10 species, 6.06%) and Mastacembelidae (5 species, 3.33%). Table 3 lists the 10 most-dominant larval fish species, each of which contributed more than 2% of the total catch. Of these 10, the top 4 were the cyprinid Esomus metallicus, the cyprinid Henicorhynchus siamensis, the clupeid Clupeichthys gonionathus and the cyprinid Henicorhynchus caudimaculatus, which accounted for 16.90%, 8.48%, 8.31% and 6.66% of the total catch, respectively. The next six species each accounted for less than 5% of the total catch: cyprinids Parachela oxygastroides (4.83%), Barbonymus gonionotus (3.41%), Amblypharyngodon chulabhornae (2.88%) and Parambassis siamensis (2.45%), the gobiid Gobiopterus chuno (3.01%) and the clupeid Clupeichthys aesarnensis (2.29%).
|
| Figure 5 Percentage of species composition of the dominant families of fish larvae sampled in the Sukhothai floodplain from 2010‒2013 |
|
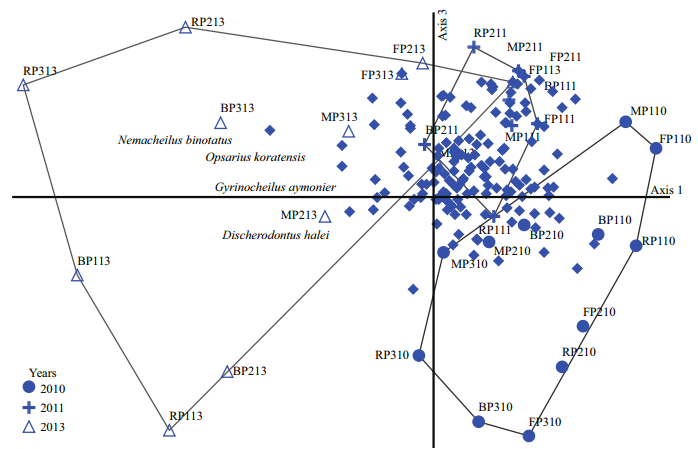
Figure 6 summarises nMDS analysis of the composition and structure of the fish larvae assemblages at each station during each period in each year. After 100 iterations, the stability criterion was met with a final stress of 14.33 (Monte Carlo test: P=0.009 91) for a three-dimensional solution. The location of the points along axis 1 (Fig. 6) identified years (temporal scale) as the main pattern in the composition and structure of the fish larvae assemblages. Samples for 2010 were positively correlated with axis 1. In contrast, samples for 2013 were negatively correlated with axis 1. It is clear that the samples plot is associated with temporal distribution among years. Figure 6 shows the species plot: cyprinid Discherodontus halei, gyrinocheilid Gyrinocheilus aymonieri, cyprinid Opsarius koratensis and balitorid Nemacheilus binotatus typically populate the samples for 2013, located on the left side of the plot. This ordination indicates that they were positively correlated with the samples for 2013. No spatial or temporal patterns were observed in axis 3.
|
| Figure 6 nMDS ordination plot of samples by taxa dissimilarities (presence/absence data), with year identified (stress=14.33%), including all 32 samples for those a faunal list was established (solid dots=fish species) |
Relationships between fish larvae abundance and abiotic variables were analysed using CCA analysis. The result of the ordination plot showed that only the discharge variable was strongly correlated with fish larvae abundance (Fig. 7). The discharge vector strongly correlated with axis 3, increasing in periods of a constant water level in the floodplain (P2) in 2011. Two cyprinid species, Rasbora aurotenia and Rasbora dusonensis, were strongly correlated with axis 3, providing values of R2=0.389 and 0.402, respectively (Fig. 7). These species were observed on the lower part of the plot, associated with the P2 samples and with high discharge values. The larvae of R. aurotenia and R. dusonensis dominated and accounted for mean values (±SD) of 32.48±11.04 and 70.19±29.70 ind./100 m3, respectively.

