Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Kok-Keong LEE, Phaik-Eem LIM, Sze-Wan POONG, Chiew-Yen WONG, Siew-Moi PHANG, John BEARDALL
- Growth and photosynthesis of Chlorella strains from polar, temperate and tropical freshwater environments under temperature stress
- Chinese Journal of Oceanology and Limnology, 36(4): 1266-1279
- http://dx.doi.org/10.1007/s00343-018-7093-x
Article History
- Received Mar. 28, 2017
- accepted in principle May. 8, 2017
- accepted for publication Jun. 21, 2017
2 Institute of Graduate Studies, University of Malaya, 50603 Kuala Lumpur, Malaysia;
3 School of Health Sciences, International Medical University, 57000 Kuala Lumpur, Malaysia;
4 National Antarctic Research Centre, University of Malaya, 50603 Kuala Lumpur, Malaysia;
5 Institute of Biological Sciences, University of Malaya, 50603 Kuala Lumpur, Malaysia;
6 School of Biological Sciences, Monash University, Clayton, Victoria, 3800, Australia
Anthropogenic influences have led to significant changes in climate. The average global temperature has risen by about 0.8℃ since 1880 and the average global surface temperature is expected to increase by 4–5℃ over the next century (IPCC, 2007). In addition to higher average temperatures, global climate change is also resulting in higher temperature variability, thus increasing the risks to species' tolerance limits. Temperature is one of the major factors affecting the distribution and productivity of microalgae.
Understanding the growth and photosynthetic response of microalgae to the changes in their thermal environment is crucial to the characterization of their ecophysiology in nature. Temperature effects on the growth rate and photosynthesis of microalgae is of great interest as the primary production of algal biomass is strongly dependent on the prevailing photosynthetic rates in a dynamic environment. Photosynthesis is known to be a heat-sensitive process, and can be inhibited by high temperature before other symptoms of stress are detected (Camejo et al., 2005). Photosystem Ⅱ (PS Ⅱ) is reported to be the most thermosensitive component of the photosynthetic apparatus compared with the electron transport chain, stromal enzymes, PSI activity and the chloroplasts envelope (Georgieva and Yordanov, 1994; Ralph, 1998). The capacity for photochemical work is influenced by the stress levels of cells and any damage to the photochemical apparatus caused by photoinhibition (Eggert et al., 2007).
Elevated temperature has a significant effect on most metabolic processes. Photoinhibition occurs before other cell functions are damaged (Harrison and Platt, 1986; Davison, 1991). Extreme temperatures induce damage to PSⅡ by limiting electron transport and carbon fixation of the algae (Levasseur et al., 1990; Anning et al., 2001). Davison (1991) suggested that elevated temperatures were able to modulate the cellular concentrations of RUBISCO and other Calvin cycle enzymes that in turn decreased the operating quantum efficiency of PSⅡ, ΔF/Fm. Elevated temperatures will thus affect the photosynthesis rate or may induce phenotypic and genotypic changes. Moreover, high temperature stress caused inactivation of PSⅡ reaction centres (Bukhov and Carpentier, 2000; Yamamoto, 2016), causes a shift of the redox equilibrium between the primary acceptor plastoquinone (QA) and the secondary acceptor plastoquinone (QB) (Pospíši and Tyystjärvi, 1999; Tóth et al., 2007), and induces dissociation of the peripheral antenna complex of PSⅡ from its core complex (Armond et al., 1980; Wen et al., 2005). When exposed to different temperatures above and below their ambient temperature, microalgae show different photosynthetic responses (Salleh and McMinn, 2011). High temperature may cause thylakoid membrane instability, specifically affecting the membrane lipid composition while extreme low temperature causes reduced flexibility of membranes which then become crystalline or freeze (Falkowski and Raven, 2013).
Chlorella species (Chlorophyta), are small, ubiquitous, coccoid phototrophic eukaryotes found in diverse habitats including hot springs and other extreme environments (Phang and Chu, 2004). In nature, these microalgae play a role as primary producers in aquatic ecosystems, but can also serve as a source of nutraceuticals, feedstocks for biofuel, and play a role in wastewater treatment (Chu et al., 2009; Lim et al., 2010). They also show potential for electricity generation (Ng et al., 2014). The biochemical responses of Chlorella to temperature stress have been studied (Teoh et al., 2004, 2013). The protein contents of polar Chlorella were markedly affected by temperature while no clear trends were observed in their lipid and carbohydrate contents (Teoh et al., 2004).
Numerous studies have been done on the various stress factors on algal cells which involve morphological and biochemical changes that are apoptosis-like (Moharikar et al., 2006; Lee and Hsu, 2013). In some cases, cells are not killed even though their photosynthetic activity may have been completely inhibited in the face of moderate to extreme temperature fluctuations. Their performance may decline to a certain extent but when the conditions are favourable, microalgal cells have the ability to fully recover (Lee and Hsu, 2013). Polar Chlorella species have shown eurythermal adaptivity and higher growth rates at temperatures higher than ambient (Teoh et al., 2004; Cao et al., 2016). Therefore, slightly warmer temperatures are predicted to accelerate the recovery of the heat-stressed Chlorella cells, within physiological limits.
In the present study, four Chlorella strains originating from different latitudes were exposed to a range of temperatures to investigate their responses based on growth and photosynthetic performance. Their ability to recover from stress caused by exposure to unfavourable temperature was also investigated.
2 MATERIAL AND METHOD 2.1 Algae cultureFour freshwater Chlorella strains were obtained from the University of Malaya Algae Culture Collection (UMACC), namely UMACC237 (Chlorella-Ant), UMACC263 (Chlorella-Arc), UMACC248 (Chlorella-Temp) and UMACC001 (Chlorella-Trop). The identification of the Chlorella spp. was done based on morphological and molecular studies (results not shown). Chlorella-Ant was isolated from a soil sample collected near a wastewater pond at Casey Station, Antarctica; Chlorella-Arc was isolated near the Arctic Research Station at Ny-Ålesund, Norway; and Chlorella-Temp (original code: LB259) was originally isolated from a freshwater lake in the Netherlands. Chlorella-Trop was isolated from a fish pond at University of Malaya (Phang, 2004). The strains were maintained at temperatures close to those of their original habitats to avoid long-term cultivation effects (Lakeman et al., 2009), although some microalgal strains may show high genetic stability despite long term cultivation (Marija and Dieter, 2014). The cultures were maintained in a controlled-environment incubator at 4℃ for the Arctic and Antarctic strains, and at 18℃ and 28℃ for the temperate and tropical strains respectively. All cultures were maintained in Bold's Basal Medium (BBM) (Nichols and Bold, 1965) and illuminated with TLD 18W/54-765 cool white fluorescent lamps (Philips, Amsterdam, the Netherlands) providing ~42 μmol photons/(m2·s) photosynthetic active radiation (PAR) on 12 h light: 12 h dark photoperiod.
