Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ÖTERLER Burak
- Comparative study of epiphytic algal communities on Typha latifolia L. and Phragmites australis (Cav.) Trin. ex Steud in the shallow Gala Lake (European Part of Turkey)
- Chinese Journal of Oceanology and Limnology, 36(5): 1615-1628
- http://dx.doi.org/10.1007/s00343-018-7128-3
Article History
- Received Apr. 27, 2017
- accepted in principle Jul. 7, 2017
- accepted for publication Sep. 25, 2017
As aquatic macrophytes in shallow lakes affect the nutrient cycle and habitat complexity, they are among the key components of inland waters (Dibble et al., 2006; Pelicice et al., 2008; Biolo and Rodrigues, 2013). They also increase the regaining of nutrients into the cycle in aquatic ecosystems and biodiversity of aquatic organisms. Macrophytes also have rhizome structures that help sediment absorption. The rhizomes and roots these organisms have are also used to store basic elements such as nitrogen and phosphorus which are synthesized with the help of the leaves (Pelicice et al., 2008; Padial et al., 2009). Such characteristics make macrophytes highly valuable for aquatic environments. In addition to these characteristics, macrophytes in the littoral zone are considered as suitable substrates as they provide wide areas for periphyton colonization and are able to improve the nutrient status of the community through nutrient secretion (Vadeboncoeur and Steinman, 2002; Santos et al., 2013). Furthermore, experimental studies have demonstrated that rooted macrophytes also serve as a source of nutrients for periphytons (Guariento et al., 2007). In some cases, macrophyte species are spread across the littoral zone and lay their shadow on the epiphyton; however, these macrophytes also periodically supply new substrates to the epiphyton near the illuminated surface (Vadeboncoeur et al., 2006). Macrophytes provide relatively rigid and generally short-term surfaces for periphyton growth. It is expected that this surface area provided by macrophytes will affect the periphyton community (Michelutti et al., 2003). These differences in the species composition among macrophytes that are substrates of epiphyton lead to different responses in the epiphytic community structure (Stevenson et al., 1985; Vinebrooke and Leavitt, 1999).
Whether it is deep or shallow, the littoral zone is accepted as a very complex area in lakes (Vadeboncoeur et al., 2006; Albay and Akçaalan, 2008). Periphyton are important in the functioning of shallow aquatic ecosystems, they may contribute to primary production, and take part in the nutritional cycle, energy flow and food chain (Vadeboncoeur and Steinman, 2002; Vadeboncoeur et al., 2008; Steinman et al., 2016). The physical heterogeneity of littoral zones gives rise to spatial variation in periphyton biomass and productivity, especially where macrophytes provide a temporally variable and structurally complex substratum for periphyton (Vadeboncoeur et al., 2014). Epiphyton in periphyton is comprehensive and important for primary producers. Epiphytic communities may be affected by the variety on the macrophytes they are on and diversity of species may also change along community structures (Algarte et al., 2009). They may affect periphyton communities on different types of macrophytes whose morphologies and physiologies are different (Cattaneo et al., 1998). Communities on macrophytes may consist of blue-green algae, diatoms, green algae or filamentous algae (Chung and Lee, 2008).
Epiphytic algae are widely used as indicators in water quality studies as they respond quickly to changes in hydrology and water quality (Gaiser et al., 2004). The responses of these organisms to pollutant sources in time may be determined on the level of communities (Albay and Akçaalan, 2008). Due to these properties, epiphytic algae have been used for years as indicator organisms in determining water quality (Albay and Akçaalan, 2003). However, as the characteristics of every aquatic ecosystem will differ, epiphyton characteristics will also be different and thus it is not possible for them to provide the same response to changes in water quality every time (Vis et al., 2007). This study was conducted with the purpose of determining the substrate preference of epiphytic algae and the environmental and hydrological factors that affect this preference in the Lake Gala, which is a shallow and eutrophic lake and important part of the Meriç Delta in the international list of Class-A aquatic areas, which was declared a National Part in 2005.
2 MATERIAL AND METHOD 2.1 Study areaLake Gala located in the coordinates of, 40°46'05″N and 26°10'59″E as an alluvial set lake, is one of the most important aquatic areas in the Meriç Delta. The lake is connected to the River Meriç in the north and the Aegean Sea in the south via a canal (Fig. 1). The depth of the lake raises up to a little more than 2 m in wet seasons and drops down to 50-60 cm during dry seasons (Elipek et al., 2010; Anonymous, 2012; Tokatli, 2014). The area, which was a nature reserve in 1991, was designated a Natural Protected Area in 1992 and a national park (Turkey's 36th national park) in 2005. The lake is located on one of the two most important bird migration routes in the western Palaearctic region. The lake is a home to 163 bird species, of which 46 are local, 27 are winter migrants and 90 are summer migrants. 16 different species of fish were found, and these include fish that have economic value such as eel, zander, carp and bowfin. However, The lake is feed by the Meriç River, which is surrounded by rice fields. The release of irrigation water from rice farming areas into Gala Lake has resulted in chemical fertilizers, especially pesticides, entering the lake and placing it under permanent anthropogenic-induced stress (Çamur-Elipek et al., 2010; Tokatli, 2015).

