Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Yang(杨阳), LIU Qinghua(刘清华), MA Daoyuan(马道远), SONG Zongcheng(宋宗诚), LI Jun(李军)
- A potential germ cell-specific marker in Japanese flounder, Paralichthys olivaceus: identification and characterization of lymphocyte antigen 75 (Ly75/CD205)
- Chinese Journal of Oceanology and Limnology, 36(4): 1342-1348
- http://dx.doi.org/10.1007/s00343-019-7390-z
Article History
- Received Dec. 19, 2017
- accepted in principle Feb. 22, 2018
- accepted for publication Feb. 27, 2017
2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Weihai Shenghang Aquatic Product Science and Technology Co. Ltd., Weihai 264200, China
Germ cells are highly specialized cells that play an extremely important role in fish reproduction (Kurokawa et al., 2007). In teleost fish, mature gametes are acquired from primordial germ cells (PGCs) through a series of basic biological processes. Among them, the PGCs, the diploid oogonia, and spermatogonia are known as special stem cells that provide the foundation for gametogenesis through self-renewal and differentiation (Schulz et al., 2010; Nakamura et al., 2011; Lacerda et al., 2012; Kagawa, 2013). However, the molecular and cellular mechanisms underlying the continuous production of mature gametes in adult fish are poorly understand. Therefore, for the molecular, cellular, and functional characterization of germ cells, germ cell marker genes such as vasa, nanos, and dead end (dnd) have been identified in recent decades (Nagasawa et al., 2010; Li et al., 2015; Wang et al., 2015b). Among them, Nanos 2, a type of nanos, has been extensively studied and is regarded as a specific marker of germline stem cells in fish (Suzuki et al., 2007). Lymphocyte antigen 75 (ly75, also called CD205) was first identified as a germ cell surface marker in rainbow trout (Nagasawa et al., 2010). Subsequently, ly75homologs have been cloned from several other fish species (Nagasawa et al., 2012, 2013; Presslauer et al., 2014). In mammals, Ly75 plays a role in the immune system and functions as an antigen-uptake receptor in dendritic cells (Jiang et al., 1995; East and Isacke, 2002). Although the role of Ly75 in fish germ cells is unknown, its amino acid sequence has a highly conserved region, and its expression is restricted specifically to spermatogonia (SG). Therefore, it is considered to be a practical mitotic germ cell-specific cell-surface marker and it has been used in SG transplantation in fish (Nagasawa et al., 2010, 2012).
Japanese flounder (Paralichthys olivaceus), a cold water benthic flatfish, is one of the most important aquaculture species in China, Korea, and Japan (Radonic and Macchi, 2009; Si et al., 2016). Due to its high commercial value, it has been intensively studied, and most research on its reproductive biology has focused on gametogenesis (Fan et al., 2014; Wang et al., 2015a). Although some germ cell markers have been identified in P. olivaceus, there is still much to learn about its germline cells, including their molecular and cellular makeup and functions. Therefore, in this study, we identified a potential germ cell-specific marker, lymphocyte antigen 75 (ly75), in P. olivaceus, and investigated its gene expression pattern using real-time quantitative PCR (RT-PCR) and in-situ hybridization (ISH) analyses.
2 MATERIAL AND METHOD 2.1 Experimental animalsNine Japanese flounders (three 8-month-old males, three adult males, and three adult females) were obtained from Oriental Ocean Sci-Tech Co., Ltd. (Shandong Province, China). The samples were collected and excised after being anesthetized with a 0.05% (w/v) solution of ethyl 3-aminobenzoate methane sulfonate (Sigma-Aldrich, Shanghai, China). To explore the function of ly75 in subsequent transplantation in marine fish, tissues including both types of testes, adult ovaries, and the gill, intestine, liver, kidney, spleen, stomach, heart, and muscle from 8-month-old males were rapidly excised and frozen in liquid nitrogen. Half of each testis and ovary sample was fixed in Bouin's fluid and 4% paraformaldehyde in Sorensen's phosphate buffer (0.1 mol/L, pH 7.2) for at least 24 h and then preserved in 70% ethanol. All animal work was conducted according to relevant national and international guidelines and was approved by the Institute of Oceanology, Chinese Academy of Sciences.