2.2 Experimental designBatch mode culturing was conducted at 4 to 33℃ for the polar strains (Chlorella-Ant and Chlorella Arc), 18 to 38℃ for Chlorella-Temp, and 18 to 43℃ for Chlorella-Trop. The cultures incubated at 4℃ for the Chlorella-Ant and Chlorella-Arc, and at 18℃ and 28℃ for the Chlorella-Temp and Chlorella-Trop strains respectively, were set as control in this experiment. The inoculum was 10% (v/v) from the exponential phase cultures standardized at an optical density of 0.2 at 620 nm (OD620). Triplicate cultures of each strain were grown in 1 L Erlenmeyer flasks with 500 mL BBM and maintained in controlled environment shaking incubators at 80 r/min (Hotech Model 718, Taiwan, China) at the range of temperatures mentioned earlier. Irradiance of ~42 μmol photons/(m2·s) PAR was provided directly from above the flasks with a 12 h light:12 h dark cycle and was measured with a Li-Cor Light Meter (Model L1-250A). Cultures were allowed to grow until stationary phase (day 10).
Growth was monitored by measuring absorbance at OD620 using a UV-1800 UV-VIS spectrophotometer (Shimadzu, Japan). Cell density was estimated by cell counting using a haemocytometer (improved Double Neubaur, Assistent, Germany). Specific growth rate (μ, /d) was calculated using the following formula:
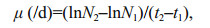
where N1 and N2 represent the cell concentrations at times t1 and t2, respectively, within the whole logarithmic phase.
Chlorophyll-a (Chl a) content was analyzed spectrophotometrically after extraction of the filtered samples (Whatman GF/C, 0.45 μm) in acetone (Strickland and Parsons, 1972). The absorbance of the pigment extract was measured at 665 nm (OD665), 645 nm (OD645) and 630 nm (OD630). The concentration of Chl a was calculated using the following formula:
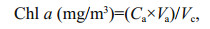
where, Ca=11.6OD665–1.31OD645–0.14OD630; Va=volume of the acetone (mL) used for extraction; Vc= volume of culture (L); Chl a (mg/L)=Chl a (mg/m3)/1 000.
The extraction and quantification of total carotenoids followed the method described by Vonshak and Borowitzka (1991). The absorbance of the pigment extract was measured at 452 nm (OD452). The total carotenoid content (μg/mL) was calculated using the following formula:
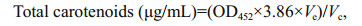
where, Ve=volume of the acetone (mL) used for extraction; Vc=volume of culture (mL).
2.3 Measurement of photosynthesis performanceNon-invasive fluorescence measurements were obtained using a high-resolution Water-Pulse Amplitude Modulated (PAM) fluorometer (Walz GmbH, Effeltrich, Germany). The cultures were dark adapted for a period of 15 min before the generation of rapid light curves (RLCs). A weak measuring light (0.15 μmol photons/(m2·s)) from the PAM fluorometer was used to measure the fluorescence yield without inducing photosynthesis, termed as minimum fluorescence (Fo, open PSⅡ reaction centers). A saturating pulse (> 3000 μmol photons/(m2·s) for 0.8 s) was used to determine maximum fluorescence (Fm in the dark) when all PSⅡ reaction centres were closed. PSⅡ quantum yield, also known as the maximal efficiency of PSⅡ, was determined as followed according to Schreiber et al. (1995):
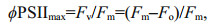
where Fm and Fo are the maximum and the minimum fluorescence yields in the dark-adapted state, respectively. RLCs were generated for all samples using the Water PAM software (WinControl, Walz). Light-emitting diodes (LEDs) provided the eight stepwise increment of actinic light used in the generation of RLCs. The actinic light levels used were 0, 48, 105, 158, 233, 358, 530, 812 and 1 216 μmol photons/(m2·s), each lasting for 10 s. While we recognise that a short exposure time, especially in dark-adapted cultures, will not provide accurate measures of steady state electron transport, all samples were processed in the same way so the changes between treatments provide a reliable measure of the relative alterations to cell performance. Photosynthetic parameters such as photosynthetic efficiency (α), maximum rate of relative electron transport (rETRmax) and photoadaptive index (Ek) were determined to compare the RLCs quantitatively (Ralph and Gademann, 2005). The relative electron transport rate (rETR) at a given irradiance was calculated by multiplying the effective photochemical efficiency and the irradiance (Genty et al., 1989). RLCs were constructed by plotting rETR against PAR data and subsequently fitted mathematically to a single exponential function (Platt et al., 1980), using a Marquardt-Levenberg regression algorithm:
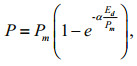
where P is the rETR at a given irradiance, Pmis the maximum potential rETR (rETRmax), α is the initial slope of the fitted RLC before the onset of saturation (light-limiting condition efficiency) and Edis the incident irradiance (400–700 nm). The function becomes an asymptotic maximum rETR value by assuming that there is no photoinhibition (Jassby and Platt, 1976). The intercept of the α value with rETRmax gave the saturation irradiance for electron transport (Ek) which is defined as:
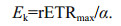
Non-Photochemical Quenching (NPQ) is a measurement of the photoprotective xanthophyll cycle whereby excessive light is dissipated as heat to avoid negative impacts to the photosynthetic electron transport chain. NPQ was calculated using the formula of Schreiber (2004):
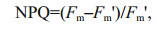
where Fm is the maximal fluorescence after a dark adaptation period and Fm' is the maximum fluorescence in the steady state light-adapted state (attained after 5–10 min). NPQ was calculated and quantified by measuring the change in Fm to the final value Fm (ΔF%). The relative inhibition of α and rETRmax in relation to the control was calculated as:
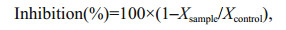
where X are the values of α or rETRmax.
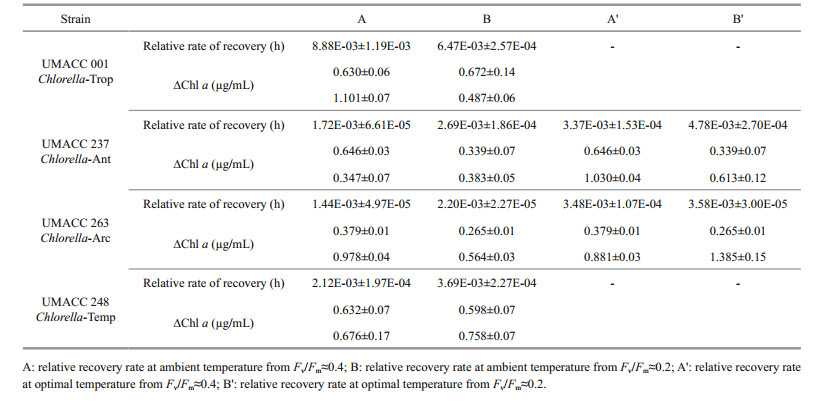
2.4 Stress and recoveryThe stress-inducing temperatures for each strain were selected based on the initial experiments with a range of increasing temperatures. Temperatures of 34℃ were used for both Chlorella-Ant and Chlorella Arc, 40℃ for Chlorella-Temp and 44℃ for Chlorella Trop. These temperatures represented the condition when the Fv/Fm decreased sharply during the growth cycle (see results), indicating a highly-stressed condition of the culture. These selected temperatures may lead to culture death with prolong exposure. The Chlorella strains were allowed to recover by returning them to their ambient temperatures after the high temperature treatments. Two threshold levels were selected; Fv/Fm≈0.4 (assigned as point 'A') and Fv/Fm≈0.2 (assigned as point 'B'). These points were selected on the basis that at Fv/Fm≈0.4, most of the culture was still able to grow while at Fv/Fm≈0.2, no net increase of biomass was observed. Biomass (Chl a) was estimated at Fv/Fm≈0.4 and Fv/Fm≈0.2. The relative rate of recovery is defined as the time taken for the culture to recover from Fv/Fm≈0.4 and Fv/Fm≈0.2, to its original Fv/Fm.