|
| Figure 7 CCA ordination plot of samples by environmental variable vector (discharge) and abundance of Rasbora aurotenia (a) (R2=0.389) and Rasbora dusonensis (b) (R2=0.402), with taxa abundance superimposed In the plot, the larger symbols denote greater values (higher abundance). |
Generally, both physico-chemical and hydrological parameters varied by year and period. Most parameters did not exceed the surface-water quality standard of Thailand and the appropriate water-quality criteria for aquatic living. The water temperature and pH varied from 23‒32℃ and from 5.0‒9.0, respectively. Most dissolved oxygen concentrations were greater than 3 mg/L, which is appropriate for freshwater aquatic resources (Pollution Control Department, 1992). In periods of a constant water level in the floodplain, the discharge profile and the water level showed increasing trends in 2011. Changing in limnological characteristics during the flooding period may be affect to fish species (Baumgartner et al., 1997). Increasing floodplain area probably reflected a direct association with floodplain utilization by migratory fish species (Tanaka et al., 2015).
4.2 Larval fish assemblagesThe dominant larval species were the cyprinids, represented mainly by the common species Esomus metallicus. This cyprinid specie is abundant in the Mekong and Chao Phraya River systems and is found in both freshwater and brackish habitats. It is the most-common and widely distributed species in Indochina and Sumatra. In all floodplains within its natural distributional range, this species is usually abundant and one of the commonest freshwater fish (Arbsuwan et al., 2012). The larvae of E. metallicus were approximately 17% of the total catch, which means it was relatively highly abundant. According to Tanaka et al. (2015) found that E. metallicus is one of four common species increased in temporarily connected survey sites in waterbodies of the Chao Phraya river basin.
The second most-abundant species of freshwater cyprinid fish, the Siamese mud carp Henicorhynchus siamensis, is a variety of Asian carp native to the Mekong and Chao Phraya Rivers in Southeast Asia. It is very common in floodplains during the wet season and migrates upstream in the Mekong, the migration pathway begins in Cambodia (Suvarnaraksha et al., 2011).
The third most-abundant species, Clupeichthys goniognathus, is a pelagic clupeid fish inhabiting the lower reaches of rivers, although it has also been recorded in reservoirs. This species also occurs in floodplains and lakes during flooding season, which agrees with the results of the present study. It is widely distributed in Thailand, Malaysia, Indonesia, Cambodia, and the Lao PDR (Vidthayanon, 2012a). Jutagate et al. (2003) and Cowx et al. (2015) have identified differences in species composition in the ichthyoplankton drift in the Lower Mekong Basin in various seasons. They found that the flooding period (maximum water level in the Mekong is from August‒ October) was dominated by Cyprinidae, which agrees with the results of this study. In the study by Cowx et al. (2015), the most abundant species were Laides longibarbis (11.2%), Cyclocheilichthys enoplos (10.3%) and Puntioplites proctozysron (8.0%), all which were found in the main channels of rivers. In the present study, all the commonly abundant fish larvae are associated with floodplain areas, and all are used locally both as food and commercially, in the aquarium trade, although pollution and large-scale overfishing may be threaten these species in the future (Mondal et al., 2010).
The spatial pattern of larval fish abundance is represented by the habitat type. The abundance of four larval fish species, Papuligobius ocellatus, Mystus bocourti, Hypophthalmichthys nobilis and Heteropneustes kemratensis, correlated with the river (R) habitat. Two native fish species, P. ocellatus and M. bocourti, are riverine fish recorded in several areas of Thailand. In addition, M. bocourti is a vulnerable fish species. Another native fish species, H. kemratensis, is distributed throughout the Irrawaddy, Sittoung and Salaween Basins in Myanmar and the Chao Phraya, Eastern and Tapi Basins in Thailand (Ratmuangkhwang, 2007). The present study also found an invasive species, a bighead carp, H. nobilis. Bighead carp are native to the large rivers and associated floodplain lakes of eastern Asia. This specie is one of the most important fish in aquaculture (Kara, 2012).