|
| Figure 1 Study area -location of the Lake Gala and sampling regions of macrophytes |
In order to determine the epiphytic algae composition of Lake Gala, whose surroundings and coasts are covered entirely with reeds, samples were collected on Tyhpa latifolia L. and Phragmites australis (Cav.) Trin. Ex Steud which are found dominant in the lake. Sampling was performed between March 2014 and November 2014 in monthly periods, except winter periods. Selected points in the lake were reached by a boat, or on foot as the lake is shallow in general. Macrophytes were harvested from sampling plots (collected using quadrats) of 1 m2 in size (in 3 to 20 replications, according to stand heterogeneity). The submerged parts of the P. australis and T. latifolia bodies in the quadrat (from above; the part contacting the water, from below; the part in the area about 5 cm over the sediment) were cut. The length of the cut piece was measured, and the area where the epiphyton gathered was measured as cm2 by a compass. The epiphytic algae on the piece were swept into 250 mL distilled water by a brush. A part of the water was used for chlorophyll-a analysis, a part was used to determine the epiphytic algae biovolume, and another part was used for counting (the Uthermohl method) and species diagnosis by fixating into a lugol + glycerol mixture (Nõges et al., 2010; Bennion et al., 2014).
The washed macrophytes and the respective washing water including epiphytic algae were placed separately in polyethylene bags for biometric and algological analyses, and carried to the laboratory at +4℃. With this sampling, it was aimed to determine the number and density of macrophytes in the lake per m2, epiphytic algae on the macrophytes, and the chlorophyll-a amounts in the epiphytic algae (Biswas and Calder, 1984; King et al., 2006). Additionally, in order to determine the physicochemical properties of the water in the lake and the changes along the study period, subsurface water samples were collected.
2.3 AnalysesWater temperature, pH, dissolved oxygen and conductivity values were measured at the sampling sites using portable equipment and probes (Lovibond - SensoDirect). Water transparency were measured using Secchi disc. Nitrate (NO3), total suspended solids (TSS) and soluble reactive phosporus (SRP) values were analyzed in the laboratory according to Apha-Awwa-Wef methods (APHA et al., 2012). Chllorophyll-a (Chl-a) concentrations were estimated according to the method done by Nush (Nusch, 1980) using a Cecil 5502 spectrophotometer. Algal species were identified with a light microscope (Olympus CX21) at magnifications 1 000× under immersion oil and using the following literature for species determination: Pestalozzi (1982); Prescott (1973); Komarek and Fott, (1983); Krammer and LangeBertalot (1986-2004), Komarek and Anagnostidis (2005), Hindák (2008) and Kristiansen and Preisig (2011). Finally, all species were checked in algaebase (Guiry and Guiry, 2017). For detail identification of diatoms, a part of the samples (50 mL) was firstly washed in a 1:1 mixture of H2SO4 and HNO3, and then rinsed with distilled water. Then, permanent preparates were obtained using Naprax (Battarbee et al., 2001).
Quantitative analyses of epiphytic algae were done by an inverted microscope (Olympus CK2) according to the Uthermöhl (1958) method. The individuals of each species (filament or colony was considered to be equal to one individual) were counted. In each sample, an average of 600-800 diatom valves were counted. The species density onin cm2 was calculated according to the methods of Ros (1979) and Bicudo and Menezes (2006). Algal biovolume calculations were done according to the methods of Hillebrand et al. (1999) and Sun and Lui (2003).
The species diversity (H') and evenness index were calculated using equations developed by Shannon and Weaver (Addinsoft, 2015). Pearson correlations between the environmental variables and species diversity, richness and evenness were determined using the SPSS 22.0 software (SPSS 22.0, 2013). To classify the algal composition on different macrophytes, Canonical correspondence analysis (CCA) was carried out using the XLSTAT-ADA statistical package (Addinsoft, 2015). CCA was carried out on the log-normal transformed abundance data to determine the relationship between the algae, environmental variables and sampling period.
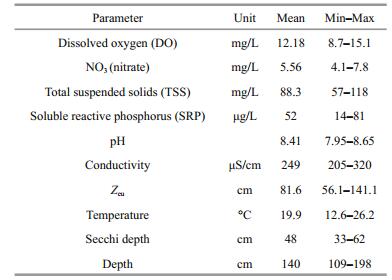
3 RESULT 3.1 Summary of environmental variablesAlthough Lake Gala is shallow, high interactions may be seen between sediment and water column due to waves that occur with the influence of the wind. Additionally, the lake shows eutrophic characteristics with its low photic depth (Zeu), high nutrient concentration and TSS values (Table 1). Dissolved oxygen showed a negative correlation with water temperature (R=-0.82, P < 0.05), Secchi disk transparency (R=-0.80, P < 0.05) and SRP (R=-0.56, P < 0.05). SRP showed a positive correlation with both water temperature (R=0.71, P < 0.05) and chllorophylla (T. latifolia) (R=0.73, P < 0.05). NO3 showed a negative correlation with both chllorophyll-a (T. latifolia) (R=-0.58, P < 0.05) and chllorophyll-a (P. australis) (R=-0.59, P < 0.05).
|
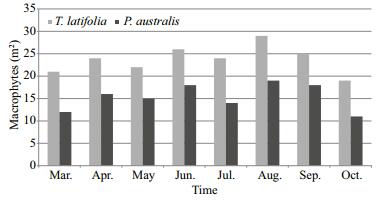
Both littoral zone of the lake and the surrounding areas are covered naturally macrophytes in the very shallow lake. While T. latifolia is generally located in the lake's south-west, west and north-west parts, P. australis is located in the south and south-east parts. Both species are mixed in the north, north-east and east parts. In the measurements (1 m2 quadrate) made in the areas covered with average macrophytes polpulations in littoral zone of the lake during the study, 24 individual T. latifolia and 15 individual P. australis were detected per m2 (Fig. 2).
|
| Figure 2 The distribution of the macrophytes found in the littoral zone of Lake Gala per m2 during the sampling period |
As a result of the study, a total of 133 taxa were identified in the epiphytic algae flora of the lake. In the distribution of these taxa, the Bacillariophyta division was at the top by 68 taxa, followed by Chlorophyta by 30, Cyanophyta by 23, Euglenophyta by 7 and Charophyta by 5 taxa (Table 2).