2.5 Statistical analysisData were analyzed by one-way analysis of variance (ANOVA) followed by a post-hoc test using Tukey's HSD test. Statistical analyses were carried out using Statistica 10 (StatSoft Inc., USA).
3 RESULT 3.1 Microalgal growth and pigment contentsThe Chlorella strains used in this study tolerated a wide range of temperatures. Chlorella-Trop displayed a higher specific growth rate (μ, /d) than the rest of the strains under various temperature treatments (Fig. 1) with the highest μ (0.579/d) attained at 28℃, but μdecreased markedly with increasing temperature above the optimum. Chlorella-Ant and Chlorella-Arc showed similar growth trends across the temperature treatments. Both polar strains could grow from 4℃ to the upper temperature limit of 33℃. The upper temperature limit is defined as the highest temperature that a culture can tolerate. The μ of polar strains increased with increasing temperatures, whereby the highest μ values for Chlorella-Ant and Chlorella-Arc were recorded as 0.405/dat 20℃ and 0.356/dat 10℃, respectively. However, there was no consistent growth trend observed for Chlorella-Temp but its highest μ was recorded as 0.469/d at 18℃. The lethal temperature (temperature at which the culture could not survive) was the lowest for Chlorella-Ant and Chlorella-Arc. Temperatures at and above 33℃, 38℃ and 43℃ were fatal to the polar, temperate and tropical Chlorella respectively.
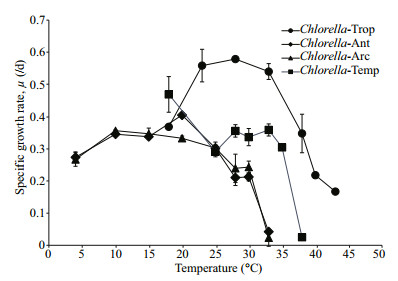
|
| Figure 1 Specific growth rates, μ (/d) of the four strains at various temperatures Data are mean±SD of triplicate samples. |
The Chl a:carotenoids ratio (μg:μg) was also used as an indicator of the stress condition of the culture (Table 1). The Chl a:car ratio (μg:μg) were significantly lower in Chlorella-Ant, Chlorella-Arc and Chlorella-Trop at temperatures above 25, 30, and 33℃, respectively. Chlorella-Temp grown at 18℃ showed the lowest Chl a:car ratio amongst the strain grown at different temperatures. The highest Chl a: car ratio in Chlorella-Ant and Chlorella-Arc were observed at 15℃ and 20℃, respectively. It was found that under the influence of cultivated temperatures, Chlorella-Ant, Chlorella-Arc and Chlorella-Trop showed a strong correlation between their specific growth rate and Chl a:car ratio (Table S1) with R2=0.933, 0.864 and 0.900 respectively (Pearson correlation, P < 0.01).
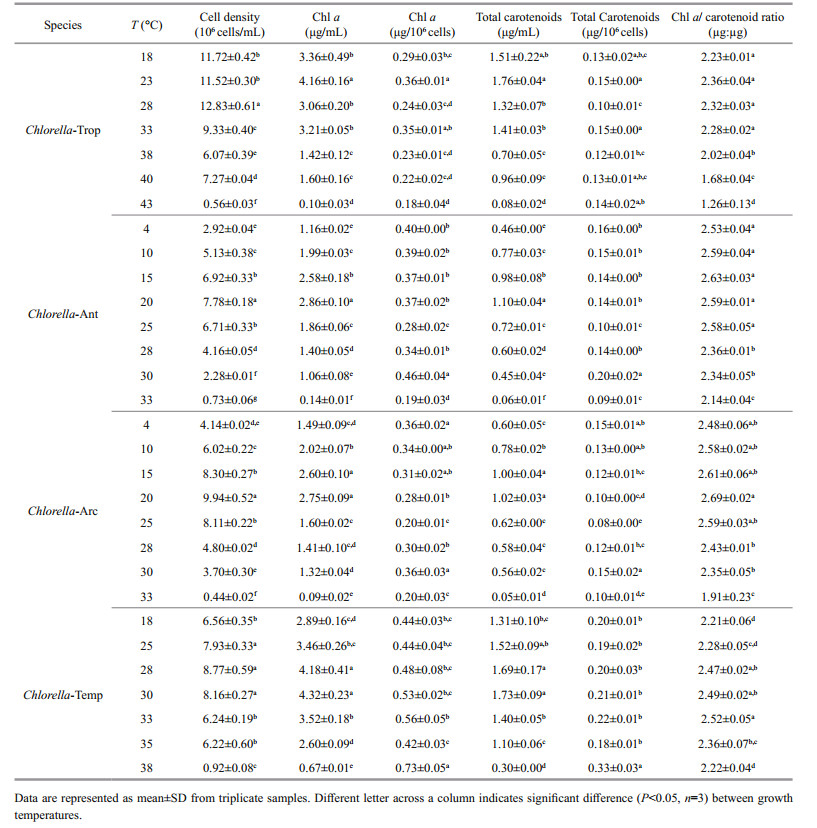
|
Effects of temperature on photosynthetic parameters varied amongst strains with regards to their latitudinal origin. During cultivation, Chlorella Ant, Chlorella-Arc and Chlorella-Trop were able to maintain a relatively stable Fv/Fm (=ϕPSⅡmax) at their ambient temperatures and at temperatures near Topt (Fig. 2). However, ϕPSⅡmax of Chlorella-Trop presented a downward trend from day 6 at 38℃ and 40℃ (Fig. 2a). For both Chlorella-Ant and Chlorella Arc, ϕPSⅡmax values were at maximum values during incubation at temperatures near 10℃, but declined as temperature rose to above 20℃. ϕPSⅡmax in both polar Chlorella were somewhat stable, albeit with comparatively lower values after day 6 at 28℃ and 30℃ (Fig. 2b, c). ϕPSⅡmax of Chlorella-Ant could still be detected on day 8 while ϕPSⅡmax of Chlorella-Arc was already zero on day 6 at 33℃. A sharp decrease in ϕPSⅡmax was observed at 33℃ for both polar Chlorella and at 43℃ for Chlorella-Trop. The variation in ϕPSⅡmax for Chlorella-Temp was temperature-independent, whereby ϕPSⅡmax fluctuated around 0.5 from 18℃ to 38℃ (Fig. 2d).