2.2 Cloning of full-length ly75 cDNATotal RNA was extracted from the testes of 8-month-old Japanese flounder using an RNA fast 200 kit (Fastagen Biotech, Shanghai, China). Firststrand cDNA was synthesized using a Transcript Firststrand cDNA synthesis kit (TransStart, TransGen, Beijing, China), according to the manufacturer's instructions. The cDNA fragment of the ly75 gene was amplified by PCR with primers (Table 1) designed according to the highly conserved regions of ly75/cd205 homologs from other fish species (Table 1). The PCRs were performed using KOD-Plus-Neo (Toyobo, Tokyo, Japan) in a PTC-100 thermal cycler (Bio-Rad, Hercules, CA, USA). Subsequently, 5′- and 3′-rapid amplification of cDNA ends (RACE) were carried out using a SMARTer RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA) using the gene-specific primers shown in Table 1. The reaction mixture contained 2 μL 10×buffer, 2 μL 2 mmol/L dNTPs, 1.4 μL 25 mmol/L MgSO4, 0.6 μL forward and reverse primers (10 μmol/L), 11 μL ddH2O, 0.4 μL KOD-Plus-Neo (1 U/μL), and 2 μL cDNA. The thermal cycling conditions were as follows: 2 min at 94℃; 10 s at 98℃, 30 s at Tm ℃, and 1 min at 68℃ for 40 cycles; and finally 10 min at 72℃. The molecular mass and pI of predicted poly75 polypeptide were estimated using the compute pI/Mw tool (http://web.expasy.org/compute_pi/).
Homology searches of the nucleotide and deduced amino acid sequences of ly75 were conducted at the National Center of Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). The deduced amino acid sequences were aligned using AlignX in Vector NTI 11.5 Suite (Life Technologies, Carlsbad, CA, USA). The percentages of similarity and identity among fish Ly75 homologs were calculated using LALIGN (http://www.ch.embnet.org/software/LALIGN-form.html). A phylogenetic tree was constructed using Mega 4.1 software with bootstrap analysis of 1 000 replicates, by the neighbor-joining method. The signal peptide sequences were determined by SignalP (http://www.cbs.dtu.dk/services/SignalP/). A domain structure analysis was carried out using SMART (http://smart.emblheidelberg.de/). Transmembrane helices in proteins were predicted by TMHMM (http://www.cbs.dtu.dk/services/TMHMM/).
2.4 Semi-quantitative RT-PCR analysisTotal RNA was extracted from tissue samples (including testis, ovary, gill, intestine, liver, kidney, spleen, stomach, heart and muscle) as described above. Specific primers (Table 1) were used to amplify ly75 and β-actin by RT-PCR, and the primer specificity for each gene was verified by sequencing. The cDNA was synthesized from 1 μg total RNA using the PrimeScript RT reagent kit (TaKaRa, Dalian, China) following the manufacturer's instructions. The reaction mixture contained 12.5 μL 2×buffer, 9.5 μL ddH2O, 0.5 μL forward and reverse primers (10 μmol/L), and 2 μL cDNA. All samples were run in triplicate. The thermal cycling conditions were as follows: 3 min at 95℃; 5 s at 95℃ and 30 s at 62℃ for 35 cycles; and 30 s at 95℃, 20 s at 60℃; and 30 s at 72℃, followed by a final elongation step at 72℃ for 3 min. The PCR products were electrophoresed on 2% (w/v) agarose gels.
2.5 HistologyThe testes and ovaries fixed in Bouin's fluid were dehydrated with an ethanol gradient, embedded in paraffin, and cut into a series of sagittal and cross sections (5 μm thick). The tissue sections were stained with hematoxylin and eosin (HE) for histological observations.
2.6 In-situ hybridizationThe localization of ly75 transcripts was analyzed by section in-situ hybridization (SISH), as described by Wang et al. (2015b). Samples were dehydrated using a methanol gradient, embedded in paraffin wax, and cut into 8-μm thick sections. Probes for ly75 (Table 1) were individually synthesized using a DIG RNA Labeling Kit (SP6/T7) (Roche, Mannheim, Germany) following the manufacturer's instructions.
