Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SUN Yanyu(孙延瑜), WANG Hui(王慧), LI Junde(李俊德), WANG Bin(王斌), QI Cancan(齐灿灿), HU Xiaoke(胡晓珂)
- Nutrient-enhanced n-alkanes biodegradation and succession of bacterial communities
- Chinese Journal of Oceanology and Limnology, 36(4): 1294-1303
- http://dx.doi.org/10.1007/s00343-018-6310-y
Article History
- Received Dec. 9, 2016
- accepted in principle Apr. 17, 2017
- accepted for publication Jun. 7, 2017
2 University of Chinese Academy of Sciences, Beijing 100049, China
Oil spills are a major source of pollution that exert obvious negative impacts on various biota, including microorganisms, vegetation, fish and animals. The potential impacts of oil spills on biogeochemical processes, ecosystem services and the ultimate link to the health of humans have recently drawn increased attention (Mendelssohn et al., 2012). After the Deepwater Horizon oil spill in the Gulf of Mexico in 2010, several large-scale oil spills occurred in China, including the Penglai 19-3 oil spill in Bohai Bay in 2011, Binzhou oil spill in 2012, and Qingdao oil pipeline explosion in 2013. Significant impacts on the environment were detected after these oil spills; accordingly, cost-effective and environmentally benign strategies are urgently needed to remediate spilled oil. Microorganisms, especially bacteria, play an important role in biodegradation of most organic pollutants (Head et al., 2006). The process of degrading hydrocarbon components is widely attributed to various microorganisms, and microorganism related bioremediation, such as phyto-microbial remediation, has been shown to be an effective method of removing residual oil from various environments (Atlas, 1995; Margesin, 2000; Röling et al., 2004a, b; Zhao et al., 2011; Martin et al., 2013).
Bacteria that can degrade hydrocarbons affiliated with different phyla, including Flexibacter Cytophaga-Bacteroides, Alpha-, Beta-, Gamma Proteobacteria, Actinobacteria, Cyanobacteria and Firmicutes, have been cultured in the laboratory (Yakimov et al., 1998; Hennessee et al., 2009; Zhao et al., 2010; Szabó et al., 2011; Luo et al., 2012). However, laboratory cultured functional bacteria only play limited roles in the in situ bioremediation of crude oil. One of the most important reasons is that environmental factors, especially nutrients (nitric salts and phosphates), could be a significant restricted parameter for the growth of functional bacteria, which vary dramatically between the in situ and laboratory environments (Wang et al., 2013).
Nitrogen and phosphorus, which are the two major limiting nutrients for all organisms on earth, are essential to synthesis of DNA, RNA, proteins, phospholipids and many other important biomolecules. In oil contaminated environments, nitrogen and phosphorus are generally insufficient to support the growth of in situ microorganisms. Indeed, considering the high carbon content of oil, the low levels of nitrogen and phosphorus are the factors limiting hydrocarbon degradation in oxic soil environments (Mohn and Stewart, 2000). Therefore, it is suggested that the addition of nitrogen and phosphorus to a contaminated environment could stimulate the growth of functional microorganisms, leading to an increase in the biodegradation rate of crude oil. McKew suggested that biostimulation with nutrients (N and P) produced a significant increase (29%; P < 0.05) in degradation of n-alkanes after 15 days, and an increase of one order of magnitude in the concentration of Thalassolituus (McKew et al., 2007). However, excessive nutrient concentrations can also inhibit the biostimulation progress. Several authors have reported the negative effects of high nutrient levels on the biodegradation of oil (Carmichael and Pfaender, 1997; Oudot et al., 1998; Chaîneau et al., 2005). A better understanding of nutrient enhancement of crude oil biodegradation is essential to monitoring of bioremediation projects. In this experiment, changes in the microbial community structure during crude oil biodegradation with nutrient enhancement rather than a single strain were investigated. The results agreed with those of the actual restoration project and therefore have the potential for use as an improved reference relative to existing data.
A discarded oil wellhead located at the oil producing city Binzhou in northern China blew out on November 15, 2012, resulting in thousands of square meters of farmland and a stream flowing through the area being badly contaminated. Additionally, more than 3 000 ducks on a farm near the wellhead were killed by the spilled oil. Accordingly, this contaminated environment was in urgent need of remediation. In this case, sediments from the contaminated stream were collected immediately after the oil spill, after which microcosms were constructed in the laboratory using the sampled sediments to investigate: 1, the capacity for bioremediation of crude oil by indigenous bacterial communities; 2, enhancement of biodegradation in response to nutrient addition; 3, changes in bacterial communities during crude oil bioremediation.
2 MATERIAL AND METHOD 2.1 Sediments and crude oil collectionSediments for microcosm construction were collected from an oil contaminated stream in Binzhou, Shandong Province, China (37°24′293″N, 118°06′767″E). The field studies did not involve endangered or protected species. Field work in this study was only conducted to sample oil contaminated soil and sediments; thus, no special permission was needed. In the field, sediments were kept in a cooler with insulation and ice packs, then transported to the laboratory within 24 hours for further analysis. The crude oil was obtained from Shengli Oilfield, Dongying, China and stored at room temperature. The API (American Petroleum Institute) gravity of the crude oil was 25.6 and the viscosity was 4 896 mPa-s when measured by a rotary viscometer (Shanghai Changji Instrument Tech. Co. Ltd., China).