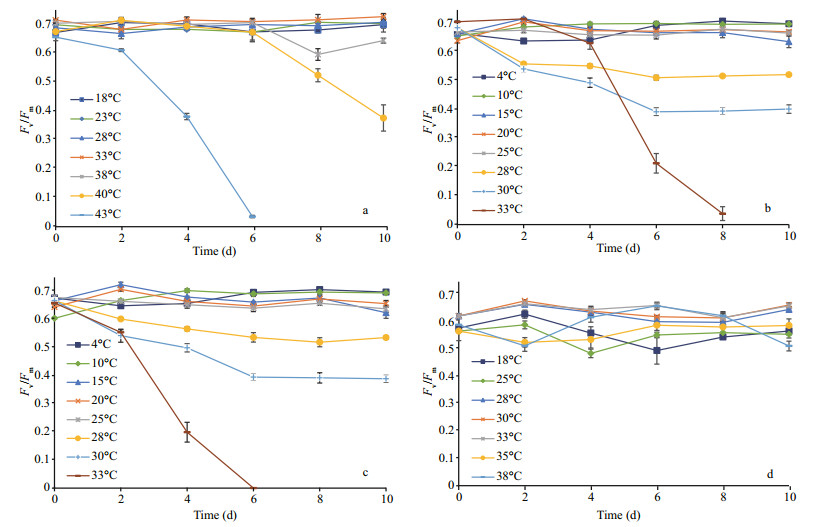
|
| Figure 2 Maximum quantum yield (Fv/Fm) of (a) Chlorella-Trop, (b) Chlorella-Ant, (c) Chlorella-Arc and (d) Chlorella-Temp grown in different temperatures Data are mean±SD of triplicate samples. |
The four Chlorella strains grown at various temperatures showed different trends in the inhibition of both rETRmax and light harvesting efficiency (α) (Fig. 3). Generally, the inhibition of rETRmax and α was increased with increasing temperature. For Chlorella-Trop, increasing temperature up to 38℃ did not inhibit rETRmax, however, temperatures beyond 38℃ lowered the rETRmax (Fig. 3a). Chlorella Ant did not show inhibition of rETRmax from 10 to 25℃, but the percentage of inhibition increased significantly (P < 0.05) with further increase in temperature (Fig. 3b). Although the inhibition of rETRmax in Chlorella-Arc was relatively higher than Chlorella-Ant, the Antarctic strain (R2=0.916, P < 0.01) displayed better correlation (Table S2) between temperature and inhibition of rETRmax than Chlorella-Arc (R2=0.775, P < 0.01) and Chlorella Trop (R2=0.604, P < 0.01). Chlorella-Arc showed 26.8%, 7.1%, 58.7%, 82.7% and complete inhibition of rETRmax at 20℃, 25℃, 28℃, 30℃ and 33℃, respectively (Fig. 3c). Inhibition of α was essentially temperature dependent especially at temperatures beyond the Topt for all strains studied. The values of α consistently declined in both polar Chlorella and Chlorella-Trop with increasing temperature. The effect of temperature on α was the most strongly correlated for Chlorella-Trop (R2=0.669, P < 0.01) followed by Chlorella-Arc (R2=0.636, P < 0.05) and Chlorella-Ant (R2=0.598, P < 0.01). Significant inhibition (P < 0.05) of rETRmax was only observed in Chlorella-Temp at 38℃ with 30.8% inhibition but α was generally suppressed across 25℃ to 38℃ (Fig. 3d).
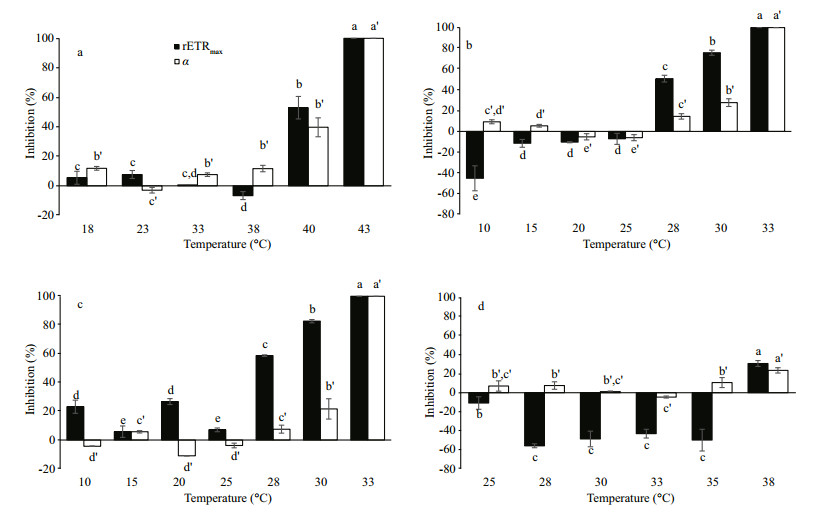
|
| Figure 3 Percentage inhibition of light harvesting efficiency (α) and maximal relative electron transport rate (rETRmax) of (a) Chlorella-Trop, (b) Chlorella-Ant, (c) Chlorella-Arc and (d) Chlorella-Temp grown at different temperatures Data were normalized against the relevant ambient temperature for each strain. Vertical bars denote standard deviations from triplicate samples and negative values represent a stimulation of the parameters. Different letters indicate significant (P < 0.05) differences. |
Generally, at 1 216 μmol photons/(m2·s) Chlorella Temp presented the lowest NPQ values ranging from 0.147 to 0.245 across the experimented temperatures (Fig. 4). NPQ in both polar Chlorella strains was activated in response to temperature stress. NPQ at 1 216 μmol photons/(m2·s) peaked at 4℃ with 0.535 followed by 0.382 at 33℃ for Chlorella-Ant. For Chlorella-Arc, NPQ at 1 216 μmol photons/(m2·s) peaked at 4℃ with 0.543 followed by 0.440 at 30℃. Chlorella-Trop recorded a largely downward trend in NPQ with increasing temperature.
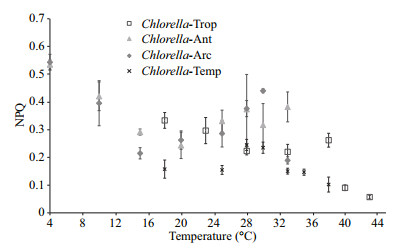
|
| Figure 4 The NPQ of Chlorella-Trop, Chlorella-Ant, Chlorella Arc and Chlorella-Temp grown at different temperatures The NPQ value is obtained at the end of each rapid light curve with the actinic irradiance of 1 216 μmol photons/(m2·s). |
All the Chlorella strains were able to recover from exposure to high temperature after being returned to their ambient or optimal temperature (Fig. 5, Table 2). The recovery rate of ϕPSⅡmax depended on the degree of damage. Recovery from Fv/Fm≈0.2 was generally faster than from Fv/Fm≈0.4 (Table 2). Only Chlorella Trop showed the inverse trend. Comparatively, Chlorella-Trop had the highest relative rate of Fv/Fm recovery, followed by Chlorella-Temp. Meanwhile, Chlorella-Ant and Chlorella-Arc showed faster relative recovery rates at their optimal temperature of 20℃ (denoted by Aꞌ and Bꞌ) than in their ambient condition (4℃) (Fig. 5b, c).
