Institute of Oceanology, Chinese Academy of Sciences
Article Information
- PANG Min(庞敏), XU Jintao(徐金涛), LIU Yunlong(刘云龙), ZHANG Xuelei(张学雷)
- Comparative study of the hemolytic and cytotoxic activities of nematocyst venoms from the jellyfish Cyanea nozakii Kishinouye and Nemopilema nomurai Kishinouye
- Chinese Journal of Oceanology and Limnology, 36(4): 1255-1265
- http://dx.doi.org/10.1007/s00343-018-7104-y
Article History
- Received Mar. 30, 2017
- accepted in principle May. 27, 2017
- accepted for publication Jul. 13, 2017
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266200, China;
3 Qinhuangdao Marine Environmental Monitoring Central Station of SOA, Qinhuangdao 066000, China;
4 Chinese Research Academy of Environmental Sciences, Beijing 100012, China
Blooms of the jellyfish Cyanea nozakii Kishinouye (C. nozakii), a cnidarian of the Class Scyphomedusae, Order Semaeostomeae, Family Cyaneidae, have occurred off coastal areas of the Northeastern China Sea, Yellow Sea, and Bohai Sea since the late 20th century (Dong et al., 2006). In addition, blooms of the jellyfish Nemopilema nomurai Kishinouye (N. nomurai), a cnidarian of the Class Scyphomedusae, Order Rhizostomeae, Family Stomolophidae (Huang and Lin, 2012) have occurred in recent years in temperate China seas, including the northern East China Sea, Yellow Sea, and Bohai Sea (Dong et al., 2010). Both C.nozakii and N. nomurai, which are often abundant in late summer to early autumn from the Bohai Sea to the Yellow Sea, are the major species causing blooms in the northern China seas. Such blooms have deleterious effects on marine ecosystems as well as fishery production, and pose a risk to human health. Humans stung by either species suffer itching, edema, muscle aches, tightness of breath, hypotension, shock, and even death (Yu et al., 2005; Li et al., 2013, 2014). These symptoms occur in response to the venom contained in nematocysts on the tentacles of the jellyfish. These venoms contain toxic polypeptides and/or proteins, which all increase the risk to human health during jellyfish blooms.
Many biological assays have been carried out to estimate the toxicity of several jellyfish venoms. Hemolytic proteins in nematocyst venoms are key markers of the toxic effects of jellyfish stings, including erythema and edema (Li et al., 2013); therefore, hemolytic and cytotoxic activities of jellyfish venoms have been the most widely reported (Nagai et al., 2000a, b; Brinkman and Burnell, 2007; Helmholz et al., 2007; Yu et al., 2007; Marino et al., 2008, 2009). The hemolytic and cytotoxic activities of venoms from C.nozakii and N. nomurai have also been evaluated. Kang et al. (2009) assessed the hemolytic activity of N. nomurai venom on cat, dog, human, rabbit, and rat erythrocytes, and evaluated its cytotoxicity using C2C12 (skeletal myoblast) and H9C2 (heart myoblast) cell lines. Feng et al. (2010) partially characterized the hemolytic activity of nematocyst venom from C. nozakii on chicken erythrocytes. Lee et al. (2011) compared the cytotoxicity of venoms extracted from N. nomurai, Rhopilema esculenta, C. nozakii, and Aurelia aurita on NIH 3T3 mouse fibroblast cells. Li et al. (2012) investigated the cytotoxicity of C. nozakii on Bel- 7402 and SMMC-7721 human hepatoma cells and on H630 human colon cancer cells. However, as far as we are aware, there is no published report comparing the activity intensity of venoms from C. nozakii and N. nomurai. Therefore, in the current study, we compared the toxic activities of crude venoms from C. nozakii and N. nomurai nematocysts using hemolytic and cytotoxic assays. The results provide a novel viewpoint for understanding the threats to human health posed by these two jellyfish species.
2 MATERIAL AND METHOD 2.1 Preparation of nematocystsSpecimens of C. nozakii and N. nomurai were collected from the near-shore area in Qingdao, China, in August 2012, and transferred to the laboratory immediately. All tentacles were removed from living jellyfish bodies, no less than ten tentacles were selected at random, and the remaining tentacles were stored at 80℃ before use. The selected tentacles were unbended on aluminum foils separately and left stretching naturally at 4℃ for 5 min. Then three 5 mm-long sections were cut randomly from each tentacle and transferred to one drop of sterilized and filtered seawater on the glass slide under microscope to count the numbers of nematocysts. All sections cut from selected tentacles of jellyfish specimens were observed, and the distribution and density of nematocysts in clusters on the hollow tubular tentacles were estimated totally.
Nematocysts were isolated from frozen tentacles following the methods described previously (Bloom et al., 1998) with a slight modification. Briefly, frozen tentacles were thawed at 4℃ and immersed for 3 days in a volume of sterilized and filtered (0.45 μm) natural seawater that was twice that of the tissue volume to enable autolysis. The mixture was stirred gently twice daily. The resulting suspension was filtered using different-sized mesh (following the order 250 μm, 160 μm, 90 μm, and 40 μm), and washed three times with sterilized and filtered natural seawater. After each round of washing, the filtrate was centrifuged at 3 412×g at 4℃ for 20 min and the debris was removed with a pipette. All The final undischarged nematocysts were collected, examined microscopically, and then stored at -80℃ until further use.
