Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HAN Mengshu(韩孟书), LI Qiuhua(李秋华), CHEN Hailong(陈海龙), XIAO Jing(肖晶), JIANG Fan(江帆)
- Spatial and temporal variations in cyanobacteria and microcystins in Aha Reservoir, Southwest China
- Chinese Journal of Oceanology and Limnology, 36(4): 1126-1131
- http://dx.doi.org/10.1007/s00343-018-7178-6
Article History
- Received Jun. 20, 2017
- accepted in principle Dec. 6, 2017
- accepted for publication Feb. 26, 2018
Cyanobacteria are considered an important water quality problem. Blooms of these organisms release toxic secondary metabolites known as cyanotoxins (Park et al., 2001; Li et al., 2008; Carmichael and Boyer, 2016), which have been shown to cause numerous animal deaths and may be a hazard to human health (Hudnell, 2010; McGregor et al., 2011; Zanchett and Oliveira-Filho, 2013; Watson et al., 2016). More than 40 species of cyanobacteria have been reported in aquatic ecosystems (Jayatissa et al., 2006; Messineo et al., 2006; Spoof et al., 2006). Hepatotoxic cyclic peptide toxins (microcystins, MCs, and nodularins), which are the most widespread cyanotoxins, are present in diverse environments. Microcystins are cyclic heptapeptides produced by different cyanobacteria genera including Microcystis, Anabaena, Plankothrix and Nostoc (Messineo et al., 2006; Spoof et al., 2006; Aráoz et al., 2010). Several incidents of wild and domestic animal poisoning and death have reportedly been caused by blooms of toxic cyanobacteria (Chapman and Schelske, 1997; Wiedner et al., 2008; Carmichael et al., 2016). Cyanobacterial blooms are currently very common in China because of eutrophication. Microcystis sp., Anabaena sp., Planktothrix sp. and Oscillatoria sp. are the most frequently reported cyanobacterial genera and microcystin-producing species that cause blooms in freshwater (Chen et al., 2008; Dong et al., 2012; Dai et al., 2012).
In southwest China, cyanobacterial blooms have been observed in reservoirs and lakes in the Yunnan-Guizhou plateau. These blooms have caused odor problems and reduced water clarity (Wu et al., 2012). The Aha Reservoir is an essential strategic water resource which, like many other water bodies in China, is primarily used for drinking purposes (He et al., 2015). The Aha Reservoir has been impacted by expansion of the surrounding city in recent years, leading to serious water quality deterioration. In this study, we will evaluate the spatial and temporal variations in cyanobacteria and cyanotoxins and the relationship of cyanobacteria with changes with environmental factors.
2 MATERIAL AND METHOD 2.1 Study areaThe Aha Reservoir (AHR), which was built in 1958, has a capacity of 0.87×l08 m3 and a water residence time of 0.325 a. The reservoir is located near Guiyang, in Guizhou Province (Li, 2018), Southwestern China. The maximum monthly precipitation in the catchment occurs between April and September (1 140–1 200 mm). Other characteristics of the reservoir are summarized in Table 1. The primary purpose of the reservoir was changed from irrigation to drinking water supply in 2000, and it is now an important source of water to residents of Guiyang (Table 1).
Four sampling sites were established: DHP (upstream of Aha Reservoir); ZCS (near the middle of the Aha Reservoir); JZR (another important tributary to the Aha Reservoir); NJ (near the dam). Each sampling point was sampled at three depths (surface, middle and bottom). Phytoplankton samples and water samples for water chemistry determination were collected biweekly or once a week from May to September in 2015. Samples were taken with a 30-lmmesh plankton net and a Van Dorn bottle (Fig. 1).