|
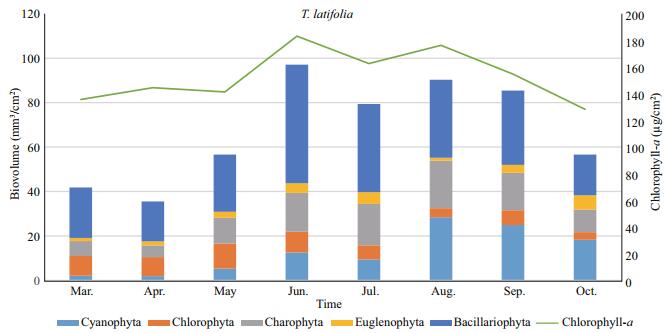
The chlorophyll-a concentrations differed a lot between the two sampled macrophytes (P < 0.05). The chlorophyll-a concentration of the epiphytic algae found on T. latifolia bodies was found to have a mean of 153.3 μg/cm2. In the samples obtained during the 2014, maximum chlorophyll-a was recorded in June as 183.2 μg/cm2 and minimum chlorophyll-a was measured in October as 128.3 μg/cm2. The temporal change of chlorophyll-a and epiphytic algal biovolume showed similar trends during the study period. A first chlorophyll-a peak was recorded on T. latifolia in June 2014, when Diatoms 56% and Charophyta (especially Conjugatophyceae=Zygnematophyceae) 17% dominated of periphyton. Second peak was recorded on T. latifolia in August 2014 when this time diatoms 38%, blue-greens 31% and Charophyta 24% of the epiphyton communities (Fig. 3).
|
| Figure 3 Monthly distributions of epiphytic chlorophyll-a and biovolume values on T. latifolia |
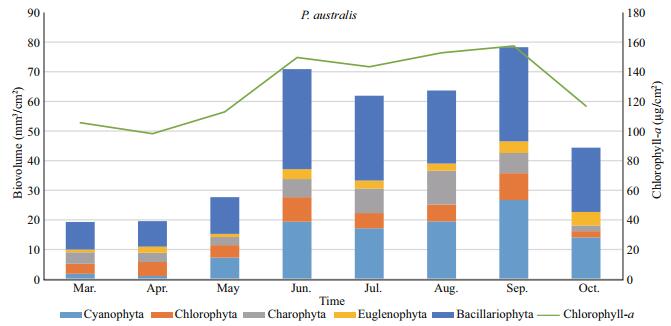
As a result of sampling activities performed along the year 2014, the chlorophyll-a concentration of the epiphytic algae found on P. australis had a mean value of 124.6 μg/cm2. The maximum chlorophyll-a amount was found in the sampling conducted in September by 152.8 μg/cm2, while the minimum values were found in the sampling in April by 98.2 μg/cm2. On P. australis, similarly, both chlorophyll-a and epiphytic algal biovolume ratios were similar. A first chlorophyll-a peak was recorded on P. australis in June 2014, when diatoms dominated 49% of periphyton and second peak was recorded on P. australis in September 2014, when this time diatoms 43% and blue-greens 37% of the epiphyton communities (Fig. 4).
|
| Figure 4 Monthly distributions of epiphytic chlorophyll-a and biovolume values on P. australis |
A total of 107 species of epiphytic algae were found on T. latifolia. The lowest species diversity was in March (30 taxa) while the highest was found in August (70 taxa). A filamentous green alga, Spirogyra affinis was the most abundant in the total algal biovolume (11.28%) followed by filamentous bluegreen algae Anabaena oscillarioides (8.05%) and Oscillatoria sancta (9.75%) as well as a branched mucilaginous stalked diatoms Gomphonema acuminatum (9.73%) and Rhoicosphenia abbreviata (7.11%). The changes in species dominance in the total algal biovolume during the investigation period were as follows: G. acuminatum in March (15.4%), O. sancta in April (12.6%), June (14.1%) and August (12.4%), S. affinis in May (14.1%), Cymbella cistula in July (10.2%), and A. oscillarioides in September (14.3%) and October (15.3%) (Fig. 5). Although Achnanthidium affine, Nitzschia palea and Tetraedron minimum were found in great abundance during the whole investigated period, they never became dominant in the total biovolume. The Shannon index (H') was changed between 2.18 in spring and 1.62 in autumn. Evenness values were between 0.450 and 0.604. (Fig. 6). There are positive correlations between Shannon diversity and number of species during the whole investigated period (R=0.62; P < 0.01). Temperature and Suspended Solids were the factors which affect the Shannon Diversity profoundly during the study period.