2.7 ImmunohistochemistryThe localization of Nanos 2 was analyzed using immunohistochemical analyses. Samples were dehydrated with a methanol gradient, embedded in paraffin wax, and cut into 8-μm thick sections. The tissue sections were treated with lead citrate (BBI, Shanghai, China) for antigen retrieval and 3% H2O2 to block endogenous peroxidase. The primary antibody against Nanos 2 protein (BBI, China) was diluted 1:200 and incubated with the samples at 4℃ overnight. The secondary antibody was goat antirabbit IgG (ABclone, China), which was diluted 1:800 and incubated with the samples for 30 min at room temperature.
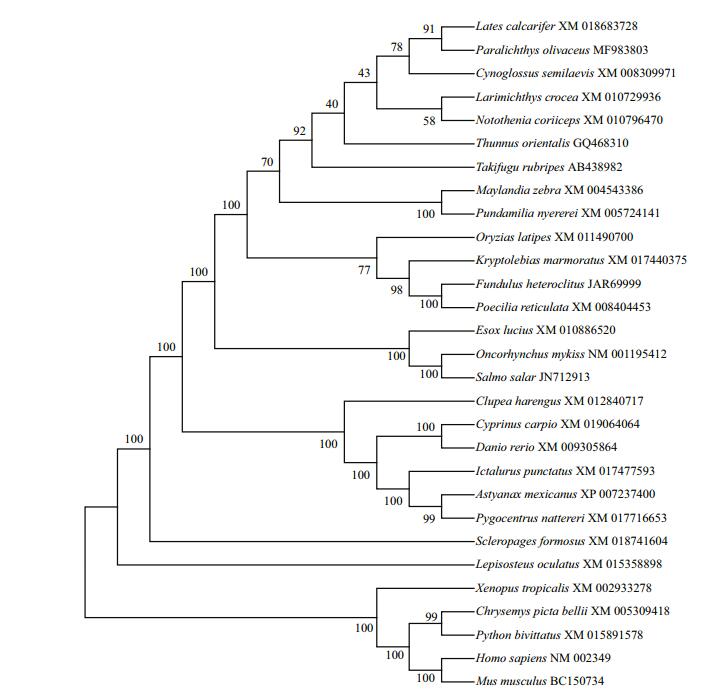
3 RESULT 3.1 Cloning of ly75 from Japanese flounderThe full-length cDNA of ly75 (GenBank accession No. MF983803) was 7 346 bp, with an open reading frame (ORF) of 5 229 bp. The ORF encoded a protein consisting of 1 742 amino acids with a predicted molecular mass of 196.89 kDa. The deduced amino acid sequence of ly75 showed 70.7% identity and 90.1% similarity with that of Cynoglossus semilaevis, 49.3% identity and 74.9% similarity with that of zebrafish, 61.4% identity and 83.9% similarity with that of medaka, and 35.8% identity and 67.1% similarity with that of mouse. A phylogenetic tree analysis (Fig. 1) revealed that P. olivaceus Ly75 was associated with teleost Ly75s and belonged to the Ly75 protein subfamily. In addition, analyses of the protein sequence of ly75 (Fig. 2) revealed a predicted N-terminal signal peptide cleavage site between amino acid positions 28 and 29. And the sequence contained a cysteine-rich domain (RICIN), a fibronectin type Ⅱ (FN2), 10 C-type lectin-like domains (CLECT), and a transmembrane domain (TM).
|
| Figure 1 Phylogenetic tree of deduced amino acid sequences for Ly75 from Japanese flounder and other vertebrates constructed by MEGA 4.1 using neighbor-joining method Numbers adjacent to nodes indicate bootstrap percentage value for 1 000 replicates (> 80%). GenBank accession numbers of sequences are given after species name. |

|
| Figure 2 Molecular characterization of Japanese flounder ly75 a. deduced amino acid sequence from Japanese flounder ly75 cDNA sequence. Amino acid residue numbers are shown on left. Signal peptide, cysteine-rich domain (RICIN), fibronectin type Ⅱ (FN2), C-type lectin-like domains (CLECT), and transmembrane domain (TM) are indicated. Sequence data have been deposited in GenBank (accession number MF983803); b. domain structure analyses of Japanese flounder Ly75 by SMART. |
Transcripts of ly75 were abundantly detected in the testis of 8-month-old fish, and in the gill, kidney, spleen, stomach, and heart. Transcripts of ly75 were detected at low levels in the adult testis and ovary, intestine, liver, and muscle (Fig. 3).

