Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Ashraf GODA, Amal SAAD, Mohamed HANAFY, Zaki SHARAWY, Ehab EL-HAROUN
- Dietary effects of Azolla pinnata combined with exogenous digestive enzyme (DigestinTM) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii (de Man 1879)
- Chinese Journal of Oceanology and Limnology, 36(4): 1434-1441
- http://dx.doi.org/10.1007/s00343-018-7019-7
Article History
- Received Feb. 18, 2017
- accepted in principle May. 12, 2017
- accepted for publication Jul. 17, 2017
2 Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 Division of Aquaculture, College of Agriculture, Food Science and Sustainable Systems, Kentucky State University, Frankfort, KY, USA
In Egypt, most of the ingredients used in aquafeed industry are exported to meet the markets requirement. In addition, wheat bran (WB) and yellow corn (YC) are the main source of energy and compete with livestock, aquaculture and poultry feeds industry which causes a price increase in the feed ingredients in Egypt (El-Sayed et al., 2015). Therefore, alternative feed ingredients being non-competitive in terms of animal and poultry consumption and locally produced is feasible solution to reduce the cost of formulated diets. Azolla has a great potential in aquaculture due to its ability to cultivate on both fresh water and waste water from agriculture and other domestic sources, Azolla is a potential source of nutrients as protein, minerals, vitamin, beta-carotene and vitamin B12 (Cherryl et al., 2014; Noor et al., 2014), which fortunately, can be used without further processing in Aquafeed industry (Balaji et al., 2009; Kumar et al., 2012; Cherryl et al., 2014). Azolla has an important contribution as a local feedstuff produced in countries such as Egypt where soybean meal (SBM) and other valuable ingredients are limited and very expensive or mostly export. However, little has been accomplished to evaluate the potential of A. pinnata as a feed ingredient for different aquatic species, particularly those species which do not easily accept fresh plants as a feed ingredient without extra processing technology such as crustaceans. Other species can efficiently use and convert A. pinnata into the live weight of carp and Nile tilapia (Gaigher et al., 1984; El-Sayed, 1992; Yılmaz et al., 2004). It's also worth to mention that most studies have investigated A. pinnata as a protein source replacing fish meals to decrease the cost (Yılmaz et al., 2004). Sustainability of Macrobrachium rosenbergii needs an economic feed formulation to be developed and raw ingredients available from local feed mill for optimal growth and cost-effective culture (Aarumugam et al., 2013). In addition, using digestive exogenous enzymes supplementation to fish diets has provided an additional powerful tool to improve growth, optimize digestibility, nutrient absorption, cost effectiveness, minimize the anti-nutritional factors (ANF) effects, effluent pollution and reduce the cost of the diets (Buchanan et al., 1997; Felix and Selvaraj, 2004; Manush et al., 2013). The present study was conducted to assess the effect of using A. pinnata as a complete replacer of WB alone or in combination with DigestinTM, in practical feeds on growth, feed efficiency ratio, and body composition of M. rosenbergii Postlarvae in terms of growth performance, nutrient utilizations, body composition and economic efficiency of prawn.