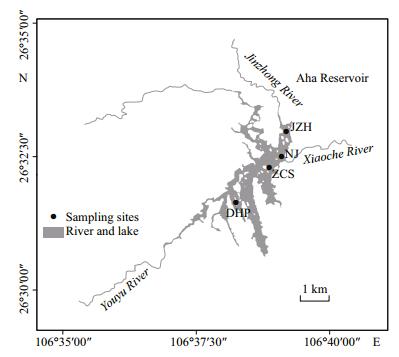
|
| Figure 1 Map of sampling sites in Aha Reservoir DHP: Dahuangpo; ZCS: Zhongcaosi; JZH: Jingzhonghe; NJ: Nanjiao, near the dam of the Aha Reservoir. |
Water samples (1 L) were collected into PE bottles from different depths of the Aha Reservoir and between various treatment steps in water treatment plants for quantitative toxin determination by PDA/ HPLC. For phytoplankton enumeration, take 1.5 L of water sample to the sample bottles from 5 L hydrophore on the spot. Samples were then preserved with 3% formaldehyde solution with migration tube. Solid phase extraction (SPE) of the filtrate was performed using C18 end-capped SPE cartridges (Agilent) conditioned with 15 mL methanol and then washed with 15 mL distillated water (H2O-MQ). Samples were loaded onto the cartridges, washed with 15 mL H2O-MQ water, and then eluted with 25 mL 80% methanol (0.02% TFA). The eluent was then dried using a rotary evaporator and resuspended in 1 mL H2O-MQ.
2.3 Analysis methodsPhytoplankton counting was performed after sedimentation of 1.5 L of samples fixed with 3% formaldehyde solution in a graduated flask. After 48– 72 h, the supernatant was syphoned off with a 2-mm diameter hose until obtaining 35 mL of residual concentrated sample. Two concentrated samples containing 0.1 mL each were allowed to settle in a Sedgewick-Rafter chamber. Taxonomical determination and counting were conducted using an Olympus-BX41 microscope at 400× magnification. One sample is made into two microscope slides, averaging, and the error should not be greater than 15%. Phytoplankton was classified at the lowest taxonomical rank possible. Species names and the definition of higher taxonomic groups of eukaryotic phytoplankton and cyanobacteria were based on the most recent literature (Krienitz and Bock 2012; Guiry and Guiry, 2016). For every individual taxon, phytoplankton biomass (PB, mg/L) were calculated from abundances (cells/mL) and specific biovolumes (μm3) estimated by approximating the phytoplankton shapes to simple geometrical solids. Specific biovolumes estimated from direct linear measurements were further verified using standard references (Druart and Rimet, 2008). External standards were prepared for MC-LR and MC-RR (both provided by ALGALCHEN Inc). Samples and standards (10 μL in 20% methanol) were injected into the HPLC (Waters e2695-2998; Column: Luna 5u C18(2) 100A, 250 mm×4.6 mm) using an auto sampler and peaks were compared to standards. The flow rate was 1.0 mL/min and a water (0.02% TFA)/acetonitrile gradient was employed as the mobile phase by transitioning from 30% acetonitrile to 70% acetonitrile over 15 min, which was then held for 5 min. With the PDA detector, the procession was set at 238 nm.
3 RESULT AND DISSCUSION 3.1 Bloom-forming cyanobacteriaAt the beginning of the sampling period in May, many phytoplankton genera were found, with the phytoplankton and cyanobacterial biomass reaching 19.2 mg/L and 17.0 mg/L, respectively. The cyanobacterial biomass was high in May, then gradually decreased until the end of the season. Toxic cyanobacteria including Aphanizomenon flos-aquae, Pseudanabaena limnetica, Cylindrospermopsis sp., and Microcystis sp. dominated the phytoplankton community in May, while the dominant genus found in the water samples was Aphanizomenon flos-aquae in May and Pseudanabaena limnetica from July to September (Table 2).
A green surface scum of Aphanizomenon flos-aquae was observed during May and June, indicating the occurrence of an Aphanizomenon flos-aquae bloom. In the early stages of the bloom, the dominant species was Aphanizomenon flos-aquae. At the surface of the JZH sampling site, the cyanobacterial biomass reached 17.0 mg/L on 18 May, while very high biomass were also observed at sampling sites ZCS and NJ. Interestingly, no Aphanizomenon flos-aquae was observed in August and September, but Pseudanabaena limnetica was. From May 14 to May 26, the dominant species was Aphanizomenon flos-aquae and the cyanobacteria bloom was single. The dominant cyanobacteria was gradually replaced by diatoms and dinoflagellates and a bloom observed from June 9–23 consisted of diatoms mixed with dinoflagellates. The water color turned from green to brown and then black with bacillariophyta and pyrrhophyta (Fig. 2).