2.2 Venom extraction and preparationVenom was extracted from nematocysts isolated from C. nozakii (VEC) or N. nomurai (VEN) using a modified version of the technique described by Carrette and Seymour (2004). In brief, nematocysts were resuspended using Hanks' balance salt solution (HBSS, NaCl 137 mmol/L, KCl 5.6 mmol/L, CaCl2 1.26 mmol/L, MgSO4 0.81 mmol/L, Na2HPO4 0.38 mmol/L, KH2PO4 0.44 mmol/L and NaHCO3 4.2 mmol/L, the pH of HBSS was adjusted to 7.4 with 0.1 mol/L HCl and NaOH) containing 1 mmol/L of phenylmethylsulfonyl fluoride (PMSF). Before extraction, 100 μL of each nematocyst resuspension was examined using microscope respectively to check the discharge condition of the nematocysts, and the number of the nematocysts prepared for venom extraction was obtained by microscope count method. One milliliter of the nematocyst resuspension solution was placed into screw-top vials with 2.5 g of glass beads (0.5 mm in diameter), and the mixture was shaken five times in a mini bead beater (Mini-Beadbeater-16, Biospec Products, Bartlesville, USA) at 3 450 r/min for 20-s intervals with intermittent cooling on ice. After ensuring the absolute discharge of the nematocysts in resuspension microscopically, the venom was then centrifuged at 6 824×g at 4℃ for 20 min and the supernatant used for the present study. The protein concentration of the venom extracts was determined following the method of Bradford (1976) and compared with bovine serum albumin (BSA) protein concentration standards, and the venoms were then used in the hemolytic and cytotoxicity assays based on their protein concentrations.
2.3 Hemolytic activity assayThe hemolytic activity of the venoms was tested using erythrocytes from chicken, pigeon, and sheep, which could easily be obtained from the local market. In brief, fresh blood samples were mixed with 1 mg/mL of heparin sodium as an anticoagulant at a 1:10 volume immediately after blood collection. Whole blood (60 mL) was centrifuged at 684×g for 10 min at 4℃, the supernatant was removed gently, and the erythrocytes were washed three times with HBSS. The erythrocytes were then resuspended in the same buffer to make a 1% (v/v) solution for the following hemolysis assay.
Various volumes of jellyfish venoms (0.1, 0.3, 0.5, 0.7 and 0.9 mL) were added to 0.5 mL of the erythrocyte suspension obtained from the three species (chicken, pigeon, and sheep). The venom erythrocyte mixtures were adjusted using HBSS to a final volume of 1.5 mL and final protein concentrations of venoms (16, 49, 82, 115, and 147 μg/mL, respectively) were checked as mentioned above. All the mixtures were incubated at 37℃ for 30 min. Then, 1 mL of each mixture was diluted with 4 mL of HBSS and centrifuged at 1 000×g for 5 min at 4℃. The supernatants were transferred to 96-well microplates and the absorbance at 410 nm was determined using a visible spectrophotometer (7230G, Shanghai, China) to measure the extent of erythrocyte lysis. The half maximal effective concentration (EC50) values of each concentration of jellyfish venom protein were calculated to evaluate the hemolytic activity of the jellyfish venoms.
2.4 Cell culture and treatmentThe A431 human epidermal carcinoma cell line was purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM Medium (Gibco, USA) with 4 mmol/L of L-glutamine, 100 μg/mL of streptomycin and 100 U/mL of penicillin, plus 15% fetal calf serum (FCS, TBD, China), and incubated at 37℃ in a humidified 5% CO2 atmosphere.
2.5 Cytotoxicity assayCytotoxicity was assessed by measuring the mitochondrial dehydrogenase activity, using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were plated on 96-well plates at a density of 4×103 cells/well and cultured under normal conditions for 24 h. Venoms were diluted with HBSS to the same concentrations described above (16, 49, 82, 115, and 147 μg/mL), and added to pre-cultured cells, respectively. All incubations with various protein concentrations were then stopped at 24, 48, 72 and 96 h. The control group was treated simultaneously with HBSS only.
After incubation, the treated cells were observed and photographed using an inverted phase-contrast microscope (Nikon, Japan). Two hundred microliters of MTT solution (2.5 mg/mL) was added to each well and the plates were incubated for another 4 h at 37℃. The medium was then removed and replaced by 200 μL of dimethyl sulfoxide (DMSO) to solubilize the formazan crystal generated in the culture plates. The absorbance at 490 nm was read using a spectrophotometric microplate reader (MultiSkan FC, Thermo Scientific, USA) and the cell survival ratio (%) was calculated using Eq.1:
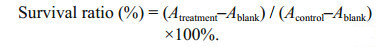 (1)
(1)The half maximal inhibitory concentration (IC50) values were calculated to evaluate the cytotoxic activity of jellyfish venoms.
2.6 Statistical analysisAll data were expressed as means±SD of three parallel measurements. The SPSS16.0 software was used for statistical analysis, and statistically significant differences between groups were determined by one way ANOVA. For further comparison between two groups, the Student-Newman-Keuls (SNK) method was used for equal variance, whereas the Games Howell test was used for unequal variances. Differences were considered significant at P < 0.05. EC50 and IC50 values were calculated using a probit regression model.
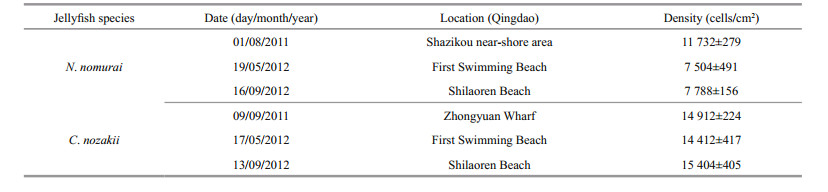
3 RESULT 3.1 Observations of nematocystsAs shown in Table 1, the density of nematocysts isolated from C. nozakii or N. nomurai was calculated after observations under an inverted microscope. There were fewer nematocysts per unit area from tentacles of N. nomurai than from tentacles of C. nozakii. Nematocysts isolated from tentacles of N. nomurai were predominant spheroidal, whereas those from C. nozakii were oval (Fig. 1).