2.2 Experimental setup for nutrient-enhanced bioremediation of crude oilTen-gram sediments and aliquots (2% w/v) of crude oil were co-incubated in 100 mL mineral solution (4 mmol/L MgSO4, 0.5 mmol/L CaCl2, 85 mmol/L NaCl) to conduct the oil bioremediation experiment. To evaluate the stimulation of bioremediation by nutrient amendments, an experimental setup designated as TON (treatments with both oil and nutrient) was conducted. Inorganic nutrients consisting of nitrate (as sodium nitrate, 82 g/L) and phosphorus (as potassium dihydrogen phosphate, 6 g/L) were added at the beginning of the bioremediation process and at 10 days and 20 days. A parallel experiment, designated as TO (treatments with oil only), was conducted without the addition of nutrients. All treatments were performed in duplicate and maintained using 250 mL Erlenmeyer flasks in a 170 r/min shaker at 30℃ for 30 days. Samples were vortexed for 10 min using a vortex mixer to ensure that the soil and solution phases were mixed well, after which 10 mL aliquots were collected from the homogenized mixture. Aliquots were collected on day 0, 10, 20, and 30, then stored at -80℃ until further analysis of crude oil composition.
2.3 Analysis of crude oil compositionResidual crude oil was extracted three times with 10 mL of n-hexane and 30 min ultrasonic treatment. The extract was then combined and dried with anhydrous sodium sulfate (Bost et al., 2001). The volume of the extract was adjusted to 25 mL after air drying, after which the components of extracts were fractionated by column chromatography (Head et al., 2006) and analyzed using a Gas Chromatography Mass Spectrometer (GC-MS, Agilent 7890-5975c) equipped with a HP-5 capillary column (Agilent Technologies, U.S.A., 60 m×0.25 mm×0.25 μm). High purity helium (99.999%) was applied as the carrier gas at a flow rate of 1 mL/min. The temperature program was as follows: 50℃–120℃ at 20℃/min, 120℃–250℃ at 4℃/min, 250℃–310℃ at 3℃/min, and then held at 310℃ for 30 min. The transfer line and injector temperatures were both set at 300℃ and the remaining hydrocarbon concentrations in the cultures were calculated according to the peak area. Anthracene (20 mg/L) was used as an internal standard to calibrate the GC-MS measurement. The recovery was 62.38%±3.68% and the RSD was 9.87%.
2.4 DNA extractionAliquots (5 mL) of samples collected at the end of the incubation period for both remediation experiments were centrifuged at 6 000×g (Thermo Scientific, Waltham, MA, USA) to collect microbial flora. A PowerSoil® DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA, USA) was then applied to extract the total genomic DNA from collected pellets and original contaminated sediments (OCS). We used two culture-independent methods, denaturing gradient gel electrophoresis (DGGE) and 16S rRNA gene based clone library analysis bacterial communities from samples before and after bioremediation (Mohamed et al., 2008; Wang et al., 2012).
2.5 DGGE analysis of bacterial communitiesFor DGGE analysis of the bacterial community, a variable region (~195-bp) corresponding to positions 341 and 534 in the 16S rRNA gene of Escherichia coli was PCR amplified from total genomic DNA using primers P2 and P3 (Mohamed et al., 2008; Wang et al., 2012). The PCR amplification was conducted in a 50-μL reaction system that included 37.8 μL of sterilized distilled water, 5 μL of 10× high fidelity PCR buffer, 2 μL of template DNA, 1 μL of 100 μmol/L of P2 and P3 primer, 1 μL of a mixture of dNTPs (2.5 mmol/L each), 0.2 μL of Platinum® Taq DNA (Invitrogen Life Technologies, Carlsbad, CA, USA) and 2 μL of MgSO4 (50 mmol/L). PCR amplification was conducted as follows. A DCode system (BioRad, Hercules, CA, USA) was applied for DGGE analysis of the bacterial communities and a 5-min initial denaturing period at 97℃ was followed by 29 cycles of 92℃ for 30 s, 52℃ for 2 min, and 72℃ for 90 s, and then final extension at 72℃ for 30 min. To separate different bacterial amplicons, 6% (w/v) polyacrylamide gel with a denaturing gradient of 40% to 70% in 1×Tris-acetate-EDTA was used. Electrophoresis was applied at 60 V and 60℃ for 16 h. The gel was then stained with SYBR green Ⅰ (diluted 1:10 000, Invitrogen) for twenty minutes and visualized with a Molecular Imager® Gel DocTM XR+ System (BioRad, Hercules, CA, USA). Scanned negative DGGE gels were analyzed using Quantity One 4.6 (BioRad) for statistical analysis of DGGE tracks. Similarities between samples were calculated using the Dice coefficient (band based).