|
| Figure 2 Total biomass and cyanobacterial biomass a. DHP; b. ZCS; c. JZH; d. NJ. |
At the DHP sampling points, the concentration of microcystins was between 0 and 1.3 μg/L, in which the MC-RR was between 0 and 1.2 μg/L, with the maximum observed on 30 June. In addition, MC-LR ranged from 0 to 0.9 μg/L, with the maximum observed on 18 May (Fig. 3a). The microcystins ranged from 0 to 2.2 μg/L, the MC-RR was between 0 and 2.2 μg/L and the MC-LR was between 0 and 0.5 μg/L (Fig. 3b). The highest concentration of microcystin was found in the water sample collected on 28 July at the JZH sampling point, where the microcystin level was 0–3.0 μg/L and the MC-RR was between 0–3.0 μg/L, the highest was at the bottom on 14 July. The MC-LR was between 0 and 0.5 μg/L, with the maximum being observed at the surface on 24 June at the JZH sampling point (Fig. 3c). Microcystin was between 0 and 3.1 μg/L, in which the MC-RR was between 0 and 3.1 μg/L, the highest was on 25 August, while the MC-LR was between 0 and 0.9 μg/L, the maximum in the surface on 18 May at the NJ sampling points (Fig. 3d).

|
| Figure 3 Concentrations of MC-LR, MC-RR and TMC a. DHP; b. ZCS; c. JZH; d. NJ. |
The total microcystin content decreased from 0.5– 2.0 μg/L in each phase of the bloom to 3.0–4.0 μg/L at the middle, and then to below the detection limit at the end of the bloom. There are several possible explanations for this phenomenon. First, the dissolved oxygen in the bottom is low because of biomass degradation. The microcystins increased gradually as dead cyanobacteria cells sank and released them during algal blooms. Second, the microcystins decreased during rainfall events and when the reservoir was drained (Fig. 3). The contents and proportions of MC-RR were much higher than those of MC-LR, especially at the end of the bloom in August. In nature, the half-life of microcystin is usually 15±5 d (Paerl et al., 2016); however, their degradation can increase under some conditions (Paerl and Otten, 2013). The MC-LR levels in the surface ranged from 0 to 1.0 μg/L, which is within the drinking water health standards (GB5749-2001), and the contents of MC-LR gradually approached zero. According to the World Health Organization (WHO) guidelines, MC-LR should not exceed 1.0 μg/L in raw water.
From May to September, the water temperature was between 21.20℃ and 26.40℃. Indeed, temperature is considered an important factor influencing cyanobacterial dominance (Paerl, 1996). Temperatures above 25.0℃ promote cyanobacterial blooms, hepatotoxic species, and production of toxins, and increases in temperature have been reported to stimulate cyanotoxin biosynthesis (El-Shehawy et al., 2012). Cyanobacterial growth rates reach their optima and continue to remain high, even when temperatures exceed 25.0℃. Moreover, cyanobacteria attain maximum growth rates at higher temperatures than green algae and diatoms (Mur et al., 1999).
Some other environmental factors have been found to have a strong relationship with the growth of cyanobacteria (Somdee et al., 2013). For example, pH and total nitrogen were associated with the growth of microcystin-producing genera in Finnish Lakes (Rantala et al., 2006). Additionally, nitric nitrogen might be a significant factor promoting Microcystis aeruginosa communities (Yoshida et al., 2007). The dominance of Aphanizomenon flos-aquae and Pseudanabaena limnetica in the Aha Reservoir agreed with its occurrence as the most common bloomforming cyanobacteria in freshwater bodies worldwide (Yılmaz et al. 2008). Aphanizomenon flos-aquae is a cylindrospermopsin producing cyanobacterium that has been identified in eutrophic water bodies worldwide. These organisms severely endanger environmental safety and human health because they produce paralytic shellfish poisons (PSPs). Microcystis sp. has been replaced by this genus as the most commonly detected toxin producing species in reservoirs in recent years (Chapman and Schelske, 1997).
4 CONCLUSIONThe results of this study clearly demonstrated that MC-LR and MC-RR were present in Aha Reservoir. The highest MC-LR content of 0.9 μg/Lwas found at the NJ sampling point on 18 May 2015, whereas the highest microcystin-RR content of 3.0 μg/L was found at the JZH sampling point on 14 July 2015. Microscopic examination of the phytoplankton samples showed the dominance of Aphanizomenon flos-aquae, Pseudanabaena limnetica, Cylindrospermopsis sp., and Microcystis sp. This is the first report providing evidence of the presence of cyanotoxins in Aha Reservoir, Southwest China. The results presented herein suggest that cyanobacterial toxins can be produced by several cyanobacterial species in Aha Reservoir; therefore, these compounds pose a risk to water ecological security and potential health risks to the human population.