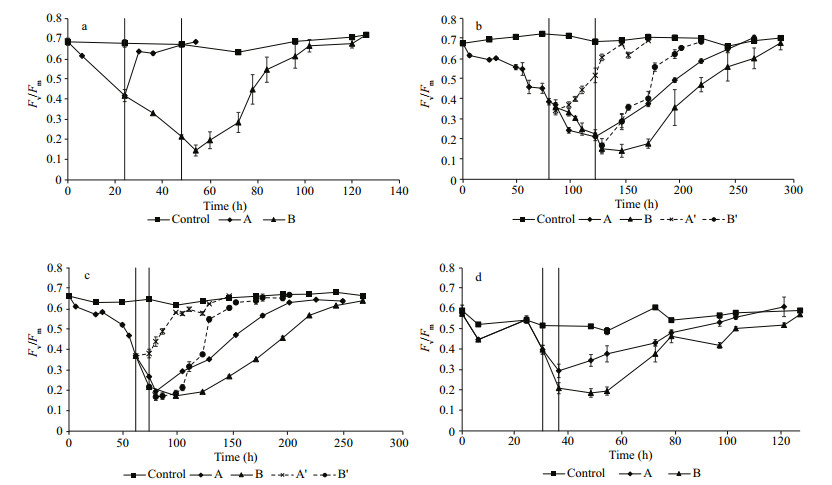
|
| Figure 5 Chlorella-Trop, (b) Chlorella-Ant, (c) Chlorella-Arc and (d) Chlorella-Temp grown at 44℃, 34℃, 34℃ and 38℃ respectively until the Fv/Fm decreased reaching the thresholds of A≈0.4 and B≈0.2 The cultures were then subjected to recovery under ambient conditions. For Chlorella-Ant and Chlorella-Arc, the recovery was additionally conducted at 20℃ (dotted line). |
|
Despite the use of a low number of strains from each latitude, which hampers our ability to draw hard conclusions about a general relationship between temperature response and latitude, the results of this study are in broad agreement with other work on various Chlorella strains from different latitudes (Teoh et al., 2013; Cao et al., 2016) and the responses of growth and photosynthesis were similar to those found in psychrophilic and temperate species of other green algae such as Chlamydomonas (Lukeš et al., 2014). The four Chlorella strains were found to be able to grow and photosynthesize at temperatures one and a half to six times higher than their ambient growth temperatures. Although the exposure time in the present study was short, at a maximum of ten days, even at the extremes of growth-permissive temperatures, the cultures were able to undergo at least one cell division over this time. For example, the μ of Chlorella-Trop under stress conditions was 0.167/d which implies that there was a turnover of at least one generation within the ten days.
4.1 The eurythermal adaptivity of polar Chlorell a strainsThis study extrapolated the possible interactions between the elevated temperatures associated with global climate change and the sensitivity of Chlorella from different latitudes, in terms of their growth and photosynthetic responses. Microalgae are able to acclimatize to changes in temperature within a short time span. The ranges of temperature used in this study varied according to the latitudinal origin of the strains and were designed to exceed their respective tolerated upper temperature limit. When exposed to a new temperature, microalgae would respond and adapt by altering their biochemical composition, pigment contents, and metabolites via regulation of gene expression (Morgan-Kiss et al., 2006; Song et al., 2014), to maintain their normal functions and prevent mortality from extreme stress. Beyond the optimal temperature range, algae would function less efficiently, and may die if the stress level is too extreme. The results showed that the polar Chlorella were able to grow at much higher temperatures, with faster growth rates than that achieved at the ambient temperature. According to Vincent (2007), the growth rates of polar species at low temperatures are less impressive compared to growth at higher temperatures. Generally, the growth rates of Chlorella increased until the respective optimum temperatures (Topt) were reached. Incubation of the Chlorella strains at supra-optimal conditions significantly affected their growth. Chlorella-Ant and Chlorella-Arc survived temperatures up to 34℃, although their optimum growth temperatures were around 20℃. Cold-adapted microorganisms include both psychrotolerant species and psychrophiles (Morgan-Kiss et al., 2006). Both polar Chlorella strains in this study are psychrotolerant as shown by the wide range of temperatures that they tolerated. As for the tropical Chlorella, an increase in temperature resulted in decreased μ and photosynthetic performance, indicating that the strain was already living at its upper limit of growth temperature. High temperature-induced stress was clearly evident when the strains were incubated at temperatures above their respective optimal temperature.
Elevated temperature had an adverse effect on the Chl a content per unit biomass, thus the photosynthetic rate per cell may decrease. Generally, Chl a decreased under temperature stress but carotenoids often remain constant or high. A reduction in Chl a might imply degradation of PSⅡ and PSI reaction centres (Luder et al., 2002). Carotenoids not only serve as accessory light-harvesting pigments but also protect photosynthetic systems as non-enzymatic antioxidant compounds against reactive oxygen species (ROS) generated during stressful conditions (Phillips et al., 1995). Photosynthetic pigments are susceptible to attack by ROS and this could lead to a decrease in the growth rate of Chlorella spp. (Takahashi et al., 2004). Chlorella cells in this study appeared to have adjusted their photoprotective carotenoids to attenuate high temperature-induced stress. The strong correlation between Chl a:car ratio and specific growth rate suggested that this ratio can be used as an indicator of the physiological status of the Chlorella species.
4.2 Photosynthetic response to temperature variationsPAM-fluorometry offers a rapid and non-invasive way to measure high temperature induced photophysiological stress in the four Chlorella strains, with respect to geographical habitat differences. The present study showed significant differences (P < 0.05) in the parameters of photosynthetic performance following exposure to various temperatures. The maximum quantum yield (Fv/Fm) of PSⅡ was found to vary significantly under incubation at different temperatures. The values of Fv/Fm observed under the ambient condition were 0.64–0.71, 0.65–0.71, 0.67– 0.71, and 0.50–0.63 for Chlorella-Ant, Chlorella Arc, Chlorella-Trop and Chlorella-Temp respectively, indicating healthily photosynthesizing cells. However, these values were significantly decreased at higher temperatures and with longer exposure periods, indicating the increase of physiological stress. The decrease in Fv/Fm suggested functional disorder of the photosynthetic apparatus and damage to PSⅡ, and cells subsequently increased their NPQ (Ralph and Gademann, 2005; Campbell et al., 2006).