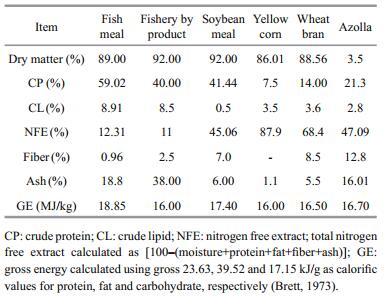
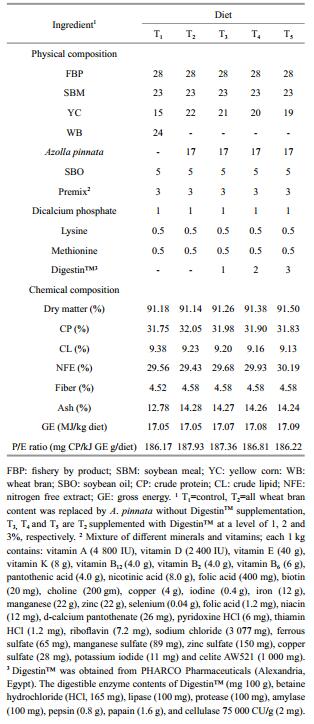
2 MATERIAL AND METHOD 2.1 Ingredients and diet formulationsAll ingredients, including imported FM, SBM, YC, WB, soybean oil (SBO) and the premix (minerals and vitamins) were purchased from a local commercial company (El-Morshady Company, Cairo, Egypt). Fresh A. pinnata was collected from Darawa Irrigation Branch, (Kalubiya, Egypt) and then sun-dried for 48 h. DigestinTM was obtained from (PHARCO Pharmaceuticals-Alexandria, Egypt). DigestinTM is a well-balanced combination of digestive enzymes containing mainly protease, amylase, lipase and cellulase. These enzymes act upon food contents of the gastrointestinal tract (GIT) and catalyze their conversion into simpler and easily digestible and absorbable components.The chemical composition of the ingredients is presented in Table 1. Five experimental diets were formulated to be isonitrogenous (32% CP and isocaloric 17 MJ/kg, Table 2). The control diet was not supplemented either with A. pinnata or with DigestinTM and classified as control (T1). In (T2) diet, all WB content was replaced by A. pinnata without DigestinTM supplementation. Diets T3, T4 and T5 were formulated as T2 diet and supplemented with increasing levels of DigestinTM (1%, 2%, and 3%, respectively). All dried ingredients were blended and homogenized to formulate 2 mm pellets using sprout Waldron lab pellet machine (Pellet Mill Co., California, USA). After the pellets dried, a diet has been crushed to a powder-like to be suitable for the PLs. During our 12 wks experiments, prawns PL were fed daily, while the diets were distributed in equal proportions of the total amount for each of the three feeding times (08:00, 12:00 and 17:00). The feeding ration was increased every second week based on prawn body weight which was determined on a fortnightly basis.
|
The grow-out production of the prawn was implemented at the Experimental fish farm of the National Institute of Oceanography and Fisheries, Kalubiya, Egypt for 12 wks. A hatch out of single female was purchased from the Marriot commercial hatchery, Alexandria, Egypt and then prawns were acclimated to trial conditions for 2 wks in a cement tank (8 m×4 m×1 m) with a daily feed control diet (32%, crude protein). At the end of the acclimation period, PLs (0.2±0.02 g) were then stocked in five cement tanks. Each tank (6 m3) was subdivided into three equal pens using nets (2 m3). Three replicates of each treatment randomly assigned to different pens in different tanks with a stocking density of 84 PL/m2 as suggested by FAO (2005). Each pen was supplied with four PVC pipes (30 cm long×16 mm diameter) to diminish the cannibalism behavior, especially during the molting process as proposed by Mariappan and Balasundaram, (2004). However, the mortalities were recorded once a day at 08:00. All pens were water supplied from the nearest agricultural irrigation branch after filtrations. Prawns were held under natural light condition (12 h:12 h, day:night) providing extra aeration using an electric blower with a daily water flow rate of 20.83 L/(min·5 tanks). Water quality parameters were measured and recorded during the experiment according to APHA et al. (1995). Water temperature (℃) was measured daily using a mercury thermometers/pen, dissolved oxygen (mg/L) was measured daily using an oxygen-meter (model: 56-YSI, Yellow Springs, Ohio, USA), whereas pH was daily measured using a pH-meter (Orion, Texas, USA), both ammonia and salinity were measured and recorded twice/wk.
2.3 Sampling and chemical analysisAt the beginning of the trial, a pooled sample of fish was collected to serve as an initial carcass sample. At the end of each trial, five fish were sampled from each pen and anaesthetized with t-amyl alcohol and killed with a cephalic blow. The five fish were pooled, autoclaved, ground into a homogeneous slurry, freezedried, reground and stored at -20℃ until analyzed. Diet, ingredients and carcass samples were analyzed for dry matter (DM) and ash according to AOAC (1995), crude protein (%N×6.25) by Kjedahl method using a Kjeltech autoanalyzer (Model 1030, Tecator, Högan s, Sweden), and total lipids according to the method of Bligh and Dyer (1959). Gross energy (GE) contents of carcass samples were measured using an automated bomb calorimeter (Model 1272, Parr Instruments Inc., Moline, IL). Uneaten food collected from each tank by syphon and dried in an oven at 105℃ until constant weight to measure feed efficiency parameters.