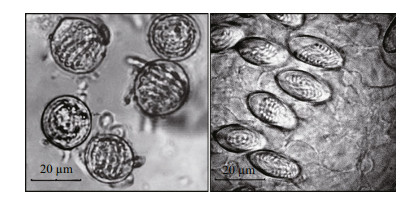
|
| Figure 1 The microscopic structure of nematocysts isolated from N. nomurai (left) and C. nozakii (right) |
The protein concentration per nematocyst was calculated based on the protein concentration of the venoms and nematocyst suspension density. As shown in Fig. 2, the protein concentration of VEN was higher than VEC at the same nematocyst density. Therefore, the protein concentration per nematocyst from N. nomurai was higher than that of C. nozakii. Interestingly, Fig. 2 (left side) shows lower concentrations of protein per nematocyst with the increasing of nematocyst densities. That was probably due to the partly discharged nematocysts in resuspension during the process of venom extraction, and protein in supernatant might induce an overestimate of protein concentration per nematocyst at lower density.
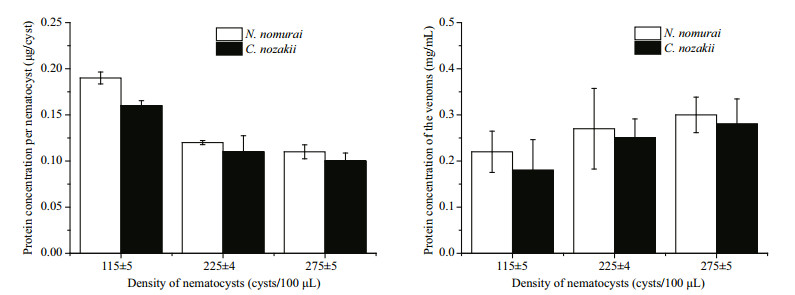
|
| Figure 2 Comparison of the protein concentration of nematocysts (left) and venoms (right) from C. nozakii (white bars) and N. nomurai (black bars) |
The hemolytic activities of venoms were assessed using erythrocyte suspensions from chicken, pigeon, and sheep. Both VEC and VEN showed strong hemolytic activities. After incubation with either VEN or VEC, all chicken, pigeon, and sheep erythrocytes showed obvious hemolytic reaction including morphological changes and obvious lysis (Fig. 3)
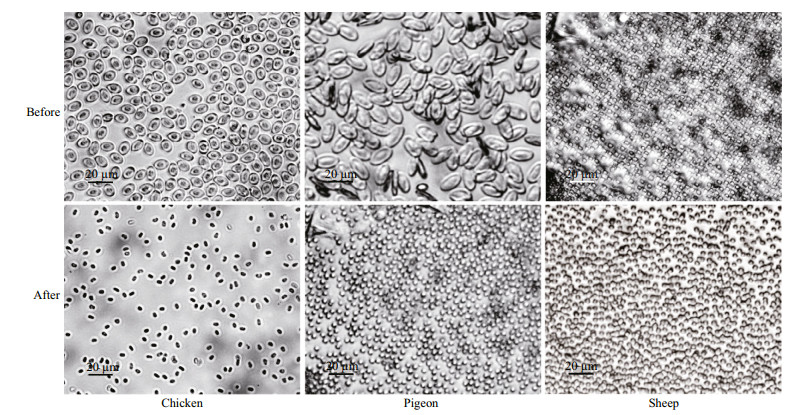
|
| Figure 3 Morphological changes of chicken, pigeon, and sheep erythrocytes before (upper) and after (lower) incubation with 147 μg/mL protein concentration of jellyfish venoms for 30 min |
Venom extracts were applied at concentrations of 16, 49, 82, 115 and 147 μg/mL. Absorbance analysis with a one-way ANOVA showed that the time- and concentration-dependent hemolytic effects were influenced significantly by the protein concentration and incubation time (P < 0.01). As shown in Figs. 4-6, treatment with 16 μg/mL of VEC for 30 min resulted in hemolysis ratios in chicken, pigeon, and sheep erythrocytes of 22.4%, 19.8% and 24.7%, respectively, whereas the hemolysis ratios of the erythrocytes treated with the same concentration of VEN were 13.8%, 12.5% and 23.8%, respectively. With an extended treatment time of 60 min, the hemolysis ratios of chicken, pigeon, and sheep erythrocytes treated with VEC increased to 26.2%, 27.6% and 33.5%, respectively, whereas the hemolysis ratios of the erythrocytes treated with VEN increased to 21.6%, 24.5% and 27.1%, respectively. The hemolytic effects of both jellyfish venoms at other protein concentrations showed similar differences, and all erythrocytes showed complete lysis after incubation with 147 μg/mL of venom (Figs. 4-6).