2.6 Cloning and phylogenetic analysis of 16S rRNA gene16S rRNA gene based clone libraries were constructed to investigate bacterial community succession. Briefly, the bacterial universal primers 27F and 1492R were used to amplify the 16S rRNA gene fragments of total genomic DNA extracted from the bacterial communities as previously described (Enticknap et al., 2006). PCR products were then ligated into the PCR-XL-TOPO vector, after which the TOPO XL PCR cloning kit (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to transform the PCR-XL-TOPO vector into One Shot TOP 10 chemically competent Escherichia coli cells. Colonies were then randomly selected from selective plates (Luria-Bertani agar plates with 50 μg/mL kanamycin), after which they were incubated in 2×YT medium (NaCl 5 g/L, tryptone 16 g/L, yeast extract 10 g/L, with 50 μg/mL kanamycin) overnight. Plasmid DNA was sequenced using primer 27F.
Chimeric sequences from clone libraries were identified using the CHECK_CHIMERA program of the Ribosomal Database Project. Sequences that were < 400 bp in length were removed from the file using the MEGA 5.0 software package (Tamura et al., 2011). We then used the BLASTn tool at the National Center for Biotechnology Information website to aid in selection of the closest reference sequences, after which phylogenetic analysis of the 16S rRNA gene sequences from clone libraries was conducted. Different phylotypes were classified using the Mothur software (Schloss et al., 2009).
2.7 Nucleotide sequence accession numbersThe 16S rRNA gene sequences from clones newly determined in this study have been deposited in GenBank under accession numbers BankIt1966583: (330).
3 RESULT AND DISCUSSION 3.1 Bioremediation of crude oilThe GC-MS analysis suggested that n-alkanes, a major component in crude oil, could be significantly degraded with/without nutrient amendments after incubation for 30 days. Depletion of n-alkanes with chain lengths of C 16 to C 30 was evaluated following treatment with oil (TO) or oil and nutrient (TON). The depletion was characterized by comparison of percentages of remaining n-alkanes with levels in the original contaminated sediments (OCS). Chemical analysis demonstrated significant differences between TO treatments and TON treatments after 30 days of incubation. As shown in Fig. 1, when crude oil was added to the OCS as the stress factor (TO treatments), the depletion of n-alkanes was chain-length dependent. Shorter n-alkanes (C 16 to C 23) showed a relatively higher depletion rate, with homogeneous values ranging from 57.16% to 60.52%. For longer n-alkanes (C 24 to C 30), percentages of remaining n-alkanes increased from 46.60% for C 24 to 99.73% for C 30. In contrast, dramatic degradation rates were observed in TON treatments. However, only C 16 –C 30 were detected in our experiment. This may have occurred because concentrations of other n-alkanes were below the detection limit. All ingredients of measured n alkanes were reduced to less than 5% of their initial value.
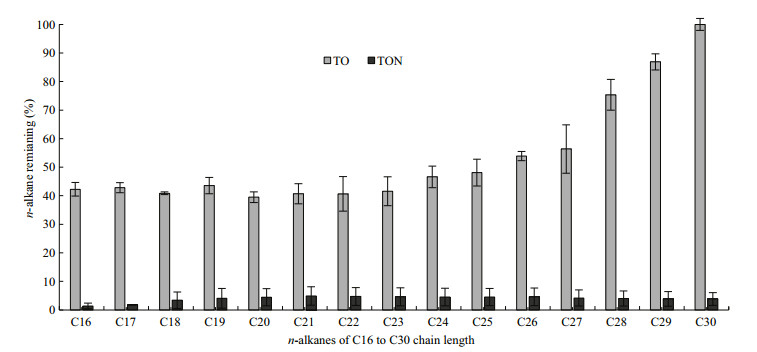
|
| Figure 1 Percentage of remaining n-alkanes during the microcosm experiment after 30 days of incubation TO: contaminated soils amended with crude oil at 30 days; TON: contaminated soils amended with crude oil and nutrients at 30 days. Each value represents the mean number from duplicate samples. |
The GC-MS analysis conducted in the present study indicated that nutrient addition dramatically stimulated the biodegradation process. Trials in which nutrients were added to various oil-contaminated environments have confirmed that nutrients, mainly nitrogen and phosphorus, are key factors influencing biodegradation of crude oil. Studies were performed successively to investigate the effects of oil degradation by addition of different values of nutrients in crude oil contaminated intertidal zone of Stert Flats, Somerset, UK (Röling et al., 2002; Röling et al., 2004a, b). All three studies demonstrated that adequate levels of nutrient addition could enhance the biodegradation of crude oil, while higher levels did not show further enhancement. After conducting a nutrient amendment in soil microcosms, Chaîneau et al. (2005) suggested that the C/N/P ratio should be considered when designing the bioremediation of crude oil polluted soils. In this study, the C/N/P ratio (C:N:P=100:10:1) was calculated based on the concentration of crude oil in collected sediments. With the addition of nutrients, n-alkane was fully degraded, while an obviously lower degrading rate was observed when nutrients were not supplemented. The other phenomenon observed in this study was that shorter-chain-length n-alkanes showed higher degrading rates than longer-chain-length ones, especially in the treatments without added nutrients. These findings were consistent with the finding that small hydrocarbon molecules were more readily biodegraded than longer ones (Harayama et al., 1999; Sei et al., 2003).