2.4 Calculation and statistical analysesAt the end of the trial, weight gains (WG, g), specific growth rates (SGR, %/day), feed conversion ratios (FCR), protein efficiency ratios (PER), protein productive values (PPV), fat retentions (FR, g), energy retentions (ER, kJ), survivals (S%) and economic conversion rates (ECR) were estimated as:
WG=FBW (g)–IBW (g),
where FBW=final body weight; IBW=initial body weight;
SGR=((lnFBW–lnIBW)/t (d))×100,
where ln=natural logarithmic; t=time;
PER=WG (g)/protein intake (g);
PPV=((protein gain (g)/protein intake (g))×100;
FR=(fat gain (g)/fat intake (g))×100;
ER=(energy gain (kJ)/energy intake (kJ))×100;
S=(final number of prawns/initial number of prawns)×100;
FCR=total weight of feed consumed/wet biomass gain;
ECR=cost of diet (kg/EGP)×FCR.
The data were analyzed applying the analysis of variance (one-way ANOVA) using MSTAT-C (1987) 4 software package. Duncan (1955) test was used to compare differences between treatments whenever significant F values (P < 0.05) were noticed. Prior to data analysis, all ratios and percentages were arc-sin transformed (Zar, 1984) and were presented untransformed to facilitate comparisons.
3 RESULT 3.1 Water qualityWater quality parameters during the experiment were within those considered to be favorable for growth, and fell under the optimal standards defined for nutritional evaluations of freshwater prawn PLs according to (Zafar et al., 2015). The mean temperature values were ranged from 28.8 to 28.5±0.8℃, while the mean value of the dissolved oxygen (DO) was expressed as 6.4 to 6.1±1.1 mg/L during the feeding experiment. Meanwhile, the mean value of pH, ammonia, and alkalinity was ranged (7.4 to 7.8±0.5), (0.27±0.1 mg/L) and (189±7.5 mg/L), respectively.
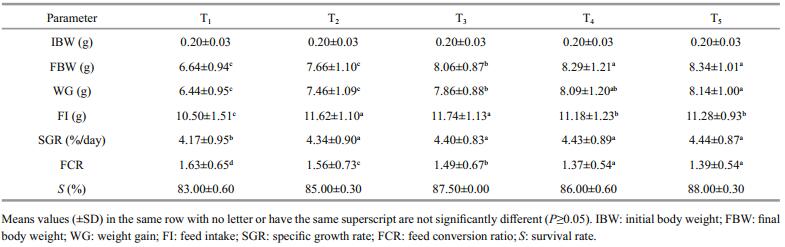
3.2 Prawn performanceGrowth performance, nutrient utilization efficiency, and survival of the juvenile of freshwater Prawn, M. rosenbergii fed the diets containing different replacement levels of wheat bran with azolla meal for 12 wks are presented in (Table 3). Survival was high in every dietary treatment, more than 80% (P≥0.05). There were no significant differences (P≥0.05) in the initial weights of prawn stocked, however, prawn fed on a T5 diet recorded highest significant (P≤0.05) weight gain, specific growth rate, feed intake and feed conversion ratio compared to other tested diets, while prawn fed the control diet (T0) recorded the lowest values of weight gain, specific growth rate, feed intake, feed conversion ratio.
|
Effects of different diets on nutrient efficiency of prawn are presented in Table 4. In general, prawn fed T1 and T5 diets recorded the best values (P≤0.05) of protein productive value, energy retention and protein efficiency ratio compared to other tested diets, while prawn fed T2 diet recorded the lowest values (P≤0.05) of protein efficiency ratio and energy retention.

|
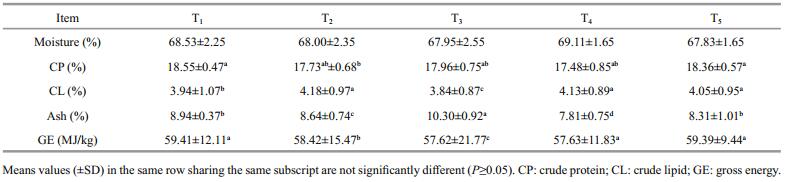
The data of whole body composition of prawn are presented in Table 5. Overall, the highest protein contents were observed on prawns fed on diets T1 and T5 with 18.55% and 18.36%, respectively, while the lowest value was recorded for T4 diet (17.48%). The results show that all prawn PLs fed the diets contain A. pinnata and supplemented with different levels of DigestinTM resulted in higher body lipid content compared to the control diet.
|
Economic analysis data are presented in (Table 6) and clearly indicated that economic performance and the cost-effectiveness improved for A. pinnata diet supplemented with DigestinTM.