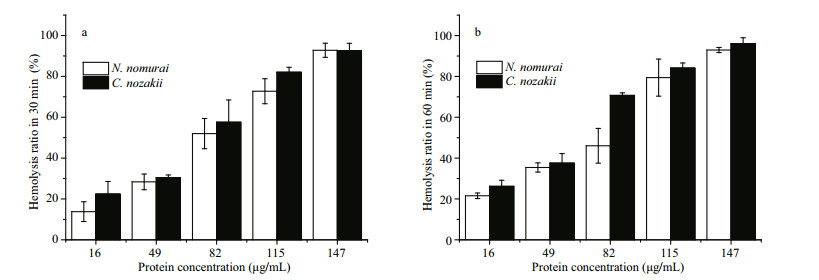
|
| Figure 4 Hemolytic effects of VEN (white bars) and VEC (black bars) on erythrocytes from chickens after 30- (a) or 60- (b) min incubation (n=3, P < 0.05) |
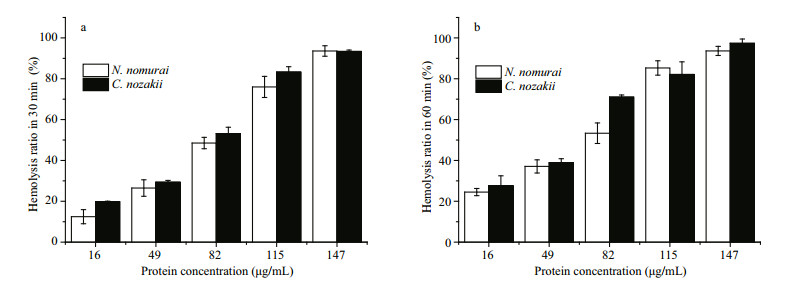
|
| Figure 5 Hemolytic effects of VEN (white bars) and VEC (black bars) on erythrocytes from pigeons after 30- (a) or 60- (b) min incubation (n=3, P < 0.05) |
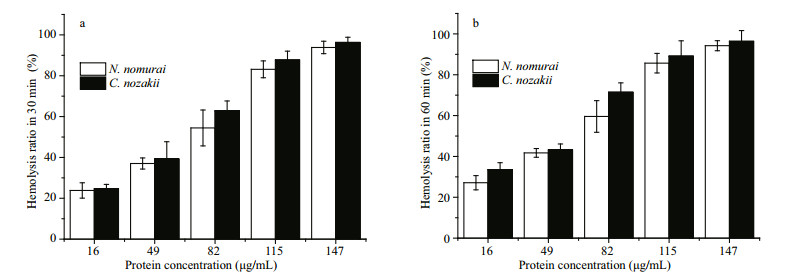
|
| Figure 6 Hemolytic effects of VEN (white bars) and VEC (black bars) on erythrocytes from sheep after 30- (a) or 60- (b) min incubation (n=3, P < 0.05) |
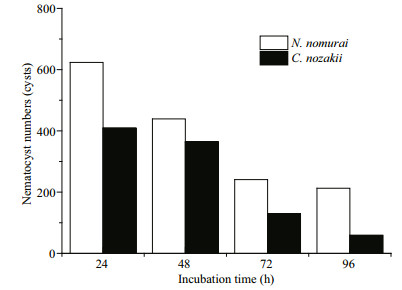
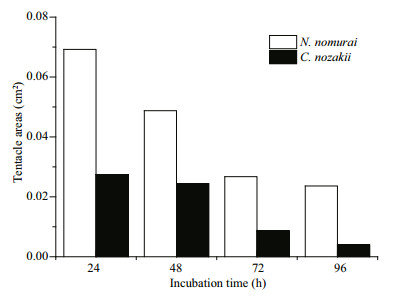
The EC50 of both VEC and VEN on erythrocytes from the three species reduced over time (P < 0.05). Comparison of the EC50 results indicated that sheep nematocysts were the most sensitive to the hemolytic effects of both jellyfish venoms, and VEC had a stronger hemolytic activity than VEN at the same protein concentrations (Fig. 7). In addition, to enable the hemolytic activity to reach the EC50 level, the number of nematocysts and tentacle area required from C. nozakii and N. nomurai were calculated according to the nematocyst densities listed above. Comparisons showed that similar numbers of nematocysts from the two species were needed to reach the EC50 level in chicken, pigeon, and sheep erythrocytes for either the 30- or 60-min exposure time (Fig. 8). Given that the number of nematocysts per unit area in tentacles of N. nomurai was lower than in C. nozakii, the tentacle area of N. nomurai corresponding to the EC50 levels on erythrocytes from the three species was larger compared with C. nozakii (Fig. 9).
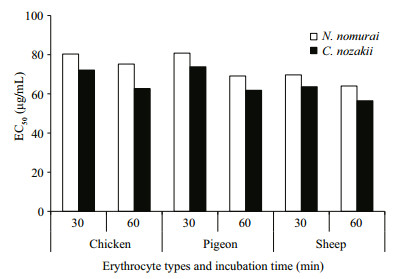
|
| Figure 7 EC50 of VEC (black bars) and VEN (white bars) on erythrocytes from chicken, pigeon, and sheep at different exposure times |
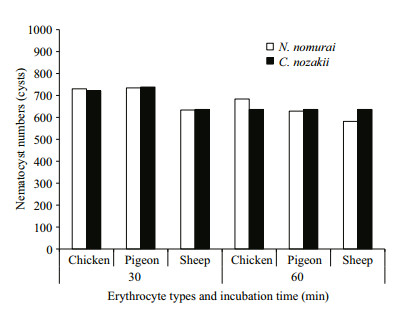
|
| Figure 8 Number of nematocysts from N. nomurai (white bars) and C. nozakii (black bars) required to meet the EC50 level on chicken, pigeon, and sheep erythrocytes over 30- and 60-min exposure times |
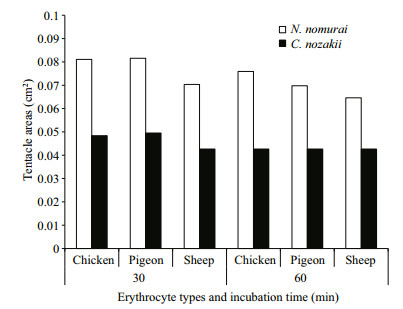
|
| Figure 9 Tentacle areas of N. nomurai (white bars) and C. nozakii (black bars) required to meet the EC50 level on chicken, pigeon, and sheep erythrocytes over 30- and 60-min exposure times |
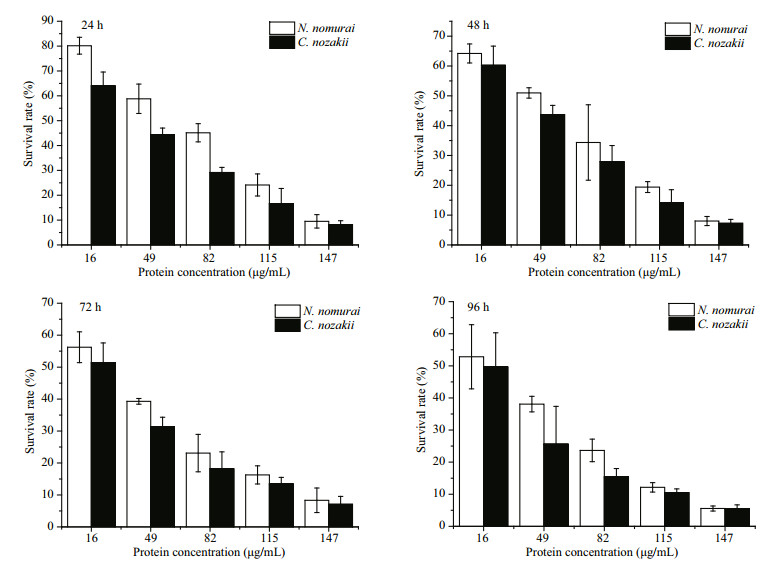
Morphological changes in A431 cells following their exposure to the jellyfish venoms were observed under a microscope. The cells of the untreated control group grew well and their nuclei were round and clear. Cells treated with either VEC or VEN were misshapen and some were broken. Nuclei were marginalized in some cells (Fig. 10). The cytotoxic activities of the venoms were evaluated using the MTT assay, and the results showed a dose-dependent inhibition of either VEN or VEC on cell viability with increasing protein concentrations (Fig. 10). After incubation with VEC and VEN for 24 h at a concentration of 16 μg/mL, the survival rates of A431 cells were 64% and 80.2%, respectively. The survival rates at 24 h reduced to 8.1% and 9.5% when the protein concentrations of venoms increased to 147 μg/mL. Meanwhile, the cytotoxic activities of both venoms were significantly related to incubation time (P < 0.05). After incubation for 96 h with VEC and VEN, the survival rates of A431 cells at a concentration of 16 μg/mL reduced to 49.7% and 52.8%, respectively. The survival rates of cells incubated with 147 μg/mL of venom reduced to 5.5% and 5.6%, respectively. Lower values of IC50 for VEC at different incubation times (Fig. 11) compared with VEN were also recorded (Figs. 12, 13).
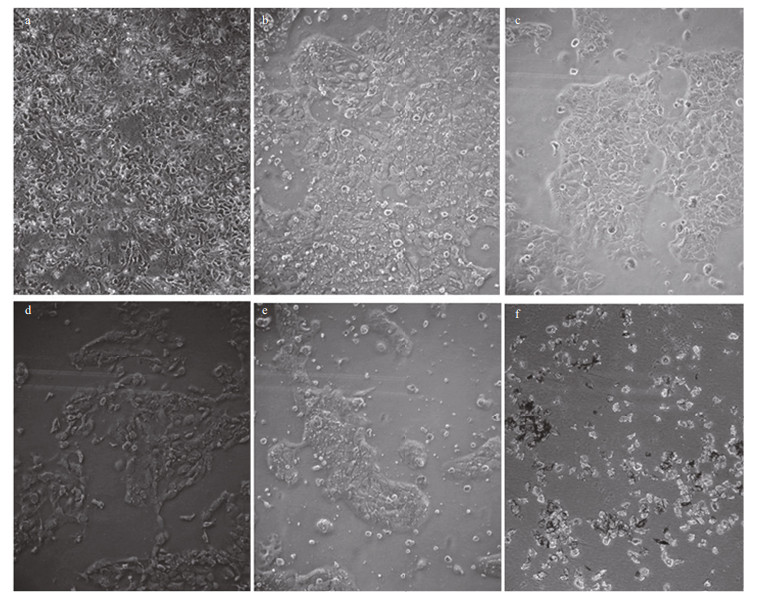
|