3.2 Changes in bacterial communities associated with oil degradationMonitoring the dynamics of microbial communities could facilitate identification of functional bacteria responsible for the biodegradation of crude oil, which in turn would be beneficial to development of new strategies for bioremediation of crude oil contamination by these bacteria (Wang et al., 2013). In this study, 16S rRNA gene based DGGE and clone library analysis were used to investigate succession of bacterial communities under the stress of oil contamination, and functional bacterial communities relevant to oil degradation were predicted.
The DGGE profile analyzed from the different microcosms revealed a significant difference between oil-amended sediments with or without nutrients and the bacterial community of the natural sediment (Fig. 2). DGGE patterns from all microcosms contaminated by crude oil for a period of ten days showed high similarity to each other and unambiguous changes compared to the natural sediment. The development of clearly different microbial communities was detected after ten days of domestication. All TO samples, except TO2 at 30 d, were grouped into a distinct cluster with 70% similarity. The four TON samples were found to be combined into one group, but significant differences were detected between individual samples. TON1 at 20 d and TON1 at 30 d showed 60% similarity, while the value for TON2 20 d and TON2 at 30 d was 65%. The lower similarity between TON1 and TON2 was observed with a value of 50%.
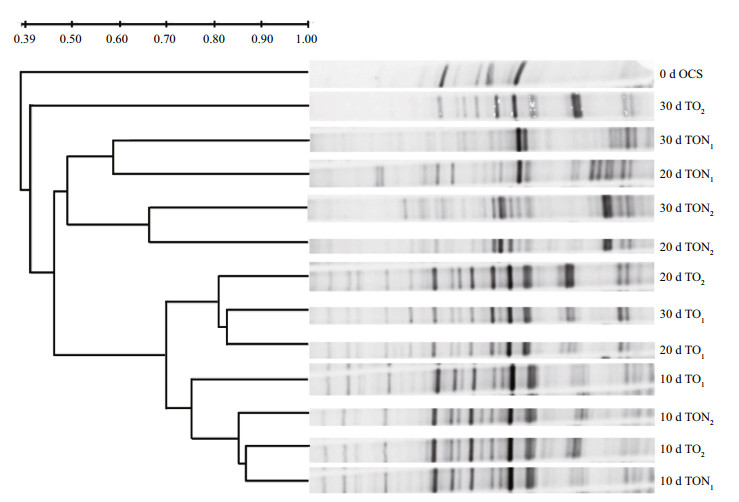
|
| Figure 2 DGGE analysis of bacterial community dynamics during n-alkane bioremediation in microcosm treatments OCS: original contaminated soils amended with crude oil at day 0; TO: contaminated soils amended with crude oil at 30 days; TON: contaminated soils amended with crude oil and nutrient at 30 days. 0 d, 10 d, 20 d, and 30 d indicate microcosms incubated for 0, 10, 20 and 30 days respectively. |
Bacterial communities in the original contaminated sediment, treatments amended with crude oil, and treatments amended with crude oil and nutrients were analyzed using a 16S rRNA gene based clone library, and six clone libraries were generated based on analysis of the DGGE profiles. Two clone libraries were constructed for the original contaminated sediment (designated as OCS1 and OCS2) used as a control to investigate bacterial communities in the original contaminated sediment. The other four clone libraries were constructed for treatments at 30 d, including two for samples amended with crude oil (designated as TO32 and TO34), and two for samples amended with both crude oil and nutrients (designated as TO31 and TO33).
Phylogenetic analysis was applied to a total of 330 sequences from the six clone libraries (55, 56, 61, 59, 48, and 51 sequences for OCS1, OCS2, TO32, TO34, TON31 and TON33, respectively). This generated 117 phylotypes affiliated with the following phyla: Acido bacteria (13), Armatimonadetes (1), Bacteroidetes (9), Cyanobacteria (1), Firmicutes (2), Gemmati monadetes (1), Nitrospirae (3), Planctomycetes (2), Proteobacteria (72), Verrucomicrobia (9) and unclassified bacterial group (4). Few changes were detected for bacteria affiliated with Acidobacteria, Bacteroidetes, and Verrucomicrobia. Diversity of bacterial communities decreased dramatically after 30 days of co-incubation with crude oil and nutrients (Fig. 3). Cyanobacteria, Fusobacteria, Gemmati monadetes, and epsilon-Proteobacteria were detected in OCS clone libraries, but not in clone libraries for TO and TON samples, which were treated with oil. Members of the Proteobacteria, especially gamma Proteobacteria, dominated the bacterial group in all six clone libraries. Different classes in Proteobacteria showed various trends after being treated with oil and nutrients. Proportions of bacterial colonies affiliated with alpha-Proteobacteria increased in all TO and TON treatments compared to OCS clone libraries, while increases in delta- and beta-Proteobacteria were only found when nutrients and oil were amended together. The ratio of colonies in beta-Proteobacteria accounted for up to 19.47%±2.35% of the total population in the TON treatments, compared to 4.38%±0.96% and 5.73%±1.53% in the OCS and TO treatments, respectively. Bacterial colonies affiliated with gamma-Proteobacteria decreased dramatically in the two clone libraries for TON treatments compared to OCS and TO treatments. For instance, 60.64%±1.96% and 58.65%±4.70% of recovered colonies were affiliated with gamma-Proteobacteria in the clone libraries constructed for samples OCS and TO, while 45.86%±2.87% of the total colonies were grouped into gamma-Proteobacteria in the two TON clone libraries.