In 2010, the main canal (M) habitat showed the highest values for fish larvae species richness, evenness and SDI, but slight differences were observed in the diversity and richness of species among the spatiotemporal scales, which may be related to the characteristics of these sites (Daga et al., 2009). The Sukhothai floodplain suffered flooding in 2011 and drought in 2013, causing changes in the pattern, quantity and intensity of flow in the area. If they continue, these condition may affect the diversity and richness of larval fish species in the Sukhothai floodplain.
In 2013, four distinctive species were observed in the larval fish assemblage. The first was the cyprinid Discherodontus colemani, which found in the upper Chao Phraya basin in northern (Vidthayanon, 2012b). The second was the cyprinid Opsarius koratensis, which is also found in clear water with sandy-rocky bottoms (Rainboth, 1996). The third was the gyrinocheilid Gyrinocheilus aymonieri, which inhabits flowing streams and tributaries with substrates of boulders, pebbles, gravel and sand (Rainboth, 1996) and occurs in medium- to largesized rivers and enters flooded fields. The fourth was the balitorid Nemacheilus binotatus, which inhabits streams and rivers with moderate current over sandy or pebbly substrate.
The study’s CCA analysis revealed that in addition to the temporal changes noted, the main environmental gradient was due to discharge. Discharge gradient is a common feature of tropical rivers and has pronounced temporal effects on fish composition and distribution. Shuai et al. (2016) found that mean water temperature, river discharge, atmospheric pressure, maximum temperature and precipitation play important roles in larval occurrence patterns. The cyprinid larval species, Megalobrama terminalis, Xenocypris davidi, and Cirrhinus molitorella, are associated with high precipitation, high river discharge, low atmospheric pressure and low DO concentrations which featured during the summer month (Shuai et al., 2016). While, no association was observed between environmental variables and spatial and temporal patterns of the ichthyoplankton assemblages of the floodplain of Upper Parana River, in Ilha Grande National Park, southern Brazil (Gogola et al., 2013).
River discharge and the timing of floods are increasingly being recognised as important causes of inter-annual variability in the recruitment success of cyprinid fish (Nunn et al., 2007). In addition, discharge variables correlate with the abundance of larval R. aurotenia and R. dusonensis. Both two species occur near the surfaces of ponds, canals and streams, are often found in turbid waters and migrate from rivers into flooded forests and swamps (Rainboth, 1996). In the present study, two Rasbora species seem not to be migratory species, however, two larval species (Hemicorhynchus siamensis and Hemicorhynchus caudimaculatus) of top 10 abundant fishes categorise as migratory fishes (Cowx et al., 2015). This presence of the larvae of migratory species indicated that the Sukhothai floodplain may be important to maintaining these species. In general, migratory species spawn in the open waters of the main channel or in tributaries, and the eggs and larvae are transported passively by currents to flooded areas and marginal lagoons, where they complete their development (Daga et al., 2009). Therefore, the larvae of migratory species in the Sukhothai floodplain possibly originated from spawning events in the Yom River.
5 CONCLUSIONThe post-larval and juvenile fish assemblages and environmental parameters in the Sukhothai floodplain were investigated in August 2010 and October 2013. The dominant larval family was Cyprinidae, accounting for 78 taxa. Esomus metallicus was the most abundant species, followed by Henicorhynchus siamensis, Clupeichthys gonionathus (Clupeidae) and Henicorhynchus caudimaculatus. The results of this study indicate that variations in larval fish assemblage are temporal in nature. The larval fish assemblage in 2013 was different from that in 2010 and 2011, and some trends were distinguishable. Four fish species, Discherodontus colemani, Gyrinocheilus aymonieri, Opsarius koratensis and Nemacheilus binotatus typically populate the samples for 2013. The water discharge was a major factor determining fish larvae composition and structure in floodplain. Specifically, there was evidence for the changes in larval fish assemblage in 2013 (the discharge decreasing, and the water level and rainfall also showed decreasing trends in 2013). Moreover, Rasbora aurotenia and Rasbora dusonensis were strongly correlated with the discharge parameter.
6 ACKNOWLEDGEMENTWe are most grateful to our colleagues for assistance during field trips. We would like to thank the Department of Fisheries and Department of Zoology for facilities and assistance.