| Figure 10 Morphological changes in A431 cells following 24-h incubation with different concentrations of jellyfish venom a. control; b. 16 μg/mL; c. 49 μg/mL; d. 82 μg/mL; e. 115 μg/mL; f. 147 μg/mL. |
|
| Figure 11 Comparison of the cytotoxic effects of VEC (black bars) and VEN (white bars) on the viability of A431 cells following their incubation with venom for 24, 48, 72, and 96 h |
|
| Figure 12 Number of nematocysts of N. nomurai (white bars) and C. nozakii (black bars) needed to meet the IC50 cytotoxic level on A431 human epidermal carcinoma cells over different incubation times |
|
| Figure 13 Tentacle area of N. nomurai (white bars) and C.nozakii (black bars) needed to meet the IC50 cytotoxic level on A431 human epidermal carcinoma cells over different incubation times |
The jellyfish C. nozakii is widely distributed along the coast of China and blooms have been reported in the northern East China Sea, Yellow Sea, and Bohai Sea (Dong et al., 2010). It can ruin fishing nets, prey on juvenile fish, crabs and mollusks, and produce toxins that are poisonous to humans and marine animals (Zhou and Huang, 1956; Peng and Zhang, 1999; Zhong et al., 2004; Dong et al., 2006). In humans, the venom of C. nozakii can produce a burning feeling that develops into severe pain, although no deaths have been attributed to this species (Tibballs, 2006; Helmholz et al., 2007). N. nomurai is also a dominant species throughout seas along East China, including Bohai Sea, Yellow Sea, and northern East China Sea (Kawahara et al., 2006). Blooms of N. nomurai were reported in the northern part of the East China Sea, Yellow Sea, and Liaodong Bay in 2003, 2005, and 2007, respectively (Cheng et al., 2004; Ding and Cheng, 2005). This species was responsible for most of the severe or fatal cases of jellyfish stings in Chinese seas (Zhang et al., 1993; Xu et al., 2007; Jiang et al., 2008). Given that hemolytic proteins in nematocyst venoms are key molecules determining the toxic effects of jellyfish stings, including erythema and edema (Li et al., 2013), the hemolytic activity of venoms extracted from jellyfish tentacle nematocysts has been widely reported against erythrocytes from different species (Bloom et al., 2001; Torres et al., 2001; Nagai et al., 2002; Tibballs, 2006). The sensitivity with which erythrocytes reflect these conditions varies from species to species (Table 2). Kang et al. (2009) assessed the hemolytic activity of N. nomurai on cat, dog, human, rabbit, and rat erythrocytes and showed a concentration-dependent activity of venom extracts starting at 10 μg/mL of protein equivalents; dog erythrocytes were the most sensitive (EC50=151 μg/mL, 30 min). The hemolytic activity of extracts from C. nozakii was also documented on human and chicken erythrocytes (Helmholz et al., 2007; Feng et al., 2009, 2010). This study compared the hemolytic activities of venoms extracted from nematocysts of C. nozakii and N. nomurai on erythrocyte suspensions from chickens, pigeons, and sheep. Sheep erythrocytes were the most sensitive to the hemolytic effects of these two jellyfish venoms, with EC50 values of VEN and VEC of 69.69 and 63.62 μg/mL over a 30-min exposure. In addition, the venom from C.nozakii showed a stronger hemolytic activity than that from N. nomurai on all three erythrocyte suspensions at the same protein concentration. Based on the same relative amount of each jellyfish nematocyst needed to reach the EC50 level on erythrocytes, the hemolytic activities per nematocyst from N. nomurai and C. nozakii were similar. However, the tentacle area of N. nomurai corresponding to the EC50 level was larger than that of C. nozakii. Thus, the hemolytic activity per unit area of tentacle from C.nozakii was stronger than that of N. nomurai.