|
In general, the increasing prices of aquatic diets, as well as feed ingredients, have been considered as the most important factor limiting profitability in prawn and shrimp cultures, while finding a relatively lowcost alternative ingredient has been an ongoing research goal (Forster et al., 2003; Hernández et al., 2008; Sharawy et al., 2016). Lower acceptance and effectiveness of fresh plant as a feed ingredient for aquatic species was the major constraint for using these ingredients in fish diets and these disadvantages could be attributed due to the lack of exogenous enzymes. Although considerable efforts have been made have been done to investigate the effects of exogenous enzymes on aquatic diets (e.g., Dabrowski and Glogowski, 1977a, b; Kolkovski et al., 1993). The current study highlighted the importance of combined exogenous digestive enzymes with A. pinnata on M. rosenbergii PL survival and growth.
Our present work clearly reveals that lower S% in control diet could not be attributed to any pensspecific effect occurred during the trial except for the severe mortality started 2 wks before the end of the experiment which might occur due to the increase of cannibalism during molting events. Generally, the molting process of crustaceans can be affected by different external factors, e.g. water temperature and salinity, light intensity as well as the internal factors that are associated with the nutritional status and the hormones (Cheng et al., 2003; Sharawy, 2012). However, inadequately formulated diet has been considered as the major factor raising the mortalities especially during the molting period (Yao et al., 2006; Goda, 2008a, b; Sharawy, 2012). The present findings suggested that Azolla meal could be readily accepted by M. rosenbergii PLs without any adverse effects on growth performance and nutrient utilization. The results showed that M. rosenbergii PLs fed with control diet (T1) exhibited significantly (P≤0.05) lower weight gain (WG) and feed intake (FI) when compared to PLs that fed on a T2 diet. The lower feed intake (FI) and growth performance values are correlated to the lower diet quality with respect to different aspects, e.g., amino acids balanced and nutrient digestibility (Hepher, 1988; Pavasovic et al., 2006, 2007a, b; Ambani, 2015). Overall, the prawns that fed on diets containing Azolla and supplemented with Digestin TM up to 3% recorded the best weight gain and feed efficiency. However, this better performance of prawn fed the diet containing Azolla meal supplemented with DigestinTM resulted from superior quality of Azolla meal which contains almost all essential amino acids, minerals such as iron, calcium, magnesium, phosphorus, etc. In addition, Azolla meal contains probiotics and biopolymers, which act as a growth promoter as reported by Pillai et al. (2002) and Chaturvedi et al. (2003). Moreover, same patterns have been recorded in the present study for other nutrient utilization indices. Meanwhile, the addition of DigestinTM improves the feed efficiency by hydrolytic enzymes including amylases and proteases, and vitamins, such as biotin and vitamin B12 (Sugita et al., 1991). Another possible explanation for increased growth performance with adding DigestinTM to Azolla meal is the improvement in digestibility, which may in turn explain the better growth and feed efficiency observed with the supplemented diets. Otherwise, the addition of DigestinTM compound to Azolla meal influence digestive processes by enhancing the digestibility and absorption of food and nutrient utilization (Bomba et al., 2002). Therefore, the values of FCR and ECR in the present results are clearly indicating that the feed cost of the diet containing A. pinnata supplemented with up to 3% DigestinTM was cheaper compared to other tested diets, and therefore it is recommended for prawn, M. rosenbergii PL's. Thus, adding such enzymes can provide additional powerful tools which enhance the nutritional value of prawn feeds, minimize the effects of ANF, effluent pollution, nutrient retention; reduce both diets costs and excretion of nutrients into the environment (Davis et al., 1998; Felix and Selvaraj, 2004). The data of whole body composition of prawn are presented in Table 5. Overall, the highest protein contents were observed on prawns fed on diets T1 and T5 with 18.55% and 18.36%, respectively, while the lowest value was recorded for T4 diet (17.48%). The results show that all prawn PLs fed the diets contain A. pinnata and supplemented with different levels of DigestinTM resulted in higher body lipid content compared to the control diet. Our results agree with the findings of previous studies (Klinnavee et al., 1990; El-Sayed, 1992) who reported that some aquatic plants including Azolla pinnata having a symbiotic relationship with nitrogen fixing cyanobacteria (Anabaena azollae), Hydrodictyon reticulatum, coontail (Ceratophyllum demersum) and chuut-nuu (Eleocharis ochrostachys) can be used as a partial replacement of standard protein for different tilapia species (El-Sayed, 1992). Therefore, Azolla appears to be a potential source of nutrients and has a considerably high feeding value (Hossiny et al., 2008; Anitha et al., 2016). These characteristic make it a suitable and powerful tool to replace expensive, inefficient and limited ingredients as wheat bran in shrimp and prawn diets and create novel ingredient to incorporate in crustacean formulation diets which could be a part to achieve the aquaculture sustainability.