Arbsuwan S, Musikasinthorn P, Marini M, Samhudi H. 2012. First record of the Cyprinid Fish, Esomus metallicus(Actinopterygii:Cyprinidae) from Sumatra. The Natural History Bulletin of the Siam Society, 58: 59-65. |
Baumgartner G, Nakatani K, Cavicchioli M, Baumgartner M T D S. 1997. Some aspects of the ecology of fish larvae in the floodplain of the high Paraná river, Brazil. Revista Brasileira de Zoologia, 14(3): 551-563. DOI:10.1590/S0101-81751997000300005 |
Choi J K, Choi J S, Beamish F W. 2005. Tropical freshwater fish fauna of central Thailand. The Korean Journal of Systematic Zoology, 21(2): 207-217. |
Cowx I G, Kamonrat W, Sukumasavin N, Sirimongkolthawon R, Suksri S, Phila N. 2015. Larval and juvenile fish communities of the lower Mekong Basin. Mekong River Commission, Phnom Penh, Cambodia: 100p. |
Daga V S, Gogola T M, Sanches P V, Baumgartner G, Baumgartner D, Piana P A, Gubiani É A, Delariva R L. 2009. Fish larvae assemblages in two floodplain lakes with different degrees of connection to the Paraná River, Brazil. Neotropical Ichthyology, 7(3): 429-438. DOI:10.1590/S1679-62252009000300010 |
Dutta D, Herath S. 2004. Trend of floods in Asia and a proposal for flood risk management with integrated river basin approach. In:Proceedings of the 2nd International Conference of Asia-Pacific Hydrology and Water Resources Association. Singapore: p.128-137. |
Gogola T M, Sanches P V, Gubiani É A, da Silva P R L. 2013. Spatial and temporal variations in fish larvae assemblages of Ilha Grande National Park, Brazil. Ecology of Freshwater Fish, 22(1): 95-105. DOI:10.1111/eff.12007 |
Górski K, de Leeuw J J, Winter H V, Vekhov D A, Minin A E, Buijse A D, Nagelkerke L A J. 2011. Fish recruitment in a large, temperate floodplain:the importance of annual flooding, temperature and habitat complexity. Freshwater Biology, 56(11): 2 210-2225. DOI:10.1111/j.1365-2427.2011.02647.x |
Hortle K G. 2009. Fisheries of the Mekong River basin. In: Campbell I C ed. The Mekong-Biophysical Environment of an International River Basin. Academic Press, Elsevier, New York. p. 197-247.
|
Hussain M G. 2010. Freshwater fishes of Bangladesh:fisheries, biodiversity and habitat. Aquatic Ecosystem Health & Management, 13(1): 85-93. DOI:10.1080/14634980903578233 |
Jutagate T, De Silva S S, Mattson N S. 2003. Yield, growth and mortality rate of the Thai river sprat, Clupeichthys aesarnensis, in Sirinthorn Reservoir, Thailand. Fisheries Management and Ecology, 10(4): 221-231. DOI:10.1046/j.1365-2400.2003.00338.x |
Kara H M. 2012. Freshwater fish diversity in Algeria with emphasis on alien species. European Journal of Wildlife Research, 58(1): 243-253. DOI:10.1007/s10344-011-0570-6 |
Kottelat M. 2013. The fishes of the inland waters of Southeast Asia:a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bulletin of Zoology, 27(S): 1-663. |
McCune B, Mefford M J. 2011. PC-ORD. Multivariate analysis of ecological data. Version 6.0. Software, Gleneden Beach, USA. |
Mondal D K, Kaviraj A, Saha S. 2010. Water quality parameters and fish biodiversity indices as measures of ecological degradation:a case study in two floodplain lakes of India. Journal of Water Resource and Protection, 2(1): 85-92. DOI:10.4236/jwarp.2010.21010 |
Moss B. 2010. Ecology of freshwaters:a view for the twentyfirst century. Wiley-Blackwell, Oxford. 470p.