5 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Aráoz R, Molǵo J, De Marsac N T. 2010. Neurotoxic cyanobacterial toxins. Toxicon, 56(5): 813-828.
DOI:10.1016/j.toxicon.2009.07.036 |
Carmichael W W, Boyer G L. 2016. Health impacts from cyanobacteria harmful algae blooms:implications for the North American Great Lakes. Harmful Algae, 54: 194-212.
DOI:10.1016/j.hal.2016.02.002 |
Chapman A D, Schelske C L. 1997. Recent appearance of Cylindrospemopsis (cyanobacteiua) in five hypereutrophic Florida Lakes. Journal of Phycology, 33(2): 191-195.
DOI:10.1111/j.0022-3646.1997.00191.x |
Chen W, Song L R, Peng L, Wan N, Zhang X M, Gan N Q. 2008. Reduction in microcystin concentrations in large and shallow lakes:water and sediment-interface contributions. Water Research, 42(3): 763-773.
DOI:10.1016/j.watres.2007.08.007 |
Dai G F, Quan C Y, Zhang X Z, Liu J, Song L R, Gan N Q. 2012. Fast removal of cyanobacterial toxin microcystinLR by a low-cytotoxic microgel-Fe(Ⅲ) complex. Water Research, 46(5): 1482-1489.
DOI:10.1016/j.watres.2011.11.010 |
Dong G F, Xie S Q, Zhu X M, Han D, Yang Y X, Song L R, Gan L Q, Chen W. 2012. Responses of yellow catfish (Pelteobagrus fulvidraco Richardson) exposed to dietary cyanobacteria and subsequent recovery. Toxicon, 60(7): 1298-1306.
DOI:10.1016/j.toxicon.2012.08.013 |
Druart J C, Rimet F. 2008. Protocoles d'analyse du phytoplancton de l'INRA: prélèvement, dénombrement et biovolumes. INRA-Thonon, Rapport SHL 283.
|
El-Shehawy R, Gorokhova E, Fernández-Piñas F, del Campo F F. 2012. Global warming and hepatotoxin production by cyanobacteria:what can we learn from experiments?. Water Research, 46(5): 1420-1429.
DOI:10.1016/j.watres.2011.11.021 |
Guiry M D, Guiry G M. 2016. AlgaeBase. World-wide electronic publication-National University of Ireland, Galway. http://www.algaebase.org.
|
He T R, Zhu Y Z, Yin D L, Luo G J, An Y L, Yan H Y, Qian X L. 2015. The impact of acid mine drainage on the methylmercury cycling at the sediment-water interface in Aha Reservoir, Guizhou, China. Environmental Science and Pollution Research, 22(7): 5124-5138.
DOI:10.1007/s11356-014-3864-x |
Hudnell H K. 2010. The state of U.S. freshwater harmful algal blooms assessments, policy and legislation. Toxicon, 55(5): 1024-1034.
DOI:10.1016/j.toxicon.2009.07.021 |
Jayatissa L P, Silva E I L, McElhiney J, Lawton L A. 2006. Occurrence of toxigenic cyanobacterial blooms in freshwaters of Sri Lanka. Systematic and Applied Microbiology, 29(2): 156-164.
DOI:10.1016/j.syapm.2005.07.007 |
Krienitz L, Bock C. 2012. Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia, 698(1): 295-326.
|
Li Q H. 2018. Characteristics and evaluation of eutrophication in Guizhou plateau reservoirs. Journal of Guizhou Normal University (Natural Science), 36(2): 1-8.
(in Chinese with English abstract) |
Li R H, Wilhelm S W, Carmichael W W, Watanabe M M. 2008. Polyphasic characterization of water bloom forming Raphidiopsis species (cyanobacteria) from central China. Harmful Algae, 7: 146-153.
DOI:10.1016/j.hal.2007.06.003 |
McGregor G B, Sendall B C, Hunt L T, Eaglesham G K. 2011. Report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis mediterranea Skuja (Cyanobacteria/Nostocales). Harmful Algae, 10(4): 402-410.