|
| Figure 3 Tissue-specific expression of ly75 mRNA as determined by RT-PCR (internal control, β-actin) |
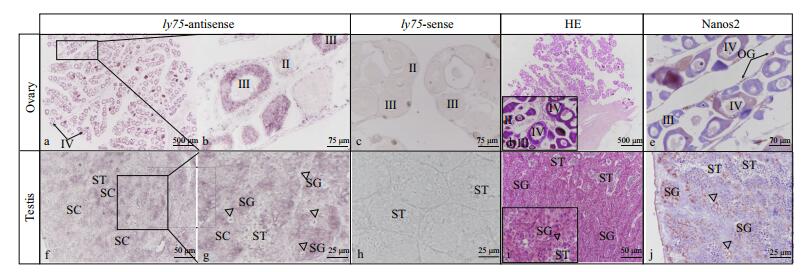
The mRNA of ly75 was detected in the testis and ovary by ISH. The localization of the Nanos 2 protein was also determined using immunohistochemical analyses. In adult ovaries, ly75 mRNA was predominantly located in the cytoplasm of oocytes at stage Ⅲ and Ⅳ, and could not be detected in oogonia and oocytes at stage Ⅱ (Fig. 4a, b). In the testis, ly75 mRNA was predominantly detected in spermatogonia, and could not be detected in spermatocytes, spermatids, or spermatozoa (Fig. 4f, g). The Nanos 2 protein was expressed in germline stem cells: spermatogonia in the testis (Fig. 4j) and oogonia in the ovary (Fig. 4d).
|
| Figure 4 Distribution of ly75 transcripts in adult P. olivaceus testis and ovary In adult ovary (a–b), ly75 mRNA was predominantly located in cytoplasm of oocytes at stage Ⅲ and Ⅳ, and could not be detected in oogonia and oocytes at stage Ⅱ. In adult testis (f–g), ly75 mRNA was located in spermatogonia and could not be detected in spermatocytes and spermatids. b and g are magnifications of a and f, respectively; c and h are negative controls (sense probes). Hematoxylin- and eosin-stained sections of ovary (d) and testis (i). Nanos 2 protein in oogonia in ovary (e) and spermatogonia in testis (j). Ⅱ: primary growth oocytes; Ⅲ: perinucleolar or pre-vitellogenic ooctyes; Ⅳ: late vitellogenic oocytes; SC: spermatocytes; ST: spermatids. |
In this study, we isolated and characterized a ly75 homolog from P. olivaceus. The deduced amino acid sequence of ly75 was similar to that of ly75s of other teleosts, showing 90.1% similarity to that of C. semilaevis, 74.9% similarity to that of zebrafish (Presslauer et al., 2014) and 67.1% similarity to that of mouse (Jiang et al., 1995). The Ly75 amino acid sequence contained typical conserved domain structures including one RICIN, one FN2, 10 CLECT, and one TM, with high identities to those of other Ly75 proteins (Nagasawa et al., 2012). The phylogenetic tree constructed using the neighborjoining method showed that the Ly75 protein was closely related to the Ly75 clade. Overall, these results revealed that the clone identified from P. olivaceus was a Ly75 homolog.
The RT-PCR analyses of ly75 transcript levels in different tissues revealed that ly75 mRNA was present in the testis, gill, and heart. Similar results were also reported for rainbow trout (Nagasawa et al., 2010), pacific bluefin tuna (Nagasawa et al., 2012), and Atlantic salmon (Nagasawa et al., 2013). This wide expression pattern was possibly related to the function of this protein in the immune system, as previously found in mammalian species (Jiang et al., 1995). Similar to the case in rainbow trout (Nagasawa et al., 2010) and Bluefin tuna (Nagasawa et al., 2012), ly75 transcripts were abundant in the testis at the early development stage. The strong expression in the gonads indicated that ly75 may play a key role in early gametogenesis in P. olivaceus.