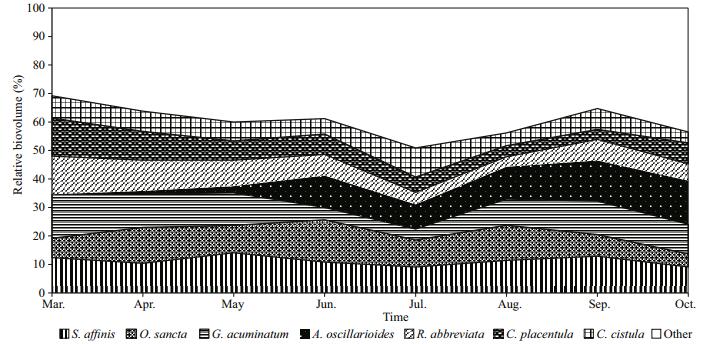
|
| Figure 5 Monthly changes in the relative biovolumes of the dominant epiphytic algae found on T. latifolia |
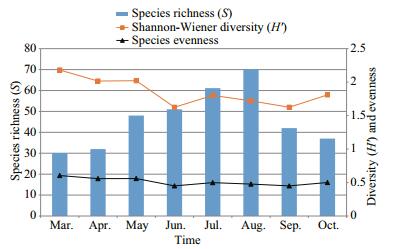
|
| Figure 6 The Shannon diversity, species richness and evenness values of the epiphytic algae found on T. latifolia |
CCA analysis was used to determine the relationship between the relative biovolume of the epiphytic algae and environmental factors. Figure 6 shows 7 taxa dominant on T. latifolia and their relationships with 8 different environmental factors. According to the CCA biplot analysis, the eigenvalues of the two axes were 0.47 and 0.53 respectively. The first axis of CCA explained 60.48% of the total variance in species, while the second axis explained 20.02% of the total variance. Spirogyra affinis on T. latifolia was placed to the origin of the ordination diagram. The position of the dominant species e.g. Rhoicosphaenia abbreviata, Cocconeis placentula and Gomphonema acuminatum were placed closed to NO3, Conductivity and pH vectors. Oscillatoria sancta was placed close to secchi disc depth vector andAnabaenaoscillarioides was placed close to SRP vector in the ordination diagram of CCA (Fig. 7).
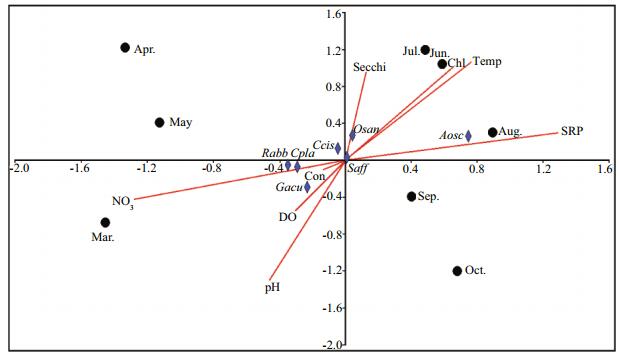
|
| Figure 7 Biplot of first and second CCA axis for epiphytic algae on T. latifolia in the Lake Gala Dominant algae: Saff: Spirogyra affinis; Osan: Oscillatoria sancta; Gacu: Gomphonema acuminatum; Aosc: Anabaena oscillarioides; Rabb: Rhoicosphenia abbreviata; Cpla: Cocconeis placentula; Ccis: Cymbella cistula. Environmental variables: temp: temperature; secchi: secchi depth; NO3: nitrate; con: conductivity; DO: dissolved oxygen; Chl: chlorophyll-a. |
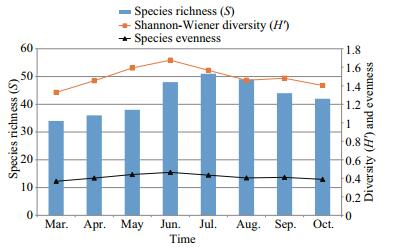
A total of 96 species of epiphytic algae were identified on P. australis in a Lake Gala. Mucilage matrixes and attached diatom, Epithemia adnata became the dominant organism on P. australis by constituting 10.08% of the total mean relative volume. It was followed by the filamentous blue-green algae Oscillatoria sancta (9.56%) and Anabaena oscillarioides (7.94%), the branched mucilaginous stalked diatom of Rhopalodia gibba (8.80%) and the attached diatom, Cocconeis placentula (8.05%). In the sampling period on P. australis, which is one of the emersed macrophytes in the lake, the highest relative biovolume was found in Oscillatoria sancta in March (13.4%), April (12.2%) and July (9.4%), in Epithemia adnata in May (11.9%), June (12.6%) and August (10.6%), in Anabaena oscillarioides in September (10.6%), and Cladophora fracta in October (10.4%) (Fig. 8). Among the epiphytic diatoms found on P. australis, Achnanthes affinis could not become dominant in terms of biovolume although its numbers were high. The epiphytic algal diversity fluctuated slightly between the sections. Shannon index (H') values changed between 1.33 and 1.68 in P. australis, while evenness values changed between 0.368 and 0.464. In P. australis, where a monthly mean of 43 epiphytic algae taxa was found, the highest number of taxa was found in July by 51 and the lowest was found in March by 34 (Fig. 9). Positive correlations were recorded between Shannon diversity and number of species on all sampling mounths were recorded (R=0.71 P < 0.01).