Apart from regulating light utilization for photosynthesis, Chlorella cells also control the amount of light absorbed. The value of α quantifies the light harvesting efficiency (Ralph and Gademann, 2005). Values of α in all strains showed different degree of inhibition under the ranges of temperature tested. In contrast to the observation by Cao (2016) and co-workers, the current finding indicated down regulation of light capture efficiency under high temperature stress. Changes in α can be attributed to the variation in pigment composition of light harvesting complexes (LHCs) and the efficiency of energy transfer from the light-harvesting antenna to PSⅡ reactions centres (Ralph and Gademann, 2005; Serôdio et al., 2006). Damage to the photosynthetic apparatus (as reflected in the decline in Fv/Fm) arising from excessively high temperature may also contribute to the drop in α.
Excessively high temperature causes a decrease in the electron transport rate, as was observed in this study. The rETRmax provides an approximation of the rate of electron flow via PSⅡ into the photosynthetic electron transport chain and is related to the overall photosynthetic capacity of microalgae (Juneau et al., 2005). The percentage inhibition of rETRmax was higher for the cultures of Chlorella-Ant, Chlorella Arc and Chlorella-Trop, but not for Chlorella-Temp, exposed to higher temperature, indicating stress to photosynthesis which intensified with increasing temperature. The rETRmaxvaluesof Chlorella-Ant and Chlorella-Arc were decreased above 25℃ and 20℃, respectively. This implied that the electron transport rates of the microalgae were higher at temperatures below their respective optimum temperatures. It has been suggested that the enzymes of the dark reaction system might operate more rapidly and thus be able to consume NADPH2 and ATP at a faster rate (Kirk, 1994) as temperatures increase until it reaches the optimum temperature. Defew et al. (2004) observed a similar pattern in a temperate microalgal community, whereby rETRmax increased with temperature until the optimal temperature (20℃) was reached and then declined as the enzymes that control the RUBISCO activity, for example RUBISCO activase and others involved in carbon fixation, were deactivated (MacIntyre et al., 1997).
It has been demonstrated that the effect of high temperature and high irradiance can be minimized if NPQ is activated (Salleh et al., 2010). NPQ helps to regulate and protect PSⅡ against photoinhibition. Normally, NPQ is associated with the xanthophyll cycle. The xanthophyll cycle in Chlorophyta consists of the pH-dependent conversion from violaxanthin to an intermediate, antheraxanthin, and subsequently to zeaxanthin (Müller et al., 2001). When the absorption of light energy exceeds the capacity for light utilization, NPQ is activated to dissipate light energy in the form of heat (Müller et al., 2001). Enhanced NPQ activities were observed in Chlorella-Ant and Chlorella-Arc at 4℃ and at a higher temperature of 33℃ for Chlorella-Ant and 30℃ for Chlorella-Arc. The marked decline in NPQ observed in Chlorella Trop as temperature increased above 28℃ suggest the diminishing ability of this strain to dissipate excess irradiance as heat. Adverse temperatures are believed to disrupt the NPQ process in cells. With regards to this, Chlorella strains are likely to show species-specific xanthophyll cycle parameters such as rate constants and the extent and kinetics of depoxidation (Lavaud et al., 2004).
4.3 Recovery from high temperature stressThe inhibition of Fv/Fm of the Chlorella species was temperature and latitude dependent. Here, Fv/Fm was used as an indicator of the physiological health of the microalgae. As the selected strains in normal conditions showed variation in Fv/Fm with values fluctuating between 0.5 and 0.7, thus this range was selected to represent a "healthy" photosynthetic state of these strains. In addition, it was observed that by exposing different stresses the Fv/Fm value began to decline below this range. Therefore, the value of 0.5 was chosen as a threshold defining the onset of stress along with the values 0.4 and 0.2 considered different levels of stress. Also as noted by Reeves et al., (2011), Fv/Fm values of ~0.1 are usually considered representative of dead cells. We noticed that at Fv/Fm 0.4, all of the strains were still able to increase biomass (Chl a), whereas most of the strains (excluding Chlorella-Trop) showed no net increase of biomass at Fv /Fm 0.2. Although the recovery process took several days, the Chlorella strains in this study were able to restore their photosynthetic fitness from two different levels of stress (Fv/Fm≈0.4 and Fv/Fm≈0.2) even at high temperatures of 34℃ for polar Chlorella, 38℃ for Chlorella-Temp and 44℃ for Chlorella-Trop. Chlorella-Trop had the highest relative rate of recovery amongst the four strains when returned to its optimum temperature. Polar strains showed higher relative rate of recovery at 20℃ than 4℃, which suggested that the protein and enzymes for repair processes are at their optimal condition at 20℃. A number of studies (Rautenberger et al., 2015; Wong et al., 2015; Rivas et al., 2016) stated that repair processes such as the turnover and re-synthesis of D1 protein are enzymatically driven, thus the repair processes are strongly dependent on temperature (within physiological limits).
Long term exposure to high temperatures above the ambient condition may result in conformation changes and denaturation of proteins (Jensen and Knutsen, 1993). Extreme temperatures decrease the repair rate of D1 protein and thus can have damaging effects on the operation of the photosynthetic apparatus. Long et al. (1994) demonstrated that photoinhibition is associated with the loss of D1 protein, and this blocks the recovery of the PSⅡ. It was predicted that longer exposure time to excessively high temperature will cause irreversible damage to the photosynthesis apparatus of the cells and may eventually be fatal if no net recovery process takes place.
5 CONCLUSIONThe growth and photosynthetic performance of Chlorella spp. incubated at different temperatures were characterized. The findings showed how Chlorella from different geographical regions responded differently to temperature stress with changes in the photosynthetic parameters and pigment contents. Different Chlorella species are adapted to grow under different thermal habitats. Chlorella-Ant and Chlorella-Arc were found to exhibit eurythermal adaptability with a wide temperature tolerance. They are presumed to have evolved from the temperate regions and possessed different mechanisms to cope with abiotic stresses. While Chlorella-Temp was rather insensitive to the range of tested temperatures, Chlorella-Trop was already living at its upper growth temperature limit. Hence further increase in temperature negatively affected the growth of this strain. The stable temperature in the tropical freshwater environment may be the reason for Chlorella-Trop's lack of ability to tolerate large temperature fluctuations. Although variations in temperature may alter the primary productivity of Chlorella spp. to a certain extent, the rise in temperature per se is unlikely to cause negative impacts on the polar Chlorella as they were able to survive at rather high temperatures. Future work should include identifying the genes and biological pathways involved in the response to heat stress. A fundamental understanding of the thermal tolerance of Chlorella from different latitudes is crucial to predict how these microalgae will fare in the future climate scenario of rising global temperatures. However, work on additional strains from each latitudinal region is required to make definitive conclusions about the relationship between latitude and temperature response.
6 DATA AVAILABILITY STATEMENTAll data generated or analysed during this study are included in this published article and its supplementary information files.
Electronic supplementary materialSupplementary material (Supplementary Tables S1–S2) is available in the online version of this article at https://doi.org/10.1007/s00343-018-7093-x.