5 CONCLUSIONUnder the present condition, enzyme supplements such as amylase, trypsin, and multi-enzyme have positive results, which play very important role in formulating eco-friendly aqua-feeds. More research toward the use of exogenous enzymes as feed supplements or as pre-treatment of foodstuffs is warranted. Based on the cost differential between the two ingredients, there are economic advantages to complete replacement of wheat bran with A. pinnata for dietary of M. rosenbergii PLs, especially since it is essentially a wild plant that provides an easy, practical and cheaper fish feedstuff. Therefore, from an economic perspective, the diet containing A. pinnata, supplemented with DigestinTM at the level of either 2% (T4) or 3% (T5) can be considered more cost effective for prawn, M. rosenbergii PLs compared to other experimental diets (Table 6).
6 ACKNOWLEDGEMENTSincere thanks to National Institute of Oceanography and Fisheries staff, technician, and scientists for providing their technical facilities and research advises.
Aarumugam P, Bhavan P S, Muralisankar T, Manickam N, Srinevasan V, Radhakrishnan S. 2013. Growth of Macrobrachium rosenbergii fed with mango seed kernel, Banana peel and papaya peel incorporated feeds. International Journal of Applied Biology and Pharmaceutical Technology, 4(2): 12-25.
|
Ambani M M. 2015. Effects of diet substitution on growth performance, energy consumption and digestive enzymes in Macrobrachium rosenbergii postlarvae. Advances in Aquaculture and Fisheries Management, 3(5): 241-248.
|
Anitha K C, Rajeshwari Y B, Prasanna S B, Shilpa S J. 2016. Nutritive evaluation of Azolla as livestock feed. Journal of Experimental Biology and Agricultural Sciences, 4(6): 670-674.
DOI:10.18006/2016.4(Issue6).670.674 |
AOAC (Association of Official Analytical Chemists). 1995. Official Methods of Analysis. 16th edn. Association of Official Analytical Chemists, Inc., Arlington, VA, USA.
|
APHA, AWWA, WPCF. 1995. Standard Methods for the Examination of Water and Wastewater. 19th edn. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, DC, USA. 1 268p.
|
Balaji K, Jalaludeen A, Churchil R R, Peethambaran P A, Senthilkumar S. 2009. Effect of dietary inclusion of azolla(Azolla pinnata) on production performance of broiler chicken. Indian Journal of Poultry Science, 44(2): 195-198.
|
Bligh E G, Dyer W J. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8): 911-917.
DOI:10.1139/o59-099 |
Bomba A, Nemcová R, Gancarcíková S, Herich R, Guba P, Mudronová D. 2002. Improvement of the probiotic effect of micro-organisms by their combination with maltodextrins, fructo-oligosaccharides and polyunsaturated fatty acids. British Journal of Nutrition, 88(S1): S95-S99.
|
Brett J R. 1973. Energy expenditure of sockeye salmon, Oncorhynchus nerka, during sustained performance. Journal of the Fisheries Research Board of Canada, 30(12): 1799-1809.
DOI:10.1139/f73-290 |
Buchanan J, Sarac H Z, Poppi D, Cowan R T. 1997. Effects of enzyme addition to canola meal in prawn diets. Aquaculture, 151(1-4): 29-35.
DOI:10.1016/S0044-8486(96)01478-0 |
Chaturvedi K M M, Langote D S, Asolekar R S. 2003. Duckweed-fed fisheries for treatment of low strength community waste water. WWWTM Newsletter-Asian Institute of Technology, India.
|
Cheng W, Liu C H, Cheng C H, Chen J C. 2003. Osmolality and ion balance in giant river prawn Macrobrachium rosenbergii subjected to changes in salinity:role of sex. Aquaculture Research, 34(7): 555-560.