There are two general mechanisms to explain this hemolytic activity. The first is an enzymatic mechanism, whereby cytolytic components bind preferentially to membrane glycolipids or glycoproteins (Burnett and Calton, 1987). The other is a stoichiometric mechanism, whereby toxin molecules bind to, and insert in, the plasma membrane followed by oligomerization to form transmembrane pores (Bhakdi and Tranum Jensen, 1988). Many studies have indicated that jellyfish venoms form pore-like structures in target membranes, causing rapid cell lysis (Rottini et al., 1995; Edwards et al., 2002), and that the pore formation only occurs at the site of envenoming, possibly serving to aid the entry of other venom components into the host (Bailey et al., 2005). Thus, the hemolytic activity is likely to occur in combination with the envenomation of the jellyfish venom. However, it is not fully understood how the venoms of C. nozakii and N. nomurai elicit their hemolytic potencies in different species, and so requires further investigation.
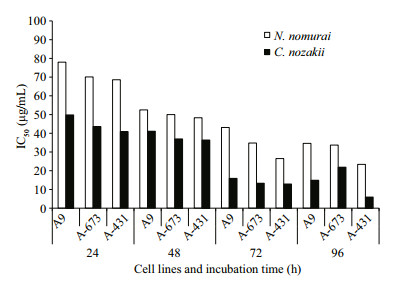
Cytotoxicity is also a common bioactivity of jellyfish venoms (Table 3). Li et al. (2012) assessed the cytotoxicity of C.nozakii venom on Bel-7402 and SMMC-7721 human hepatoma cells and H630 human colon cancer cells. H630 cells were most sensitive to the venom (IC50=15.9, 8.8, and 5.1 μg/mL after incubation for 12, 24, and 48 h, respectively) followed by Bel-7402 (17.9 μg/mL) and SMMC-7721 (24.3 μg/mL). C2C12 (muscle myoblast) and H9C2 (heart myoblast) cell lines were used to assess the cytotoxicity of crude venom from N. nomurai, which showed high cytotoxic effects against H9C2 heart myoblasts (IC50=2 μg/mL; Kang et al., 2009). Lee et al. (2011) assessed the cytotoxicity of venom from four scyphozoan jellyfish (Nemopilema nomurai, Rhopilema esculenta, C. nozakii, and Aurelia aurita) on NIH 3T3 mouse fibroblast cells, and reported a cytotoxic potency scale of C. nozakii > N. nomurai > A. aurita > R. esculenta. We had previously compared the cytotoxicity of venoms from C. nozakii and N. nomurai on A9 (mouse subcutaneous connective tissue) and A673 (human rhabdomyosarcoma) (Xu et al., 2014). The results from both our studies demonstrated that venom from C. nozakii nematocysts was more cytotoxically potent than that from N. nomurai nematocysts at the same protein concentration. Further calculations also indicated the stronger cytotoxic activities per nematocyst and per unit area of tentacle from C. nozakii compared with N. nomurai. In addition, a comparison of IC50 values of C. nozakii and N. nomurai nematocyst venoms on A9, A673, and A431 cells showed that A431 cells were most sensitive to the cytotoxic effects of both jellyfish venoms (Fig. 14).
|
| Figure 14 IC50 of N. nomurai (white bars) and C. nozakii (black bars) nematocyst venoms on A9, A673, and A431 cells following 24-96-h exposure |
In conclusion, our results showed that both C. nozakii and N. nomurai jellyfish venoms have strong hemolytic and cytotoxic activities, which might be attributed to the protein content of the venoms, with venoms from C. nozakii showing stronger toxic activities than those from N. nomurai. Sheep erythrocytes were the most sensitive to the hemolytic effects of both jellyfish venoms, and the EC50 values of venoms from N. nomurai and C. nozakii were 69.69 and 63.62 μg/mL following 30-min exposure, respectively. The IC50 values of venoms from N. nomurai and C. nozakii were 68.6 and 40.9 μg/mL, respectively, following 24-h incubation with A431 human epidermal carcinoma cells. Given that jellyfish nematocysts contain at least one toxic component, further biochemical investigations are required to characterize the different components of jellyfish nematocyst extracts and to clarify their mechanism of action.