|
Nunn A D, Harvey J P, Britton J R, Frear P A, Cowx I G. 2007. Fish, climate and the Gulf Stream:the influence of abiotic factors on the recruitment success of cyprinid fishes in lowland rivers. Freshwater Biology, 52: 1576-1586. DOI:10.1111/j.1365-2427.2007.01789.x |
Phomikong P, Fukushima M, Sricharoendham B, Nohara S, Jutagate T. 2015. Diversity and community structure of fishes in the regulated versus unregulated tributaries of the Mekong river. River Research and Applications, 31(10): 1262-1275. DOI:10.1002/rra.2816 |
Pollution Control Department. 1992. Water quality management bureau. http://infofile.pcd.go.th/mgt/WaterQuality.pdf. Accessed on 2016-05-20.
|
Rainboth W J. 1996. Fishes of the Cambodian Mekong. FAO, Rome: 256p. |
Ratmuangkhwang S. 2007. Taxonomic review of the southern Asian airsac catfish Heteropneustes fossilis (Siluriformes:Heteropneustidae). Kasetsart University, Bangkok.
|
Royal Irrigation Department Thailand. 2014. Hydrology irrigation for lower Northern region. http://www.hydro-2.com/. Accessed on 2014-01-10.
|
Shuai F M, Li X H, Li Y F, Li J, Yang J P, Lek S. 2016. Temporal patterns of larval fish occurrence in a large subtropical river. PLoS One, 11(1): e0146441. DOI:10.1371/journal.pone.0146441 |
Suvarnaraksha A, Lek S, Lek-Ang S, Jutagate T. 2011. Life history of the riverine cyprinid Henicorhynchus siamensis(Sauvage, 1881) in a small reservoir. Journal of Applied Ichthyology, 27(4): 995-1. DOI:10.1111/j.1439-0426.2010.01619.x |
Tanaka W, Wattanasiriserekul R, Tomiyama Y, Yamasita T, Phinrub W, Chamnivikaipong T, Suvarnaraksha A, Shimatani Y. 2015. Influence of floodplain area on fish species richness in waterbodies of the Chao Phraya river basin, Thailand. Open Journal of Ecology, 5(9): 434-451. DOI:10.4236/oje.2015.59036 |
Termvidchakorn A, Buanak T, Soonthornvipat S, Hunpongkittikul A, Suksri S. 2005. The development tribe and subtribe identification of the fish larvae in subfamily Cyprininae. Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok. 77p.
|
Termvidchakorn A, Hortle K G. 2013. A guide to larvae and juveniles of some common fish species from the Mekong River Basin. Mekong River Commission, Phnom Penh. 234p.
|
Termvidchakorn A, Soonthornvipat P, Soonthornvipat S, Hunpongkittikul A, Suksri S. 2007. The development and family identification of the catfish larvae in order siluriformes. Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok. 54p.
|
Termvidchakorn A. 2003. Freshwater fish larvae. Inland Fisheries Resources Research and Development Institute, Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok. 130p.
|
Termvidchakorn A. 2005. Freshwater fish larvae in Thailand Ⅱ. Inland Fisheries Resources Research and Development Institute, Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok. 125p.
|
Thailand Water Partnership. 2014. Yom River Basin, Thailand. http://gwpseatoolbox.net/wapi/mctweb.dll/. Accessed on 2014-01-10.
|
Vidthayanon C. 2012a. Clupeichthys goniognathus. The IUCN red list of threatened species 2012: e. T180731A1656641. https://doi.org/10.2305/IUCN.UK.2012-1.RLTS.T180731A1656641.en. Accessed on 2016-05-20.
|
Vidthayanon, C. 2012b. Discherodontus colemani. The IUCN Red List of Threatened Species 2012: e. T181286A1717373. https://doi.org/10.2305/IUCN.UK.2012-1.RLTS.T181286A1717373.en. Accessed on 2016-12-03.
|
Wootton R J. 1992. Fish Ecology. Chapman and Hall Publishing, New York. 121p.