Anning T, Harris G, Geider R J. 2001. Thermal acclimation in the marine diatom Chaetoceros calcitrans(Bacillariophyceae). Eur. J. Phycol., 36(3): 233-241.
DOI:10.1080/09670260110001735388 |
Armond P A, Björkman O, Staehelin L A. 1980. Dissociation of supramolecular complexes in chloroplast membranes A manifestation of heat damage to the photosynthetic apparatus. Biochim. Biophys. Acta, 601: 433-442.
DOI:10.1016/0005-2736(80)90547-7 |
Bukhov N G, Carpentier R. 2000. Heterogeneity of photosystem Ⅱ reaction centers as influenced by heat treatment of barley leaves. Physiol. Plantarum, 110(2): 279-285.
DOI:10.1034/j.1399-3054.2000.110219.x |
Camejo D, Rodríguez P, Morales MA, Dell'Amico J M, Torrecillas A, Alarcón J J. 2005. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol., 162(3): 281-289.
DOI:10.1016/j.jplph.2004.07.014 |
Campbell S J, McKenzie L J, Kerville S P. 2006. Photosynthetic responses of seven tropical seagrasses to elevated seawater temperature. J. Exp. Mar. Biol. Ecol., 330(2): 455-468.
DOI:10.1016/j.jembe.2005.09.017 |
Cao K W, He M L, Yang W N, Chen B, Luo W, Zou S M, Wang C H. 2016. The eurythermal adaptivity and temperature tolerance of a newly isolated psychrotolerant Arctic Chlorella sp. J. Appl. Phycol., 28(2): 877-888.
DOI:10.1007/s10811-015-0627-0 |
Chu W L, See Y C, Phang S M. 2009. Use of immobilised Chlorella vulgaris for the removal of colour from textile dyes. J. Appl. Phycol., 21(6): 641-648.
DOI:10.1007/s10811-008-9396-3 |
Davison I R. 1991. Environmental effects on algal photosynthesis:temperature. J. Phycol., 27(1): 2-8.
DOI:10.1111/j.0022-3646.1991.00002.x |
Defew E C, Perkins R G, Paterson D M. 2004. The influence of light and temperature interactions on a natural estuarine microphytobenthic assemblage. Biofilms, 1(1): 21-30.
DOI:10.1017/S1479050503001054 |
Eggert A, Raimund S, Michalik D, West J, Karsten U. 2007. Ecophysiological performance of the primitive red alga Dixoniella grisea (Rhodellophyceae) to irradiance, temperature and salinity stress:growth responses and the osmotic role of mannitol. Phycologia, 46(1): 22-28.
DOI:10.2216/06-12.1 |
Falkowski P G, Raven J A. 2013. Aquatic Photosynthesis:Second Edition. Princeton University Press, Princeton, USA.
|
Genty B, Briantais J M, Baker N R. 1989. The relationship between the quantum yield of photosynthetic electrontransport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta, 990(1): 87-92.
DOI:10.1016/S0304-4165(89)80016-9 |
Georgieva K, Yordanov I. 1994. Temperature dependence of photochemical and non-photochemical fluorescence quenching in intact pea leaves. J. Plant Physiol., 144(6): 754-759.
DOI:10.1016/S0176-1617(11)80673-5 |
Harrison W G, Platt T. 1986. Photosynthesis-irradiance relationships in polar and temperate phytoplankton populations. Polar Biol., 5(3): 153-164.
DOI:10.1007/BF00441695 |
IPCC. 2007. Climate Change 2007:Synthesis Report.Contribution of Working Groups Ⅰ, Ⅱ and Ⅲ to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland.
|
Jassby A D, Platt T. 1976. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr., 21(4): 540-547.
DOI:10.4319/lo.1976.21.4.0540 |
Jensen S, Knutsen G. 1993. Influence of light and temperature on photoinhibition of photosynthesis in Spirulina platensis. J. Appl. Phycol., 5(5): 495-504.
DOI:10.1007/BF02182508 |
Juneau P, Green B R, Harrison P J. 2005. Simulation of PulseAmplitude-Modulated (PAM) fluorescence:limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica, 43(1): 75-83.
DOI:10.1007/s11099-005-5083-7 |
Kirk J T O. 1994. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. p.509.
|
Lakeman M B, von Dassow P, Cattolico R A. 2009. The strain concept in phytoplankton ecology. Harmful Algae, 8(5): 746-758.
DOI:10.1016/j.hal.2008.11.011 |
Lavaud J, Rousseau B, Etienne A L. 2004. General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae). J. Phycol., 40(1): 130-137.
DOI:10.1046/j.1529-8817.2004.03026.x |
Lee T C, Hsu B D. 2013. Characterization of the decline and recovery of heat-treated Scenedesmus vacuolatus. Bot.Stud., 54: 3.
DOI:10.1186/1999-3110-54-3 |
Levasseur M E, Morissette J C, Popovic R, Harrison P J. 1990. Effects of long term exposure to low temperature on the photosynthetic apparatus of Dunaliella tertiolecta(Chlorophyceae). J. Phycol., 26(3): 479-484.
DOI:10.1111/j.0022-3646.1990.00479.x |
Lim S L, Chu W L, Phang S M. 2010. Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour.Technol., 101(19): 7 314-7322.
DOI:10.1016/j.biortech.2010.04.092 |
Long S P, Humphries S, Falkowski P G. 1994. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol.Plant Mol. Biol., 45(1): 633-662.
DOI:10.1146/annurev.pp.45.060194.003221 |
Luder U H, Wiencke C, Knoetzel J. 2002. Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipiens (Rhodophyta):a study to simulate Antarctic winter sea ice cover. J. Phycol., 38(5): 904-913.
DOI:10.1046/j.1529-8817.2002.t01-1-01071.x |
Lukeš M, Procházková L, Shmidt V, Nedbalová L, Kaftan D. 2014. Temperature dependence of photosynthesis and thylakoid lipid composition in the red snow alga Chlamydomonas cf.nivalis (Chlorophyceae). FEMS Microbiol. Ecol., 89(2): 303-315.
DOI:10.1111/fem.2014.89.issue-2 |
MacIntyre H L, Sharkey T D, Geider R J. 1997. Activation and deactivation of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) in three marine microalgae. Photosynth. Res., 51(2): 93-106.
DOI:10.1023/A:1005755621305 |
Marija S, Dieter H. 2014. Sensitivity of photosynthesis to UV radiation in several Cosmarium strains(Zygnematophyceae, Streptophyta) is related to their geographical distribution. Photochem. Photobiol. Sci., 13(7): 1066-1081.
DOI:10.1039/C3PP50192B |
Moharikar S, D'Souza J S, Kulkarni A B, Rao B J. 2006. Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (Chlorophyceae)cells following UV irradiation:detection and functional analyses. J. Phycol., 42(2): 423-433.
DOI:10.1111/jpy.2006.42.issue-2 |
Morgan-Kiss R M, Priscu J C, Pocock T, Gudynaite-Savitch L, Huner N P A. 2006. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev., 70(1): 222-252.