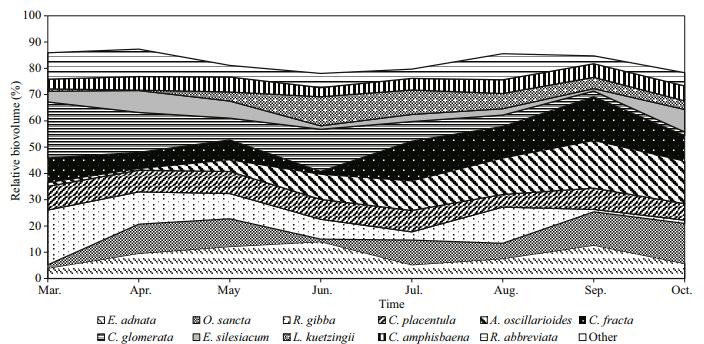
|
| Figure 8 Monthly changes in the relative biovolumes of the dominant epiphytic algae found on P. australis |
|
| Figure 9 The Shannon diversity, species richness and evenness values of the epiphytic algae found on P. australis |
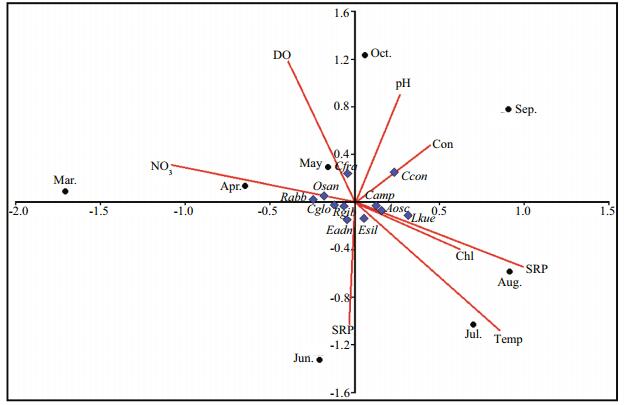
CCA analysis was used to determine the relationship between the relative biovolume of the epiphytic algae and environmental factors. Figure 9 shows 12 taxa dominant on the epiphyton and their relationships with 7 different environmental factors. According to the CCA biplot analysis, the eigenvalues of the two axes were 0.79 and 0.41 respectively. The first axis of CCA explained 48.69% of the total variance in species, while the second axis explained 22.13% of the total variance. According to the CCA analysis results of dominant epiphytic algae found on P. australis, the analyzed organisms were located in proximity to the center of ordination.
The position of the dominant species e.g. Oscillatoria sancta, Rhoicosphenia abbreviata, Cladophora glomerata, Rhopalodia gibba and Epithemia adnata were placed to near nutrients vectors. Cladophora fracta was placed closed to DO vector, Calothrix confervicola was placed closed to conductivity vector, Caloneis amphisbaena, Anabaena oscillarioides and Lyngbya kuetzingii were placed to near Secchi vector. Encyonema silesiacum was placed closed to temperature vector in the ordination diagram of CCA (Fig. 10).
|
| Figure 10 Biplot of first and second CCA axis for epiphytic algae on P. australis in the Lake Gala Dominant algae: Eadn: Epithemia adnata: Osan: Oscillatoria sancta: Rgib: Rhopalodia gibba: Cpla: Cocconeis placentula: Aosc: Anabaena oscillarioides: Cfra: Cladophora fracta: Cglo: Cladophora glomerata: Esil: Encyonema silesiacum: Lkue: Lyngbya kuetzingii: Camp: Caloneis amphisbaena: Rabb: Rhoicosphenia abbreviata: Ccon: Calothrix confervicola. Environmental variables: temp: temperature: secchi: secchi depth: NO3: nitrate: con: conductivity: DO: dissolved oxygen: chl: chlorophyll-a. |
Lake Gala, which is on important migratory routes for birds and an important part of the Meriç Delta that is listed among Class-A aquatic areas, may be considered as a very shallow and eutrophic lake with its depth not exceeding 2 m. The macrophyte intensity continues all year long in the lake whose sides and surroundings are covered by high rates of macrophytes. The dense macrophyte cover of the inside and surroundings of the lake provides a good shelter for water birds.
Like many shallow lakes, Lake Gala is under the pressure of the agricultural activity in its proximity (Coops and Hosper, 2002; Albay and Akçaalan, 2003, 2008; Schippers et al., 2006; Tunca et al., 2014). Temperatures, mixture of water as a result of waves, mixture of water for agricultural usage, create seasonal variations in the physical and chemical characteristics of the lake (Çamur-Elipek et al., 2010; Nivolianitou and Synodinou, 2012; Tokatli et al., 2014; Öterler et al., 2015). It is known that nutrients are primarily important in the growth of algae (Graham et al., 2009). Because of the effects of the agricultural lands near the lake, nutrient levels constantly change through the year. In the lake where the average physicochemical values are almost reaching eutrophication values, the pH was found to be slightly alkaline during the study period. The DO levels were found to be high, though not as high as found in previous studies (Çamur-Elipek et al., 2010; Öterler et al., 2015). Decreasing concentrations of both NO3 and SRP could be attributed to the dense macrophyte vegetation during the agricultural activity period.
The shallow nature of the lake, its domination by macrophytes around it, and the cloudiness due to waving, led to low water transparency levels. This situation provides suitable conditions for growth of especially diatoms (Messyasz et al., 2009). In the epiphyton, diatoms were the dominant group of the epiphytic community in terms of number of taxonomic species, abundance and biovolume. This group was followed by cyanobacteria, which had much lower numbers of species but reached high numbers in the lake in terms of number of colonies and cells. This case was also observed in similar shallow lake studies (Albay and Akçalan, 2008; Tunca et al., 2014).