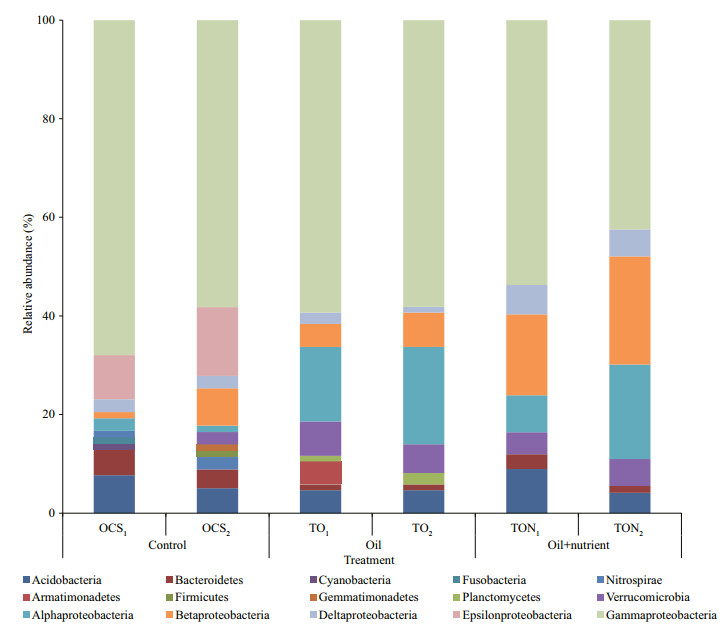
|
| Figure 3 Successions of bacterial communities (phylum level) in the process of n-alkanes degradation based on 16S rRNA gene clone library analysis |
The 12 most dominant phylotypes recovered from all six clone libraries were selected to analyze the succession of bacterial communities (Table 1). These phylotypes possessed 328 colonies, which accounted for 68.62% of colonies from all six clone libraries. The results indicated that degradation related bacteria were selected with the addition of crude oil, while bacterial communities with other functions were eliminated after 30 days of incubation. As shown in Table 1, colonies affiliated with the unclassified genus in the family of Comamonadaceae in beta Proteobacteria, unclassified genus in the class PYR10d3 in gamma-Proteobacteria, the genus of Hydrocarboniphaga in gamma-Proteobacteria were only detected or showed dramatic increases in clone libraries for samples treated with oil (TO and TON). For example, the phylotypes grouped into the genus Hydrocarboniphaga were not recovered from the original oil samples (OCS), while they reached 3.04%±1.01% and 13.19%±0.46% after treatment with oil and oil/nutrients, respectively. Two cultured species in this genus, H. effuse (Palleroni et al., 2004) and H. daqingensis (Liu et al., 2011), were reportedly capable of degrading n-alkanes. Similar results were also reported for the phylotype affiliated with the family Comamonadaceae, which contained functional genera capable of degrading n-alkanes and cyclic alkanes (Brzostowicz et al., 2005; Mattes et al., 2008). Bacterial colonies were affiliated with the class PYR10d3, an uncultured bacterial class, also showed the aforementioned trend. By applying DNA-based stable-isotope probing (SIP) and quantification by real-time quantitative PCR analysis, Singleton et al. (2006) demonstrated that PYR10d3 was involved in the process of degrading pyrene. These findings indicated that functional bacteria capable of degrading PAHs also existed in the contaminated environment. Unfortunately, no dramatic biodegradation of PAHs was detected.