5 CONCLUSIONComparative study on the toxic activities of crude venoms from C.nozakii and N. nomurai nematocysts shows that nematocyst venom from C. nozakii had stronger hemolytic and cytotoxic activities than that from N. nomurai. We concluded that C. nozakii might be more harmful to the health of humans than be N. nomurai, and, thus, people are advised to pay more attention on the risk of sting by C. nozakii.
Bailey P M, Bakker A J, Seymour J E, Wilce J A. 2005. A functional comparison of the venom of three Australian jellyfish-Chironex fleckeri, Chiropsalmus sp., and Carybdea xaymacana-on cytosolic Ca2+, haemolysis and Artemia sp. lethality. Toxicon, 45(2): 233-242.
DOI:10.1016/j.toxicon.2004.10.013 |
Bhakdi S, Tranum-Jensen J. 1988. Damage to cell membranes by pore-forming bacterial cytolysins. Prog. Allergy, 40: 1-43.
|
Bloom D A, Burnett J W, Alderslade P. 1998. Partial purification of box jellyfish (Chironex fleckeri) nematocyst venom isolated at the beachside. Toxicon, 36(8): 1075-1085.
DOI:10.1016/S0041-0101(98)00096-8 |
Bloom D A, Radwan F F Y, Burnett J W. 2001. Toxinological and immunological studies of capillary electrophoresis fractionated Chrysaora quinquecirrha (Desor) fishing tentacle and Chironex fleckeri Southcott nematocyst venoms. Comp. Biochem. Physiol. C Toxicol.Pharmacol., 128(1): 75-90.
DOI:10.1016/S1532-0456(00)00180-0 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Brinkman D, Burnell J. 2007. Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri. Toxicon, 50(6): 850-860.
DOI:10.1016/j.toxicon.2007.06.016 |
Burnett J W, Calton G J. 1987. Venomous pelagic coelenterates:chemistry, toxicology, immunology and treatment of their stings. Toxicon, 25(6): 581-602.
DOI:10.1016/0041-0101(87)90105-X |
Carrette T, Seymour J. 2004. A rapid and repeatable method for venom extraction from Cubozoan nematocysts. Toxicon, 44(2): 135-139.
DOI:10.1016/j.toxicon.2004.04.008 |
Cheng J H, Li S F, Ding F Y, Yan L P. 2004. Primary analysis on the jellyfish blooms and its cause in the East China Sea and the Yellow Sea. Mod. Fish. Inf., 19(5): 10-12.
(in Chinese) |
Ding F Y, Cheng J H. 2005. The analysis on fish stock characteristics in the distribution areas of large jellyfish during summer and autumn in the East China Sea region. Mar. Fish., 27(2): 120-128.
(in Chinese with English abstract) |
Dong J, Liu C Y, Wang Y Q, Wang B. 2006. Laboratory observations on the life cycle of Cyanea nozakii(Semeostomida, Scyphozoa). Acta Zool. Sinica, 52(2): 389-395.
|
Dong Z J, Liu D Y, Keesing J K. 2010. Jellyfish blooms in China:dominant species, causes and consequences. Mar.Pollut. Bull., 60(7): 954-963.
DOI:10.1016/j.marpolbul.2010.04.022 |
Edwards L P, Whitter E, Hessinger D A. 2002. Apparent membrane pore-formation by Portuguese Man-of-war(Physalia physalis) venom in intact cultured cells. Toxicon, 40(9): 1 299-1305.
DOI:10.1016/S0041-0101(02)00138-1 |
Feng J H, Yu H H, Xing R E, Liu S, Wang L, Cai S B, Li P C. 2010. Partial characterization of the hemolytic activity of the nematocyst venom from the jellyfish Cyanea nozakii Kishinouye. Toxicol. In Vitro, 24(6): 1 750-1756.
DOI:10.1016/j.tiv.2010.02.010 |
Helmholz H, Ruhnau C, Schütt C, Prange A. 2007. Comparative study on the cell toxicity and enzymatic activity of two northern scyphozoan species Cyanea capillata (L.) and Cyanea lamarckii (Péron & Léslieur). Toxicon, 50(1): 53-64.
DOI:10.1016/j.toxicon.2007.02.014 |
Huang Z, Lin M. 2012. The Living Species and Their Illustrations in China's Seas (Part Ⅰ). The Living Species in China's Seas. Ocean Press, Beijing, China. p.317-318.
(in Chinese)
|
Jiang Z G, Liu W B, Yue X A. 2008. Three cases of jellyfishsting induced death. Chin. J. Dermatol., 41(10): 707.
(in Chinese) |
Kang C, Munawir A, Cha M, Sohn E T, Lee H, Kim J S, Yoon W D, Lim D, Kim E. 2009. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa:Rhizostomeae) venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 150(1): 85-90.