|
| Arbsuwan S, Musikasinthorn P, Marini M, Samhudi H, 2012. First record of the Cyprinid Fish, Esomus metallicus(Actinopterygii:Cyprinidae) from Sumatra. The Natural History Bulletin of the Siam Society, 58: 59–65. |
| Baumgartner G, Nakatani K, Cavicchioli M, Baumgartner M T D S, 1997. Some aspects of the ecology of fish larvae in the floodplain of the high Paraná river, Brazil. Revista Brasileira de Zoologia, 14(3): 551–563. Doi: 10.1590/S0101-81751997000300005 |
| Choi J K, Choi J S, Beamish F W, 2005. Tropical freshwater fish fauna of central Thailand. The Korean Journal of Systematic Zoology, 21(2): 207–217. |
| Cowx I G, Kamonrat W, Sukumasavin N, Sirimongkolthawon R, Suksri S, Phila N, 2015. Larval and juvenile fish communities of the lower Mekong Basin. Mekong River Commission, Phnom Penh, Cambodia: 100p. |
| Daga V S, Gogola T M, Sanches P V, Baumgartner G, Baumgartner D, Piana P A, Gubiani É A, Delariva R L, 2009. Fish larvae assemblages in two floodplain lakes with different degrees of connection to the Paraná River, Brazil. Neotropical Ichthyology, 7(3): 429–438. Doi: 10.1590/S1679-62252009000300010 |
| Dutta D, Herath S, 2004. Trend of floods in Asia and a proposal for flood risk management with integrated river basin approach. In:Proceedings of the 2nd International Conference of Asia-Pacific Hydrology and Water Resources Association. Singapore: p.128–137. |
| Gogola T M, Sanches P V, Gubiani É A, da Silva P R L, 2013. Spatial and temporal variations in fish larvae assemblages of Ilha Grande National Park, Brazil. Ecology of Freshwater Fish, 22(1): 95–105. Doi: 10.1111/eff.12007 |
| Górski K, de Leeuw J J, Winter H V, Vekhov D A, Minin A E, Buijse A D, Nagelkerke L A J, 2011. Fish recruitment in a large, temperate floodplain:the importance of annual flooding, temperature and habitat complexity. Freshwater Biology, 56(11): 2 210–2225. Doi: 10.1111/j.1365-2427.2011.02647.x |
| Hortle K G. 2009. Fisheries of the Mekong River basin. In: Campbell I C ed. The Mekong-Biophysical Environment of an International River Basin. Academic Press, Elsevier, New York. p. 197-247. |
| Hussain M G, 2010. Freshwater fishes of Bangladesh:fisheries, biodiversity and habitat. Aquatic Ecosystem Health & Management, 13(1): 85–93. Doi: 10.1080/14634980903578233 |
| Jutagate T, De Silva S S, Mattson N S, 2003. Yield, growth and mortality rate of the Thai river sprat, Clupeichthys aesarnensis, in Sirinthorn Reservoir, Thailand. Fisheries Management and Ecology, 10(4): 221–231. Doi: 10.1046/j.1365-2400.2003.00338.x |
| Kara H M, 2012. Freshwater fish diversity in Algeria with emphasis on alien species. European Journal of Wildlife Research, 58(1): 243–253. Doi: 10.1007/s10344-011-0570-6 |
| Kottelat M, 2013. The fishes of the inland waters of Southeast Asia:a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bulletin of Zoology, 27(S): 1–663. |
| McCune B, Mefford M J, 2011. PC-ORD. Multivariate analysis of ecological data. Version 6.0. Software, Gleneden Beach, USA. |
| Mondal D K, Kaviraj A, Saha S, 2010. Water quality parameters and fish biodiversity indices as measures of ecological degradation:a case study in two floodplain lakes of India. Journal of Water Resource and Protection, 2(1): 85–92. Doi: 10.4236/jwarp.2010.21010 |
| Moss B, 2010. Ecology of freshwaters:a view for the twentyfirst century. Wiley-Blackwell, Oxford470p. |