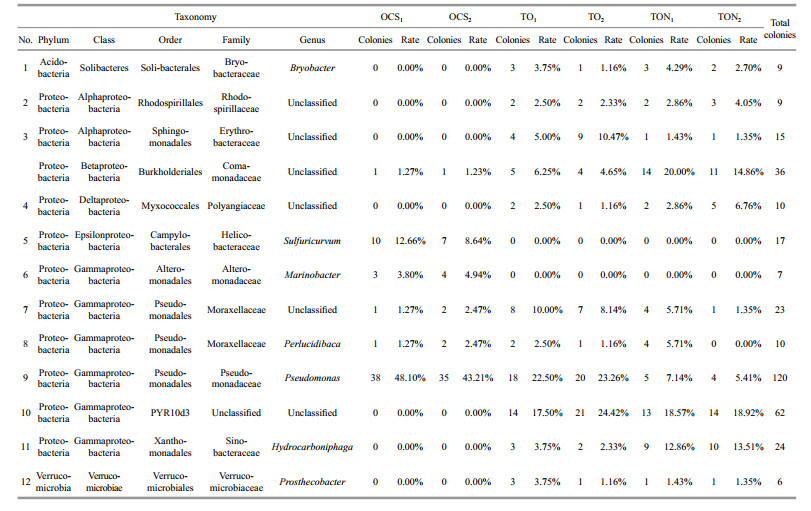
|
In contrast, colonies affiliated with the genera Sulfuricurvum, Marinobacter, and Pseudomonas showed obvious decreasing trends after being treated with oil or oil and nutrients. For instance, 10.65%±2.84% of the total recovered colonies were affiliated with the genus Sulfuricurvum in the clone libraries constructed for OCS samples, while no colonies were grouped into this phylotype in the four clone libraries for TO and TON samples. The newly proposed genus Sulfuricurvum was acknowledged to be responsible for oxidation of reduced sulfur compounds (Kodama and Watanabe, 2004; Haaijer et al., 2008). Even the type strain Sulfuricurvum kujiense YK-1T was isolated from an underground crude-oil storage cavity, while there was no evidence that the genus could degrade n-alkane. Similarly, 45.66%±3.46% of the recovered colonies were grouped into the genus Pseudomonas before being treated with oil, but the ratio dropped to 22.88%±0.53% and 6.27%±1.23% after being treated with oil and oil/ nutrient, respectively.
The results of this study indicated that the addition of nutrients could greatly stimulate changes in bacterial communities. As shown in Table 1, TON treatments resulted in more abundant colonies being affiliated into the genus Hydrocarboniphaga and the unclassified genus family Comamonadaceae when compared to TO treatments. Additionally, 13.19%±0.46% of the recovered colonies belonged to the genus Hydrocarboniphaga for the TON treatments, while the ratio was only 3.04%±1.01% for the TO treatments. The two ratios for colonies affiliated into the unclassified genus in the family Comamonadaceae were 17.43%±3.63% and 5.45%±1.13%, respectively. In contrast, TON treatments possessed less abundant bacterial colonies affiliated with the genus Pseudomonas. The aim of bioremediation is to stimulate pollutant-degrading functional microorganisms to speed the recovery of contaminated ecosystems (Röling et al., 2002). In the present study, bacteria grouped into phylotypes Hydrocarboniphaga and Comamonadaceae were promoted along with notable biodegradation of n-alkanes. Nutrient amendments could be considered a factor influencing the succession of bacterial communities as changes in bacterial communities were more closely related to the stress of crude oil and the degrading process. These findings are consistent with the conclusions raised by Röling et al. (2002), who found remarkable changes in bacterial communities, even without nutrients being amended. Alcanivorax/Fundibacter like sequences, which were closely-related to oil degradation, were found to be dominant in the corresponding clone library. The role that nutrient amendments played could be interpreted as stimulating the growth of functional bacteria that were responsible for oil-degradation.
4 CONCLUSIONMicrocosm experiments in this study demonstrated that bioremediation of crude oil could be enhanced by amendment with inorganic nutrients. Additionally, nutrient amendments showed a substantial impact on succession of bacterial communities. Efficient bioremediation was closely related to dramatic changes in bacterial communities when nutrients were supplied. n-alkanes degrading bacteria became more abundant during the biodegradation process. This study provides a deep understanding of nutrient enhancement of bioremediation of n-alkanes and bacterial communities associated with the process that could be helpful for assisting recovery of oil contaminated environments.
5 ACKNOWLEDGEMENTWe acknowledge critical review of the manuscript by Dr. Roy Bonnette from Southeastern Louisiana University.
Atlas R M. 1995. Bioremediation of petroleum pollutants. Int.Biodeter. Biodegr., 35(1-3): 317-327.
DOI:10.1016/0964-8305(95)00030-9 |
Bost F D, Frontera-Suau R, McDonald T J, Peters K E, Morris P J. 2001. Aerobic biodegradation of hopanes and norhopanes in Venezuelan crude oils. Org. Geochem., 32(1): 105-114.
DOI:10.1016/S0146-6380(00)00147-9 |
Brzostowicz P C, Walters D M, Jackson R E, Halsey K H, Ni H, Rouvière P E. 2005. Proposed involvement of a soluble methane monooxygenase homologue in the cyclohexanedependent growth of a new Brachymonas species. Environ. Microbiol., 7(2): 179-190.
DOI:10.1111/emi.2005.7.issue-2 |
Carmichael L M, Pfaender F K. 1997. The effect of inorganic and organic supplements on the microbial degradation of phenanthrene and pyrene in soils. Biodegradation, 8(1): 1-13.