The ISH analyses showed that ly75 mRNA was present in oocytes at stage Ⅲ and Ⅳ, but not in oogonia and oocytes at stage Ⅱ and Ⅴ in ovaries. In contrast, ly75 transcripts were detected in oogonia and nucleolus-stage oocytes in rainbow trout (Nagasawa et al., 2010), and in oocytes at stages Ⅰ, Ⅱ, and Ⅲ in zebrafish (Presslauer et al., 2014). Most fishes release eggs, and fish embryos rely upon the maternal provision of immunity molecules for protection against invading pathogens before their own immune system fully develops (Zhang et al., 2013). Therefore, in oogenesis, maternally transferred immunity molecules including lysozyme, lgM, and the egg yolk protein phosvitin are accumulated and stored in oocytes (Wang et al., 2017). Based on the present results, Ly75 as an immunity-related protein expressed in oocytes, may be related to maternally derived immunity molecules. In the adult testis, ly75 mRNA was predominantly detected in SG, just its homologs were only detected in type A SG in rainbow trout (Nagasawa et al., 2010), zebrafish (Presslauer et al., 2014), and Thunnus orientalis (Nagasawa et al., 2012).
Immunohistochemical analyses revealed that the Nanos 2 protein was predominantly expressed in germline stem cells: spermatogonia in the testis and oogonia in the ovary, as in other fish (Suzuki et al., 2007; Huang et al., 2017). Several other genes have been reported as germ cell-specific markers in fish. In our previous study, dnd was identified as a gene essential for PGCs migration and oocytes and spermatocytes (Wang et al., 2015b). The results of the present study indicate that ly75 could be a potential germ cell-specific marker in P. olivaceus, since it is specifically expressed in germ cells. In future research, we intend to functionally characterize this protein.
5 CONCLUSIONWe cloned Japanese flounder ly75 and analyzed its expression pattern. In RT-PCR analyses, ly75 transcripts were detected in all analyzed tissues but abundantly in the testis. The ISH analyses showed that ly75 mRNA was predominantly localized in oocytes in the ovary and spermatogonia in the testis. We did not detect ly75 mRNAs in the oogonia, spermatids, spermatocytes, or spermatozoa. Therefore, our results indicate that ly75, like dnd, could be a potential germ cell-specific marker in P. olivaceus.
7 DATA AVAILABILITY STATEMENTThe datasets generated and analyzed during the current study are not publicly available from NCBI (https://www.ncbi.nlm.nih.gov/) until Sep. 3, 2018 due to author request, but are available from the corresponding author on reasonable request.
East L, Isacke C M. 2002. The mannose receptor family. Biochimica et Biophysica Acta (BBA)-General Subjects, 1572(2-3): 364-386.
DOI:10.1016/S0304-4165(02)00319-7 |
Fan Z F, You F, Wang L J, Weng S D, Wu Z H, Hu J W, Zou Y X, Tan X G, Zhang P J. 2014. Gonadal transcriptome analysis of male and female olive flounder (Paralichthys olivaceus). BioMed Research International, 2014: 291067.
|
Huang J Q, Li Y J, Shao C W, Wang N, Chen S L. 2017. Identification, characterization and functional analysis of regulatory region of nanos gene from half-smooth tongue sole (Cynoglossus semilaevis). Gene, 617: 8-16.
DOI:10.1016/j.gene.2017.03.033 |
Jiang W P, Swiggard W J, Heufler C, Peng M, Mirza A, Steinman R M, Nussenzweig M C. 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature, 375(6527): 151-155.
DOI:10.1038/375151a0 |
Kagawa H. 2013. Oogenesis in teleost fish. Aqua-BioScience Monographs (ABSM), 6(4): 99-127.
DOI:10.5047/absm.2013.00604.0099 |
Kurokawa H, Saito D, Nakamura S, Katoh-Fukui Y, Ohta K, Baba T, Morohashi K, Tanaka M. 2007. Germ cells are essential for sexual dimorphism in the medaka gonad. Proceedings of the National Academy of Sciences of the United States of America, 104(43): 16 958-16 963.
DOI:10.1073/pnas.0609932104 |
Lacerda S M S N, Aponte P M, Costa G M J, Campos-Junior P H A, Segatelli T M, Silva M A, Franca L R. 2012. An overview of spermatogonial stem cell physiology, niche and transplantation in fish. Animal Reproduction, 9(4): 798-808.
|
Li M J, Tan X G, Jiao S, Wang Q, Wu Z H, You F, Zou Y X. 2015. A new pattern of primordial germ cell migration in olive flounder (Paralichthys olivaceus) identified using nanos3. Development Genes and Evolution, 225(4): 195-206.