Because of the mixing in the water column due to waves and as a result of this, resuspension of the sediment, there is an important relationship between limnetic and benthic habitats (Goldsborough and Robinson, 1996; Schallenberg and Burns, 2004). Therefore, as indicated by some researchers, species in the epiphyton may have substrate preference. Macrophytes with different morphological characteristics may naturally harbor different epiphytic communities. In this study, a difference was found between the species compositions of submersed and emerged macrophytes. Thus, it may be stated that epiphytic algae in the lake have substrate preference. As a result of our study, it was observed that some species in the epiphyton had a substrate preference between two emerged macrophytes.
While Oscillatoria sancta, Anabaena oscillarioides, Rhoicosphenia abbreviata, Cocconeis placentula and Encyonema silesiacum among the dominant organisms of the epiphyton were present on both macrophytes in significant numbers and biovolumes, the species Spirogyra affinis, Gomphonema acuminatum and Cymbella cistula preferred to be on T. latifolia epiphytically and the species Epithemia adnata, Rhopalodia gibba, Cladophora fracta, Cladophora glomerata, Lyngbya kuetzingi and Caloneis amphisbaena preferred P. australis. While the epiphyton community on T. latifolia was relatively more homogeneously distributed, some species were found noticeably dominant in the epiphyton community on P. australis.
Generally, filamentous green algae on both emersed macrophytes showed good development in spring and fall months, diatoms developed in spring and summer months, blue-green algae developed especially in summer and fall months. Spirogyra affinis on T. latifolia had the highest biovolume in the epiphytic flora. In addition to this species, Cocconeis placentula, Rhoicosphenia curvata and Gomphonema acuminatum were constantly found abundant. While diatoms were abundant on P. australis, the diatom Epithemia adnata was the dominant organism in the epiphytic flora. Additionally, the diatoms Cocconeis placentula, Rhopalodia gibba, Encyonema silesiacum, Caloneis amphisbaena and Rhoicosphenia abbreviata, the blue-greens Oscillatoria sancta and Anabaena oscillarioides, and the greens Cladophora fracta and Cladophora glomerata were found to have high numbers and biovolumes during the entire sampling.
The species richness and diversity values of the epiphytic algae found in Lake Gala increased from the end of spring through the middle of summer. Thus, no noticeable difference was found between T. latifolia and P. australis. Diversity values also varied within both macrophytes. Several epiphytic algae species except dominant species were represented with low values. Ács et al. (2000), and Albay and Akçalan (2008) stated that changes in the number of species in the periphyton and evenness rates are connected to the algae's rates of migration and reproduction. They reached the conclusion that migration on macrophytes increased the number of species, and protect species richness by reducing losses caused by reproduction and death. The periphyton community was dominated by species which is tolerant to the eutrophic conditions in Lake Gala. According to the results, the substrate preference in the periphyton shows that it is possible to use these as bioindicators of anthropogenic contamination in lakes. More data is needed to investigate factors related to periphyton colonization in lakes with different mixing and trophic levels (Flynn et al., 2002).
According to the results of the CCA analysis, there was a significant relationship between water quality parameters and time-based changes in dominant epiphytic algae. For T. latifolia; according to the CCA results, dissolved oxygen, NO3, conductivity and pH were effective on the epiphyton in spring months, while chlorophyll-a, secchi depth and water temperature were influential in summer months. For P. australis; dissolved oxygen and NO3 were effective in spring months, while water temperature, SRP and chlorophyll-a were effective in summer months and pH and conductivity were effective in fall months. Albay and Akçaalan (2003) indicated that filamentous cyanophytes have a broad range of tolerance to physical disturbance including water level fluctuation, large amounts of suspended solids, and low Secchi disk transparency. While similar results were found by Tunca et al. (2014), the relationship between the dominant filamentous blue-green algae species and secchi was not very strong in our study. Moreover, while the shading effects that take place especially in spring and summer months due to the increased number of macrophytes and their larger sizes may create suppression on the growth of some species, this may be an advantage for species that are less in need of light to develop in the ecosystem. Among the dominant species found epiphytically in the lake, Spirogyra affinis had a negative correlation with secchi depth (R=-0.59, P < 0.05), Oscillatoria sancta had a negative correlation with DO (R=-0.78, P < 0.05) but positive correlations with chlorophyll-a (R=0.79, P < 0.05) and water temperature (R=0.62, P < 0.05). Among the dominant diatoms, Gomphonema acuminatum, Rhoicosphenia abbreviata and Cocconeis placentula had a positive correlation with NO3 (respectively, R=0.66, R=0.77 and R=0.83; P < 0.05), while they had a negative correlation with SRP (respectively, R=-0.62, R=-0.81 and R=-0.72; P < 0.05) and temperature (respectively, R=-0.70, R= -0.62 and R=-0.68; P < 0.05).
Benthic communities in inland waters are highly affected by biotic and abiotic factors (Letáková et al., 2016). Distribution of benthic algae in lakes is based on microhabitats and this distribution is influenced by factors such as hydrological status, lake bathymetry, light, nutrients and grazing (Cantonati et al., 2012; Cano et al., 2012; Neif et al., 2013). Furthermore, according to Blanco et al. (2014), environmental factors are primarily effective on benthic algae. In the lake, whose western and northern sides are generally covered by Typha, Phragmites were localized in the southern and eastern parts. In the very shallow lake, both macrophytes have reached significant populations. Epiphytic algae on richness, diversity, and composition differed significantly between these two main host plants. Because of the macrophytes' morphologies, distribution of epiphytic algae (such as nutrient-based substrate preference) may have been seasonally influenced. Seasonal changes have been found to be significant in shallow lowland ponds (Kitner et al., 2005). On both macrophytes, the epiphytic algae colonization was found higher in summer months than in spring and autunm months.