DOI:10.1016/j.cbpc.2009.03.003 |
Kawahara M, Uye S, Burnett J, Mianzan H. 2006. Stings of edible jellyfish (Rhopilema hispidum, Rhopilema esculentum and Nemopilema nomurai) in Japanese waters. Toxicon, 48(6): 713-716.
DOI:10.1016/j.toxicon.2006.06.015 |
Lee H, Jung E S, Kang C, Yoon W D, Kim J S, Kim E. 2011. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon, 58(3): 277-284.
DOI:10.1016/j.toxicon.2011.06.007 |
Li C P, Li P C, Feng J H, Li R F, Yu H H. 2012. Cytotoxicity of the venom from the nematocysts of jellyfish Cyanea nozakii Kishinouye. Toxicol. Ind. Health, 28(2): 186-192.
DOI:10.1177/0748233711410910 |
Li R F, Yu H H, Xing R E, Liu S, Qing Y K, Li K C, Li B, Meng X T, Cui J H, Li P C. 2013. Isolation and in vitro partial characterization of hemolytic proteins from the nematocyst venom of the jellyfish Stomolophus meleagris. Toxicol. in Vitro, 27(6): 1620-1625.
DOI:10.1016/j.tiv.2013.04.004 |
Li R F, Yu H H, Yue Y, Liu S, Xing R E, Chen X L, Wang X Q, Li P C. 2014. In depth analysis of the in vivo toxicity of venom from the jellyfish Stomolophus meleagris. Toxicon, 92: 60-65.
DOI:10.1016/j.toxicon.2014.10.002 |
Marino A, Morabito R, La Spada G. 2009. Factors altering the haemolytic power of crude venom from Aiptasia mutabilis(Anthozoa) nematocysts. Comp. Biochem. Physiol. A Mol. Integr. Physiol., 152(3): 418-422.
DOI:10.1016/j.cbpa.2008.11.016 |
Marino A, Morabito R, Pizzata T, La Spada G. 2008. Effect of various factors on Pelagia noctiluca (Cnidaria, Scyphozoa) crude venom-induced haemolysis. Comp.Biochem. Physiol. A Mol. Integr. Physiol., 151(1): 144-149.
DOI:10.1016/j.cbpa.2008.06.013 |
Nagai H, Takuwa K, Nakao M, Ito E, Miyake M, Noda M, Nakajima T. 2000a. Novel proteinaceous toxins from the box jellyfish (sea wasp) Carybdea rastoni. Biochem.Biophys. Res. Commun., 275(2): 582-588.
DOI:10.1006/bbrc.2000.3353 |
Nagai H, Takuwa K, Nakao M, Sakamoto B, Crow G L, Nakajima T. 2000b. Isolation and characterization of a novel protein toxin from the Hawaiian box jellyfish (Sea Wasp) Carybdea alata. Biochem. Biophys. Res. Commun., 275(2): 589-594.
DOI:10.1006/bbrc.2000.3352 |
Nagai H, Takuwa-Kuroda K., Nakao M, Oshiro N, Iwanaga S, Nakajima T. 2002. A novel protein toxin from the deadly box jellyfish (Sea Wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem., 66(1): 97-102.
DOI:10.1271/bbb.66.97 |
Peng L S, Zhang X Q. 1999. Cyanea sp.endanger marine fishery resources. Mar. Inf., 8: 25.
(in Chinese) |
Rottini G, Gusmani L, Parovel E, Avian M, Patriarca P. 1995. Purification and properties of a cytolytic toxin in venom of the jellyfish Carybdea marsupialis. Toxicon, 33(3): 315-326.
DOI:10.1016/0041-0101(94)00174-7 |
Tibballs J. 2006. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon, 48(7): 830-859.
DOI:10.1016/j.toxicon.2006.07.020 |
Torres M, Aguilar M B, Falcón A, Sánchez L, Radwan F F Y, Burnett J W, Heimer-De La Cotera E P, Arellano R O. 2001. Electrophysiological and hemolytic activity elicited by the venom of the jellyfish Cassiopea xamachana. Toxicon, 39(9): 1 297-1307.
DOI:10.1016/S0041-0101(01)00081-2 |
Xu J T, Zhang X L, Pang M. 2014. Comparative study on cytotoxicity of Nemopilema nomurai and Cyanea nozakii nematocyst venoms. Adv. Mar. Sci., 32(4): 543-550.
(in Chinese with English abstract) |
Xu Y, Li H H, Li Z Z. 2007. Clinical analysis and treatment experience of 530 cases of jellyfish dermatitis. J. Clin.Dermatol., 36(5): 294-295.
(in Chinese) |
Yu H H, Liu X G, Dong X L, Li C P, Xing R E, Liu S, Li P C. 2005. Insecticidal activity of proteinous venom from tentacle of jellyfish Rhopilema esculentum Kishinouye. Bioorg. Med. Chem. Lett., 15(22): 4 949-4 952.
DOI:10.1016/j.bmcl.2005.08.015 |
Yu H H, Xing R E, Liu S, Li C P, Guo Z Y, Li P C. 2007. Studies on the hemolytic activity of tentacle extracts of jellyfish Rhopilema esculentum Kishinouye:application of orthogonal test. Int. J. Biol. Macromol., 40(3): 276-280.
DOI:10.1016/j.ijbiomac.2006.06.020 |
Zhang M L, Qin S D, Li M, Chen G Z. 1993. Investigation of coelenterare stings in North China Sea. Acta Acad. Med.Qingdao, 29(4): 263-268.
(in Chinese with English abstract) |
Zhong X M, Tang J H, Liu P T. 2004. A study on the relationship between Cyanea nozakii Kisninouye breaking out and ocean ecosystem. Mod. Fish. Inf., 19(3): 15-17.
(in Chinese with English abstract) |
 2018, Vol. 36
2018, Vol. 36