| Nunn A D, Harvey J P, Britton J R, Frear P A, Cowx I G, 2007. Fish, climate and the Gulf Stream:the influence of abiotic factors on the recruitment success of cyprinid fishes in lowland rivers. Freshwater Biology, 52: 1576–1586. Doi: 10.1111/j.1365-2427.2007.01789.x |
| Phomikong P, Fukushima M, Sricharoendham B, Nohara S, Jutagate T, 2015. Diversity and community structure of fishes in the regulated versus unregulated tributaries of the Mekong river. River Research and Applications, 31(10): 1262–1275. Doi: 10.1002/rra.2816 |
| Pollution Control Department. 1992. Water quality management bureau. http://infofile.pcd.go.th/mgt/WaterQuality.pdf. Accessed on 2016-05-20. |
| Rainboth W J, 1996. Fishes of the Cambodian Mekong. FAO, Rome: 256p. |
| Ratmuangkhwang S, 2007. Taxonomic review of the southern Asian airsac catfish Heteropneustes fossilis (Siluriformes:Heteropneustidae). Kasetsart University, Bangkok. |
| Royal Irrigation Department Thailand. 2014. Hydrology irrigation for lower Northern region. http://www.hydro-2.com/. Accessed on 2014-01-10. |
| Shuai F M, Li X H, Li Y F, Li J, Yang J P, Lek S, 2016. Temporal patterns of larval fish occurrence in a large subtropical river. PLoS One, 11(1): e0146441. Doi: 10.1371/journal.pone.0146441 |
| Suvarnaraksha A, Lek S, Lek-Ang S, Jutagate T, 2011. Life history of the riverine cyprinid Henicorhynchus siamensis(Sauvage, 1881) in a small reservoir. Journal of Applied Ichthyology, 27(4): 995–1. Doi: 10.1111/j.1439-0426.2010.01619.x |
| Tanaka W, Wattanasiriserekul R, Tomiyama Y, Yamasita T, Phinrub W, Chamnivikaipong T, Suvarnaraksha A, Shimatani Y, 2015. Influence of floodplain area on fish species richness in waterbodies of the Chao Phraya river basin, Thailand. Open Journal of Ecology, 5(9): 434–451. Doi: 10.4236/oje.2015.59036 |
| Termvidchakorn A, Buanak T, Soonthornvipat S, Hunpongkittikul A, Suksri S, 2005. The development tribe and subtribe identification of the fish larvae in subfamily Cyprininae. Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok77p. |
| Termvidchakorn A, Hortle K G, 2013. A guide to larvae and juveniles of some common fish species from the Mekong River Basin. Mekong River Commission, Phnom Penh234p. |
| Termvidchakorn A, Soonthornvipat P, Soonthornvipat S, Hunpongkittikul A, Suksri S, 2007. The development and family identification of the catfish larvae in order siluriformes. Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok54p. |
| Termvidchakorn A, 2003. Freshwater fish larvae. Inland Fisheries Resources Research and Development Institute, Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok130p. |
| Termvidchakorn A, 2005. Freshwater fish larvae in Thailand Ⅱ. Inland Fisheries Resources Research and Development Institute, Inland Fisheries Research and Development Bureau, Department of Fisheries, Bangkok125p. |
| Thailand Water Partnership. 2014. Yom River Basin, Thailand. http://gwpseatoolbox.net/wapi/mctweb.dll/. Accessed on 2014-01-10. |
| Vidthayanon C. 2012a. Clupeichthys goniognathus. The IUCN red list of threatened species 2012: e. T180731A1656641. https://doi.org/10.2305/IUCN.UK.2012-1.RLTS.T180731A1656641.en. Accessed on 2016-05-20. |
| Vidthayanon, C. 2012b. Discherodontus colemani. The IUCN Red List of Threatened Species 2012: e. T181286A1717373. https://doi.org/10.2305/IUCN.UK.2012-1.RLTS.T181286A1717373.en. Accessed on 2016-12-03. |
| Wootton R J, 1992. Fish Ecology. Chapman and Hall Publishing, New York121p. |
 2018, Vol. 36
2018, Vol. 36