DOI:10.1023/A:1008258720649 |
Chaîneau C H, Rougeux G, Yéprémian C, Oudot J. 2005. Effects of nutrient concentration on the biodegradation of crude oil and associated microbial populations in the soil. Soil Biol. Biochem., 37(8): 1490-1497.
DOI:10.1016/j.soilbio.2005.01.012 |
Enticknap J J, Kelly M, Peraud O, Hill R T. 2006. Characterization of a culturable alphaproteobacterial symbiont common to many marine sponges and evidence for vertical transmission via sponge larvae. Appl. Environ.Microbiol., 72(5): 3 724-3 732.
DOI:10.1128/AEM.72.5.3724-3732.2006 |
Haaijer S C M, Harhangi H R, Meijerink B B, Strous M, Pol A, Smolders A J P, Verwegen K, Jetten M S M, den Camp H J M O. 2008. Bacteria associated with iron seeps in a sulfur-rich, neutral pH, freshwater ecosystem. ISME J., 2(12): 1231-1242.
DOI:10.1038/ismej.2008.75 |
Harayama S, Kishira H, Kasai Y, Shutsubo K. 1999. Petroleum biodegradation in marine environments. J. Mol. Microbiol.Biotechnol., 1(1): 63-70.
|
Head I M, Jones D M, Röling W F M. 2006. Marine microorganisms make a meal of oil. Nat. Rev. Microbiol., 4(3): 173-182.
DOI:10.1038/nrmicro1348 |
Hennessee C T, Seo J S, Alvarez A M, Li Q X. 2009. Polycyclic aromatic hydrocarbon-degrading species isolated from Hawaiian soils:Mycobacterium crocinum sp. nov., Mycobacterium pallens sp. nov., Mycobacterium rutilum sp. nov., Mycobacterium rufum sp. nov. and Mycobacterium aromaticivorans sp. nov. Int. J. Syst.Evol. Microbiol., 59(Pt 2): 378-387.
|
Kodama Y, Watanabe K. 2004. Sulfuricurvum kujiense gen.nov., sp. nov., a facultatively anaerobic, chemolithoautotrophic, sulfur-oxidizing bacterium isolated from an underground crude-oil storage cavity. Int.J. Syst. Evol. Microbiol., 54(Pt 6): 2297-2300.
|
Liu Y, Song X F, Jiang J T, Liu Y H, Xu C J, Li H, Liu Z P. 2011. Hydrocarboniphaga daqingensis sp. nov., isolated from a freshwater lake. Int. J. Syst. Evol. Microbiol., 61(2): 408-411.
DOI:10.1099/ijs.0.019380-0 |
Luo Y R, Tian Y, Huang X, Kwon K, Yang S H, Seo H S, Kim S J, Zheng T L. 2012. Sphingomonas polyaromaticivorans sp. nov., a polycyclic aromatic hydrocarbon-degrading bacterium from an oil port water sample. Int. J. Syst. Evol.Microbiol., 62(6): 1223-1227.
|
Margesin R. 2000. Potential of cold-adapted microorganisms for bioremediation of oil-polluted Alpine soils. Int.Biodeterior. Biodegrad., 46(1): 3-10.
DOI:10.1016/S0964-8305(00)00049-4 |
Martin F, Malagnoux L, Violet F, Jakoncic J, Jouanneau Y. 2013. Diversity and catalytic potential of PAH-specific ring-hydroxylating dioxygenases from a hydrocarboncontaminated soil. Appl. Microbiol. Biotechnol., 97(11): 5 125-5135.
DOI:10.1007/s00253-012-4335-2 |
Mattes T E, Alexander A K, Richardson P M, Munk A C, Han C S, Stothard P, Coleman N V. 2008. The genome of Polaromonas sp. strain JS666:insights into the evolution of a hydrocarbon-and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl. Environ.Microbiol., 74(20): 6 405-6 416.
DOI:10.1128/AEM.00197-08 |
McKew B A, Coulon F, Yakimov M M, Denaro R, Genovese M, Smith C, Osborn A M, Timmis K N, McGenity T J. 2007. Efficacy of intervention strategies for bioremediation of crude oil in marine systems and effects on indigenous hydrocarbonoclastic bacteria. Environ. Microbiol., 9(6): 1562-1571.
DOI:10.1111/emi.2007.9.issue-6 |
Mendelssohn I A, Andersen G L, Baltz D M, Caffey R H, Carman K R, Fleeger J W, Joye S B, Lin Q X, Maltby E, Overton E B, Rozas L P. 2012. Oil impacts on coastal wetlands:implications for the Mississippi River Delta ecosystem after the Deepwater horizon oil spill. BioScience, 62(6): 562-574.
DOI:10.1525/bio.2012.62.6.7 |
Mohamed N M, Enticknap J J, Lohr J E, McIntosh S M, Hill R T. 2008. Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl. Environ. Microbiol., 74(4): 1209-1222.
DOI:10.1128/AEM.02047-07 |
Mohn W W, Stewart G R. 2000. Limiting factors for hydrocarbon biodegradation at low temperature in arctic soils. Soil Biol. Biochem., 32(8-9): 1161-1172.