5 CONCLUSIONIn conclusion, this study determined the relationships of the epiphyton communities on two different macrophytes in Lake Gala with water quality and hydrologic drivers. According to the results of the study, epiphytic colonization is controlled by seasonal variations that take place in major environmental gradients (such as temperature, dissolved oxygen and water level fluctuation). Another important result is that some species of algae in Lake Gala had substrate preference between T. latifolia and P. australis. However, substrate type affected the colonization of epiphytic algal seasonality. Species composition of epiphytic algae was diverse as a result of macrophyte morphology, but diversity values were similar.
6 DATA AVAILABILITY STATEMENTThe datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Ács É, Kiss K T, Szabó K, Makk J. 2000. Short-term colonization sequence of periphyton on glass slides in a large river (River Danube, near Budapest). Algological Studies, 100: 135-156.
|
Addinsoft, Xlstat. 2015. Data analysis and statistics with MS Excel®. Addinsoft, NY, USA. xlstat available at http://www.xlstat.com/en/home.
|
Albay M, Akçaalan R. 2003. Comparative study of periphyton colonisation on common reed (Phragmites australis) and artificial substrate in a shallow lake, Manyas, Turkey. Hydrobiologia, 506(1-3): 531-540.
DOI:10.1023/B:HYDR.0000008606.69572.f6 |
Albay M, Akçaalan R. 2008. Effects of water quality and hydrologic drivers on periphyton colonization on Sparganium erectum ın two turkish lakes with different mixing regimes. Environmental Monitoring and Assessment, 146(1-3): 171-181.
DOI:10.1007/s10661-007-0069-5 |
Algarte V M, Siqueira N S, Murakami E A, Rodrigues L. 2009. Effects of hydrological regime and connectivity on the interannual variation in taxonomic similarity of periphytic algae. Braz. J. Biol., 62(2S): 609-616.
DOI:10.1590/S1519-69842009000300015 |
Anonymous. 2012. İpsala Vision Plan. Trakya Development Agency, 2012. (ın Turkish)
|
APHA, AWWA, WEF. 2012. 10300 Periphyton. Standard Methods for the Examination of Water and Wastewater. 22nd edn. American Public Health Association, Washington, D.C.
|
Battarbee R W, Jones V J, Flower R J, Cameron N G, Bennion H, Carvalho L, Juggins S. 2001. Diatoms. In: Smol J P, Birks H J, Last W M eds. Tracking Environmental Change Using Lake Sediments. Volume 3: Terrestrial, Algal, and Siliceous İndicators. Springer, The Netherlands. p.155-202.
|
Bennion H, Kelly M G, Juggins S, Yallop M L, Burgess A, Jamieson J, Krokowski J. 2014. Assessment of ecological status in UK lakes using benthic diatoms. Freshwater Science, 33(2): 639-654.
DOI:10.1086/675447 |
Bicudo C E M, Menezes M. 2006. Gêneros de algas de águas continentais do Brasil. (Chave de identificação e descrições). 2nd edn. Rıma, São Carlos. p.1-489.
|
Biolo S, Rodrigues L. 2013. Comparison of the structure of the periphytic community in distinct substrates from a neotropical floodplain. International Research Journal of Plant Science, 4(3): 64-75.
|
Biswas K, Calder C C. 1984. Hand-Book of Common Water and Marsh Plants of India and Burma. p.1-216.
|
Blanco S, Cejudo-Figueiras C, Álvarez-Blanco I, van Donk E, Gross E M, Hansson L A, Irvine K, Jeppesen E, Kairessalo T, Moss B, Nõges T, Bécares E. 2014. Epiphytic diatoms along environmental gradients in western european shallow lakes. CLEAN-Soil, Air, Water, 42(3): 229-235.
DOI:10.1002/clen.201200630 |
Çamur-Elipek B, Arslan N, Kırgız T, Öterler B, Güher H, Özkan N. 2010. Analysis of benthic macroinvertebrates in relation to environmental variables of Lake Gala, a National Park of Turkey. Turkish Journal of Fisheries and Aquatic Sciences, 10(1): 235-243.
DOI:10.4194/trjfas.2010.0212 |
Cano M G, Casco M A, Claps M C. 2012. Effect of environmental variables on epiphyton in a pampean lake with stable turbid-and clear-water states. Aquatic Biology, 15(1): 47-59.
DOI:10.3354/ab00409 |
Cantonati M, Angeli N, Bertuzzi E, Spitale D, Lange-Bertalot H. 2012. Diatoms in springs of the Alps: spring types, environmental determinants, and substratum. Freshwater Science, 31(2): 499-524.
DOI:10.1899/11-065.1 |
Cattaneo A, Galanti G, Gentinetta S, Romo S. 1998. Epiphytic algae and macro invertebrates on submerged and floatingleaved macrophytes in an Italian lake. Freshwater Biology, 39(4): 725-740.
DOI:10.1046/j.1365-2427.1998.00325.x |
Chung M H, Lee K S. 2008. Species composition of the epiphytic diatoms on the leaf tissues of three Zostera species distributed on the southern coast of Korea. Algae, 23(1): 75-81.
DOI:10.4490/ALGAE.2008.23.1.075 |
Coops H, Hosper S H. 2002. Water-level management as a tool for the restoration of shallow lakes in the Netherlands. Lake and Reservoir Management, 18(4): 293-298.