DOI:10.1016/j.hal.2011.02.002 |
Messineo V, Mattei D, Melchiorre S, Salvatore G, Bogialli S, Salzano R, Mazza R, Capelli G, Bruno M. 2006. Microcystin diversity in a Planktothrix rubescens population from Lake Albano (Central Italy). Toxicon, 48(2): 160-174.
DOI:10.1016/j.toxicon.2006.04.006 |
Mur L R, Skulberg O M, Utkilen H. 1999. Cyanobacteria in the environment. In: Chorus I, Bartran J eds. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. E&FN Spon, London. p. 427-433.
|
Paerl H W, Gardner W S, Havens K E, Joyner A R, McCarthy M J, Newell S E, Qin B Q, Scott J T. 2016. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae, 54: 213-222.
DOI:10.1016/j.hal.2015.09.009 |
Paerl H W, Otten T G. 2013. Harmful cyanobacterial blooms:causes, consequences, and controls. Microbial Ecology, 65(4): 995-1.
|
Paerl H W. 1996. Microscale physiological and ecological studies of aquatic cyanobacteria:macroscale implications. Microscopy Research & Technique, 33(1): 47-72.
|
Park H, Namikoshi M, Brittain S M, Carmichael W W, Murphy T. 2001. [D-Leu1] microcystin-LR, a new microcystin isolated from waterbloom in a Canadian prairie lake. Toxicon, 39(6): 855-862.
DOI:10.1016/S0041-0101(00)00224-5 |
Rantala A, Rajaniemi-Wacklin P, Lyra C, Lepistö I, Rintala J, Mankiewicz-Boczek J, Sivonen K. 2006. Detection of microcystin-producing cyanobacteria in Finnish lakes with genus-specific microcystin synthetase gene E (mcyE)PCR and associations with environmental factors. Applied and Environmental Microbiology, 72(9): 6101-6110.
DOI:10.1128/AEM.01058-06 |
Somdee T, Kaewsan T, Somdee A. 2013. Monitoring toxic cyanobacteria and cyanotoxins (microcystins and cylindrospermopsins) in four recreational reservoirs(Khon Kaen, Thailand). Environmental Monitoring and Assessment, 185(11): 9521-9529.
|
Spoof L, Berg K A, Rapala J, Lahti K, Lepistö L, Metcalf J S, Codd G A, Meriluoto J. 2006. First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environmental Toxicology, 21(6): 552-560.
DOI:10.1002/(ISSN)1522-7278 |
Watson S B, Miller C, Arhonditsis G, Boyer G L, Carmichael W, Charlton M N, Confesor R, Depew D C, Höök T O, Ludsin S A, Matisoff G, McElmurry S P, Murray M W, Richards R P, Rao Y R, Steffen M M, Wilhelm S W. 2016. The re-eutrophication of Lake Erie:Harmful algal blooms and hypoxia. Harmful Algae, 56: 44-66.
DOI:10.1016/j.hal.2016.04.010 |
Wiedner C, Rücker J, Fastner J, Chorus I, Nixdorf B. 2008. Seasonal dynamics of cylindrospermopsin and cyanobacteria in two German lakes. Toxicon, 52(6): 677-686.
DOI:10.1016/j.toxicon.2008.07.017 |
Wu Y L, Li L, Gan N Q, Zheng L L, Ma H Y, Shan K, Liu J, Xiao B D, Song L R. 2014. Seasonal dynamics of water bloom-forming Microcystis morphospecies and the associated extracellular microcystin concentrations in large, shallow, eutrophic Dianchi Lake. Journal of Environmental Sciences, 26(9): 1921-1929.
DOI:10.1016/j.jes.2014.06.031 |
Yılmaz M, Phlips E J, Szabo N J, Badylak S. 2008. A comparative study of Florida strains of Cylindrospermopsis and Aphanizomenon for cylindrospermopsin production. Toxicon, 51(1): 130-139.
DOI:10.1016/j.toxicon.2007.08.013 |
Yoshida M, Yoshida T, Takashima Y, Hosoda N, Hiroishi S. 2007. Dynamics of microcystin-producing and nonmicrocystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiology Letters, 226(1): 49-53.
|
Zanchett G, Oliveira-Filho E C. 2013. Cyanobacteria and cyanotoxins:from impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins, 5(10): 1896-1917.
|
 2018, Vol. 36
2018, Vol. 36