DOI:10.1016/S0038-0717(00)00032-8 |
Oudot J, Merlin F X, Pinvidic P. 1998. Weathering rates of oil components in a bioremediation experiment in estuarine sediments. Mar. Environ. Res., 45(2): 113-125.
DOI:10.1016/S0141-1136(97)00024-X |
Palleroni N J, Port A M, Chang H K, Zylstra G J. 2004. Hydrocarboniphaga effusa gen. nov., sp. nov., a novel member of the γ-Proteobacteria active in alkane and aromatic hydrocarbon degradation. Int. J. Syst. Evol.Microbiol., 54(4): 1203-1207.
DOI:10.1099/ijs.0.03016-0 |
Röling W F M, de Brito I R C, Swannell R P J, Head I M. 2004a. Response of archaeal communities in beach sediments to spilled oil and bioremediation. Appl.Environ. Microbiol., 70(5): 2 614-2620.
DOI:10.1128/AEM.70.5.2614-2620.2004 |
Röling W F M, Milner M G, Jones D M, Fratepietro F, Swannell R P J, Daniel F, Head I M. 2004b. Bacterial community dynamics and hydrocarbon degradation during a field-scale evaluation of bioremediation on a mudflat beach contaminated with buried oil. Appl.Environ. Microbiol., 70(5): 2 603-2 613.
DOI:10.1128/AEM.70.5.2603-2613.2004 |
Röling W F M, Milner M G, Jones D M, Lee K, Daniel F, Swannell R J P, Head I M. 2002. Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl.Environ. Microbiol., 68(11): 5 537-5 548.
DOI:10.1128/AEM.68.11.5537-5548.2002 |
Schloss P D, Westcott S L, Ryabin T, Hall J R, Hartmann M, Hollister E B, Lesniewski R A, Oakley B B, Parks D H, Robinson C J, Sahl J W, Stres B, Thallinger G G, Van Horn D J, Weber C F. 2009. Introducing mothur:open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol., 75(23): 7 537-7 541.
DOI:10.1128/AEM.01541-09 |
Sei K, Sugimoto Y, Mori K, Maki H, Kohno T. 2003. Monitoring of alkane-degrading bacteria in a sea-water microcosm during crude oil degradation by polymerase chain reaction based on alkane-catabolic genes. Environ.Microbiol., 5(6): 517-522.
DOI:10.1046/j.1462-2920.2003.00447.x |
Singleton D R, Sangaiah R, Gold A, Ball L M, Aitken M D. 2006. Identification and quantification of uncultivated Proteobacteria associated with pyrene degradation in a bioreactor treating PAH-contaminated soil. Environ.Microbiol., 8(10): 1736-1745.
DOI:10.1111/emi.2006.8.issue-10 |
Szabó I, Szoboszlay S, Kriszt B, Háhn J, Harkai P, Baka E, Táncsics A, Kaszab E, Privler Z, Kukolya J. 2011. Olivibacter oleidegradans sp. nov., a hydrocarbondegrading bacterium isolated from a biofilter clean-up facility on a hydrocarbon-contaminated site. Int. J. Syst.Evol. Microbiol., 61(12): 2 861-2 865.
DOI:10.1099/ijs.0.026641-0 |
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol.Evol., 28(10): 2 731-2 739.
DOI:10.1093/molbev/msr121 |
Wang H, Laughinghouse IV H D, Anderson M A, Chen F, Willliams E, Place A R, Zmora O, Zohar Y, Zheng T L, Hill R T. 2012. Novel bacterial isolate from Permian groundwater, capable of aggregating potential biofuelproducing microalga Nannochloropsis oceanica IMET1. Appl. Environ. Microbiol., 78(5): 1445-1453.
DOI:10.1128/AEM.06474-11 |
Wang H, Wang C X, Lin M, Sun X N, Wang C Y, Hu X K. 2013. Phylogenetic diversity of bacterial communities associated with bioremediation of crude oil in microcosms. Int. Biodeterior. Biodegrad., 85: 400-406.
DOI:10.1016/j.ibiod.2013.07.015 |
Yakimov M M, Golyshin P N, Lang S, Moore E R B, Abraham W R, Lünsdorf H, Timmis K N. 1998. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbondegrading and surfactant-producing marine bacterium. Int. J. Syst. Evol. Microbiol, 48(2): 339-348.
|
Zhao B S, Wang H, Li R R, Mao X W. 2010. Thalassospira xianhensis sp. nov., a polycyclic aromatic hydrocarbondegrading marine bacterium. Int. J. Syst. Evol. Microbiol., 60(5): 1125-1129.
DOI:10.1099/ijs.0.013201-0 |
Zhao D F, Liu C S, Liu L H, Zhang Y B, Liu Q Y, Wu W M. 2011. Selection of functional consortium for crude oilcontaminated soil remediation. Int. Biodeterior.Biodegrad., 65(8): 1244-1248.
DOI:10.1016/j.ibiod.2011.07.008 |
 2018, Vol. 36
2018, Vol. 36