DOI:10.1007/s00427-015-0503-6 |
Nagasawa K, Fernandes J M O, Yoshizaki G, Miwa M, Babiak I. 2013. Identification and migration of primordial germ cells in Atlantic salmon, Salmo salar:characterization of Vasa, Dead End, and Lymphocyte antigen 75 genes. Molecular Reproduction and Development, 80(2): 118-131.
DOI:10.1002/mrd.v80.2 |
Nagasawa K, Miwa M, Yazawa R, Morita T, Takeuchi Y, Yoshizaki G. 2012. Characterization of lymphocyte antigen 75 (Ly75/CD205) as a potential cell-surface marker on spermatogonia in Pacific bluefin tuna Thunnus orientalis. Fisheries Science, 78(4): 791-800.
DOI:10.1007/s12562-012-0501-9 |
Nagasawa K, Shikina S, Takeuchi Y, Yoshizaki G. 2010. Lymphocyte antigen 75 (Ly75/CD205) is a surface marker on mitotic germ cells in rainbow trout. Biology of Reproduction, 83(4): 597-606.
DOI:10.1095/biolreprod.109.082081 |
Nakamura S, Kobayashi K, Nishimura T, Tanaka M. 2011. Ovarian germline stem cells in the teleost fish, medaka(Oryzias latipes). International Journal of Biological Sciences, 7(4): 403-409.
DOI:10.7150/ijbs.7.403 |
Presslauer C, Nagasawa K, Dahle D, Babiak J, Fernandes J M O, Babiak I. 2014. Induced autoimmunity against gonadal proteins affects gonadal development in juvenile zebrafish. PLoS One, 9(12): e114209.
DOI:10.1371/journal.pone.0114209 |
Radonic M, Macchi G J. 2009. Gonadal sex differentiation in cultured juvenile flounder, Paralichthys orbignyanus(Valenciennes, 1839). Journal of the World Aquaculture Society, 40(1): 129-133.
DOI:10.1111/jwas.2009.40.issue-1 |
Schulz R W, de França L R, Lareyre J J, Le Gac F, ChiariniGarcia H, Nobrega R H, Miura T. 2010. Spermatogenesis in fish. General and Comparative Endocrinology, 165(3): 390-411.
DOI:10.1016/j.ygcen.2009.02.013 |
Si Y F, Ding Y X, He F, Wen H S, Li J F, Zhao J L, Huang Z J. 2016. DNA methylation level of cyp19a1a and Foxl2 gene related to their expression patterns and reproduction traits during ovary development stages of Japanese flounder(Paralichthys olivaceus). Gene, 575(2): 321-330.
DOI:10.1016/j.gene.2015.09.006 |
Suzuki A, Tsuda M, Saga Y. 2007. Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development, 134(1): 77-83.
DOI:10.1242/dev.02697 |
Wang L J, You F, Weng S D, Wen A Y, Wu Z H, Zou Y X, Xin M J, Zhang P J. 2015a. Molecular cloning and sexually dimorphic expression patterns of nr0b1 and nr5a2 in olive flounder, Paralichthys olivaceus. Development Genes and Evolution, 225(2): 95-104.
DOI:10.1007/s00427-015-0495-2 |
Wang P, Jiang C Y, Liu S S, Cui P F, Zhang Y, Zhang S C. 2017. Trans-generational enhancement of C-type lysozyme level in eggs of zebrafish by dietary β-glucan. Developmental & Comparative Immunology, 74: 25-31.
|
Wang X Y, Liu Q H, Xiao Y S, Yang Y, Wang Y F, Song Z C, You F, An H, Xiao Z Z, Xu S H, Ma D Y, Li J. 2015b. The dnd RNA identifies germ cell origin and migration in olive flounder (Paralichthys olivaceus). BioMed Research International, 2015: 428591.
|
Zhang S C, Wang Z P, Wang H M. 2013. Maternal immunity in fish. Developmental & Comparative Immunology, 39(1-2): 72-78.
|
 2018, Vol. 36
2018, Vol. 36