DOI:10.1128/MMBR.70.1.222-252.2006 |
Müller P, Li X P, Niyogi K K. 2001. Non-photochemical quenching. A response to excess light energy. Plant Physiol., 125(4): 1 558-1 566.
DOI:10.1104/pp.125.4.1558 |
Ng F L, Phang S M, Periasamy V, Yunus K, Fisher A C. 2014. Evaluation of algal biofilms on indium tin oxide (ITO) for use in biophotovoltaic platforms based on photosynthetic performance. PLoS One, 9(5): e97643.
DOI:10.1371/journal.pone.0097643 |
Nichols H W, Bold H C. 1965. Trichosarcina polymorpha gen.et sp. nov. J. Phycol., 1(1): 34-38.
DOI:10.1111/jpy.1965.1.issue-1 |
Phang S M. 2004. The University of Malaya Algae Culture Collection (UMACC) and potential applications of a unique Chlorella from the collection. Jap. J. Phycol., 52: 221-224.
|
Phillips L G, Cowan A K, Rose P D, Logie M R R. 1995. Operation of the xanthophyll cycle in non-stressed and stressed cells of Dunaliella salina teod. In response to diurnal changes in incident irradiation:a correlation with intracellular β-carotene content. J. Plant Physiol., 146(4): 547-553.
DOI:10.1016/S0176-1617(11)82022-5 |
Platt T, Gallegos C L, Harrison W G. 1980. Photoinhibition of photosynthesis in natural assemblages of marinephytoplankton. J. Mar. Res., 38(4): 687-701.
|
Pospíšil P, Tyystjärvi E. 1999. Molecular mechanism of hightemperature-induced inhibition of acceptor side of Photosystem Ⅱ. Photosynth. Res., 62(1): 55-66.
DOI:10.1023/A:1006369009170 |
Ralph P J, Gademann R. 2005. Rapid light curves:a powerful tool to assess photosynthetic activity. Aquat. Bot., 82(3): 222-237.
DOI:10.1016/j.aquabot.2005.02.006 |
Ralph P J. 1998. Photosynthetic response of laboratorycultured Halophila ovalis to thermal stress. Mar. Ecol.Prog. Ser., 171: 123-130.
DOI:10.3354/meps171123 |
Rautenberger R, Huovinen P, Gómez I. 2015. Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae. Mar. Biol., 162(5): 1 087-1 097.
DOI:10.1007/s00227-015-2651-7 |
Reeves S, McMinn A, Martin A. 2011. The effect of prolonged darkness on the growth, recovery and survival of Antarctic sea ice diatoms. Polar Biol., 34(7): 1 019-1032.
DOI:10.1007/s00300-011-0961-x |
Rivas C, Navarro N, Huovinen P, Gómez I. 2016. Photosynthetic UV stress tolerance of the Antarctic snow alga Chlorella sp. modified by enhanced temperature?. Rev. Chil. Hist.Nat., 89: 7.
DOI:10.1186/s40693-016-0050-1 |
Salleh S, McMinn A, Mohammad M, Yasin Z, Tan S. 2010. Effects of temperature on the photosynthetic parameters of antarctic benthic microalgal community. ASM Sci. J., 4(1): 81-88.
|
Salleh S, McMinn A. 2011. The effects of temperature on the photosynthetic parameters and recovery of two temperate benthic microalgae, Amphora cf. coffeaeformis and Cocconeis cf. sublittoralis (Bacillariophyceae). J. Phycol., 47(6): 1 413-1424.
DOI:10.1111/jpy.2011.47.issue-6 |
Schreiber U, Endo T, Mi H L, Asada K. 1995. Quenching analysis of chlorophyll fluorescence by the saturation pulse method:particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol., 36(5): 873-882.
DOI:10.1093/oxfordjournals.pcp.a078833 |
Schreiber U. 2004. Pulse-amplitude-modulation (PAM)fluorometry and saturation pulse method. In: Papageorgiou G, Govindjee eds. Chlorophyll a Fluorescence: a Signature of Photosynthesis. Advances in Photosynthesis and Respiration Series. Kluwer Academic Publishers, Dordrecht, the Netherlands. p. 279-319.
|
Serôdio J, Vieira S, Cruz S, Coelho H. 2006. Rapid lightresponse curves of chlorophyll fluorescence in microalgae:relationship to steady-state light curves and nonphotochemical quenching in benthic diatom-dominated assemblages. Photosynth. Res., 90(1): 29-43.
|
Song Y P, Chen Q Q, Ci D, Shao X N, Zhang D Q. 2014. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol., 14: 111.
DOI:10.1186/1471-2229-14-111 |
Strickland J D, Parsons T R. 1972. A Practical Handbook of Seawater Analysis. 2nd edn. Fisheries Research Board of Canada, Ottowa, Canada.
|
Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H. 2004. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol., 45(2): 251-255.
DOI:10.1093/pcp/pch028 |
Teoh M L, Chu W L, Marchant H, Phang S M. 2004. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J. Appl. Phycol., 16(6): 421-430.
DOI:10.1007/s10811-004-5502-3 |
Teoh M L, Phang S M, Chu W L. 2013. Response of Antarctic, temperate, and tropical microalgae to temperature stress. J. Appl. Phycol., 25(1): 285-297.
DOI:10.1007/s10811-012-9863-8 |
Tóth S Z, Schansker G, Garab G, Strasser R J. 2007. Photosynthetic electron transport activity in heat-treated barley leaves:the role of internal alternative electron donors to photosystem Ⅱ. Biochim. Biophys. Acta, 1767(4): 295-305.
DOI:10.1016/j.bbabio.2007.02.019 |
Vincent W F. 2007. Cold tolerance in cyanobacteria and life in the cryosphere. In: Seckbach J ed. Algae and Cyanobacteria in Extreme Environments. Springer, Dordrecht, Netherlands. p. 287-301.
|
Vonshak A, Borowitzka M. 1991. Laboratory manual:research seminar and workshop on mass cultures of microalgae. Slipakorn University, Thailand: 40p.
|
Wen X G, Gong H M, Lu C M. 2005. Heat stress induces an inhibition of excitation energy transfer from phycobilisomes to photosystem Ⅱ but not to photosystem Ⅰ in a cyanobacterium Spirulina platensis. Plant Physiol.Biochem., 43(4): 389-395.
DOI:10.1016/j.plaphy.2005.03.001 |
Wong C Y, Teoh M L, Phang S M, Lim P E, Beardall J. 2015. Interactive effects of temperature and UV radiation on photosynthesis of Chlorella strains from polar, temperate and tropical environments:differential impacts on damage and repair. PLoS One, 10(10): e0139469.
DOI:10.1371/journal.pone.0139469 |
Yamamoto Y. 2016. Quality control of photosystem Ⅱ:the mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front Plant Sci., 7: 1 136.
|
 2018, Vol. 36
2018, Vol. 36