DOI:10.1046/j.1365-2109.2003.00853.x |
Cherryl D M, Prasad R M V, Jagadeeswara R S, Jayalaxmi P, Kumar D S. 2014. A study on the nutritive value of Azolla pinnata. Livestock Research International, 2(1): 13-15.
|
Dabrowski K, Glogowski J. 1977a. Studies on the role of exogenous proteolytic enzymes in digestion processes in fish. Hydrobiologia, 54(2): 129-134.
DOI:10.1007/BF00034986 |
Dabrowski K, Glogowski J. 1977b. A study of the application of proteolytic enzymes to fish food. Aquaculture, 12(4): 349-360.
DOI:10.1016/0044-8486(77)90213-7 |
Davis D A, Johnston W L, Arnold C R. 1998. The use of enzyme supplements in shrimp diets. In: Ⅳ International Symposium on Aquatic Nutrition. La Paz, B. C. S., Mexico.
|
Duncan D B. 1955. Multiple ranges and multiple F test. Biometrics, 11(1): 1-42.
DOI:10.2307/3001478 |
El-Sayed A F M, Dickson M W, El-Naggar G O. 2015. Value chain analysis of the aquaculture feed sector in Egypt. Aquaculture, 437: 92-101.
DOI:10.1016/j.aquaculture.2014.11.033 |
El-Sayed A F M. 1992. Effects of substituting fish meal with Azolla pinnata in practical diets for fingerling and adult Nile tilapia, Oreochromis niloticus (L). Aquaculture Research, 23(2): 167-173.
DOI:10.1111/are.1992.23.issue-2 |
FAO (Food and Agricultural Organization of the United Nations). 2005. The State of the World Fisheries and Aquaculture (SOFIA). Data extracted from the FAO Fisheries Global Aquaculture Production, Database for freshwater crustaceans. Food and Agricultural Organization, Rome. http://www.faostat.fao.org/faostat./notes/units-e.html.
|
Felix N, Selvaraj S. 2004. Enzyme for sustainable aquaculture. Aquaculture Asia Magazine, 1: 5-6.
|
Forster I P, Dominy W, Obaldo L, Tacon A G J. 2003. Rendered meat and bone meals as ingredients of diets for shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture, 219(1-4): 655-670.
DOI:10.1016/S0044-8486(02)00457-X |
Gaigher I G, Porath D, Granoth G. 1984. Evaluation of duckweed (Lemna gibba) as feed for tilapia (Oreochromis niloticus×O.aureus) in a recirculating unit. Aquaculture, 41(3): 235-244.
|
Goda A M A S. 2008a. Effect of dietary protein and lipid levels and protein-energy ratio on growth indices, feed utilization and body composition of freshwater prawn, Macrobrachium rosenbergii (de Man 1879) post larvae. Aquaculture Research, 39(8): 891-901.
DOI:10.1111/j.1365-2109.2008.01947.x |
Goda A M A S. 2008b. Effect of dietary ginseng herb (Ginsana® G115) supplementation on growth, feed utilization, and hematological indices of Nile Tilapia, Oreochromis niloticus (L.), Fingerlings. Journal of the World Aquaculture Society, 39(2): 205-214.
|
Hepher B. 1988. Nutrition of Pond Fishes. Cambridge University Press, New York.
|
Hernández C, Olvera-Novoa M A, Aguilar-Vejar K, GonzálezRodríguez B, De La Parra I A. 2008. Partial replacement of fish meal by porcine meat meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture, 277(3-4): 244-250.
DOI:10.1016/j.aquaculture.2008.02.016 |
Hossiny H, Setoudeh M, Rokni H, Dehghanzadeh H, Cheraghcheshm M. 2008. Using of silage azollain Guilan male calves nutrition. In: Proceedings of Third National Congress of Recycling and Reuse of Renewable Organic Resources in Agriculture Islamic Azad University. Khorasgan Branch (Isfshan) Agricultural Faculty, Waste and Water Research Centre.
|
Klinnavee S, Tansakul R, Promkuntong W. 1990. Growth of Nile tilapia (Oreochromis niloticus) fed with aquatic plant mixtures. In: Hirano R, Hanyu I eds. 2nd edn. Asian Fisheries Forum. Asian Fisheries Society, Manila, Philippines. p. 283-286.
|
Kolkovski S, Tandler A, Kissil G W, Gertler A. 1993. The effect of dietary exogenous digestive enzymes on ingestion, assimilation, growth and survival of Gilthead seabream (Sparus aurata, Sparidae, Linnaeus) larvae. Fish Physiology and Biochemistry, 12(3): 203-209.
DOI:10.1007/BF00004368 |
Kumar D S, Prasad R M V, Kishore K R, Rao E R. 2012. Effect of azolla (Azolla pinnata) based concentrate mixture on nutrient utilization in buffalo bulls. Indian Journal of Animal Research, 46(3): 268-271.
|
Manush S M, Srivastava P P, Kohli M P S, Jain K K, Ayyappan S, Metar S Y. 2013. Combined effect of papain and vitamin-C levels on growth performance of freshwater giant prawn, Macrobrachium rosenbergii. Turkish Journal of Fisheries and Aquatic Sciences, 13: 479-486.
|
Mariappan P, Balasundaram C. 2004. Effect of shelters, densities, and weight groups on survival, growth and limb loss in the freshwater prawn, Macrobrachium nobilii(Henderson and Matthai, 1910). Journal of Applied Aquaculture, 15(3-4): 51-62.
DOI:10.1300/J028v15n03_04 |
MSTAT-C. 1987. Software Program for the Design and Analysis of Agronomic Research Experiments. Michigan State University, East Lansing.
|
Noor N A S, Syed J, Dileep N, Rakesh K N, Prashith Kekuda T R. 2014. Antioxidant activity of Azolla pinnata and Azolla rubra- a comparative study. Scholars Academic Journal of Biosciences, 2(10): 719-723.
|
Pavasovic A, Anderson A J, Mather P B, Richardson N A. 2007a. Influence of dietary protein on digestive enzyme activity, growth and tail muscle composition in redclaw crayfish, Cherax quadricarinatusi (von Martens). Aquaculture Research, 38(6): 644-652.
DOI:10.1111/are.2007.38.issue-6 |
Pavasovic A, Anderson A J, Mather P B, Richardson N A. 2007b. Effect of a variety of animal, plant and single cellbased feed ingredients on diet digestibility and digestive enzyme activity in redclaw crayfish, Cherax quadricarinatusi (Von Martens 1868). Aquaculture, 272(1-4): 564-572.
|
Pavasovic A, Richardson N A, Mather P B, Anderson A J. 2006. Influence of insoluble dietary cellulose on digestive enzyme activity, feed digestibility and survival in the red claw crayfish, Cherax quadricarinatusi (von Martens). Aquaculture Research, 37(1): 25-32.
DOI:10.1111/are.2006.37.issue-1 |
Pillai P K, Premalatha S, Rajamony S. 2002. Azolla:a sustainable feed substitute for livestock. Leisa India, 4(1): 15-17.
|
Sharawy Z, Goda A M A S, Hassaan M S. 2016. Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, Postlarvae. Animal Feed Science and Technology, 212: 90-99.
DOI:10.1016/j.anifeedsci.2015.12.009 |
Sharawy Z. 2012. Investigations into Growth and Nutritional Condition of Crangon crangon (L). University of Hamburg, Hamburg.
|
Sugita H, Miyajima C, Deguchi Y. 1991. The vitamin B12-producing ability of the intestinal microflora of freshwater fish. Aquaculture, 92: 267-276.
DOI:10.1016/0044-8486(91)90028-6 |
Yao J J, Zhao Y L, Wang Q, Zhou Z L, Hu X C, Duan X W, An C G. 2006. Biochemical compositions and digestive enzyme activities during the embryonic development of prawn, Macrobrachium rosenbergii. Aquaculture, 253(1-4): 573-582.
DOI:10.1016/j.aquaculture.2005.08.011 |
Yılmaz E, Akyurt İ, Günal G. 2004. Use of duckweed, Lemna minor, as a protein feedstuff in practical diets for common carp, Cyprinus carpio, fry. Turkish Journal of Fisheries and Aquatic Sciences, 4: 105-109.
|
Zafar M A, Haque M M, Aziz M S B, Alam M M. 2015. Study on water and soil quality parameters of shrimp and prawn farming in the southwest region of Bangladesh. Journal of the Bangladesh Agricultural University, 13(1): 153-160.
|
Zar J H. 1984. Biostatistical Analysis. 2nd edn. Prentice-Hall, Englewood Hills, New Jersey, UAS.
|
 2018, Vol. 36
2018, Vol. 36