DOI:10.1080/07438140209353935 |
Dibble E D, Thomaz S M, Padial A A. 2006. Spatial complexity measured at a multi-scale in three aquatic plant species. Journal of Freshwater Ecology, 21(2): 239-247.
DOI:10.1080/02705060.2006.9664992 |
doa Santos T R, Ferragut C, de Mattos Bicudo C E. 2013. Does macrophyte architecture influence periphyton? Relationships among Utricularia foliosa, periphyton assemblage structure and its nutrient (C, N, P) status. Hydrobiologia, 714(1): 71-83.
DOI:10.1007/s10750-013-1531-8 |
Flynn N J, Snook D L, Wade A J, Jarvie H P. 2002. Macrophyte and periphyton dynamics in a UK Cretaceous chalk stream: the River Kennet, a tributary of the Thames. The Science of the Total Environment, 282-283: 143-157.
DOI:10.1016/S0048-9697(01)00949-4 |
Gaiser E E, Scinto L J, Richards J H, Jayachandran K, Chiders D L, Trexler J C, Jones R D. 2004. Phosphorus in periphyton mats provides the best metric for detecting low-level P enrichment in an oligotrophic wetland. Water Research, 38(3): 507-516.
DOI:10.1016/j.watres.2003.10.020 |
Goldsborough L G, Robinson G C. 1996. Patterns in wetlands. In: Stevenson R J, Bothwell M L, Lowe R L eds. Algal Ecology: Freshwater Benthic Ecosystems. Academic, London. p.78-117.
|
Graham L E, Graham J M, Wilcox L W. 2009. Algae. 2nd edn. Prentice-Hall, Inc., Upper Saddle River, New Jersey. p.1-616.
|
Guariento R D, Caliman A, Esteves F A, Enrich-Prast A, Bozelli R L, Farjalla V F. 2007. Substrate-mediated direct and indirect effects on periphytic biomass and nutrient content in a tropical coastal lagoon, Rio de Janeiro, Brazil. Acta Limnologica Brasiliensia, 19: 331-340.
|
Guiry M D, Guiry G M. 2017. AlgaeBase. World-wide Electronic Publication, National University of Ireland, Galway. http://www.algaebase.org.
|
Hillebrand H, Dürselen C D, Kirschtel D, Pollingher U, Zohary T. 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35(2): 403-424.
DOI:10.1046/j.1529-8817.1999.3520403.x |
Hindák F. 2008. Colour Atlas of Cyanophytes. VEDA, Bratislava. p.1-253.
|
IBM Corp, 2013. IBM SPSS Statistics for Windows (Version 22.0). IBM Corp, Armonk, NY.
|
King L, Clarke G, Bennion H, Kelly M, Yallop M. 2006. Recommendations for sampling littoral diatoms in lakes for ecological status assessments. Journal of Applied Phycology, 18: 15-25.
DOI:10.1007/s10811-005-9009-3 |
Kitner M, Poulíčková A, Hašler P. 2005. Algal colonization process in fishponds of different trophic status. Algological Studies, 115: 115-127.
DOI:10.1127/1864-1318/2005/0115-0115 |
Komarek J, Anagnostidis K. 2005. Cyanoprokariota. 2. Teil: Oscillatoriales. In: Büdel B, Gärtner G, Krienitz L, Schagerl M eds. Süßwasserflora von Mitteleuropa. Elsevier, Heidelberg. p.1-759.
|
Komarek J, Fott B. 1983. Die binnnengewässer. Band 26, Das phytoplankton des süßwassers. 7 Teil, 1. Hälfte, Chlorophyceae (Grünalgen), Ordnung: Chlorococcales. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, p.1-1044.
|
Krammer K, Lange-Bertalot H. 1986–2004. Bacillariophyceae. 1-4 Teil. Süsswasserflora von Mitteleuropa. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D eds. Fischer-Verlag, Stuttgart, Germany.
|
Kristiansen J, Preisig H R. 2011. Phylum Chrysophyta (Golden Algae). In: John D M, Whitton B A, Brook A J eds. The Freshwater Algal Flora of the British Isles: An İdentification Guide to Freshwater and Terrestrial Algae. 2nd edn. Cambridge University Press, Cambridge. p.1-878.
|
Letáková M, Cantonati M, Hašler P, Nicola A, Poulíčková A. 2016. Substrate specificity and fine-scale distribution of epiphytic diatoms in a shallow tarn in the Brenta Dolomites (south-eastern Alps). Plant Ecology and Evolution, 149(2): 144-156.
DOI:10.5091/plecevo.2016.1206 |
Messyasz B, Kuczyńska-Kippen N, Nagengast B. 2009. The epiphytic communities of various ecological types of aquatic vegetation of five pastoral ponds. Biologia, 64(1): 88-96.
DOI:10.2478/s11756-009-0006-x |
Michelutti N A, Holtham J, Douglas M S V, Smol J P. 2003. Periphytic diatom ssemblages from ultraoligotrophic and UV transparent lakes and ponds on Victoria Island and comparisons with other diatom surveys in the Canadian Arctic. Journal of Phycology, 39(3): 465-480.
DOI:10.1046/j.1529-8817.2003.02153.x |
Neif É M, de Lima Behrend R D, Rodriguez L. 2013. Seasonal dynamics of the structure of epiphytic algal community on different substrates from a Neotropical floodplain. Brazilian Journal of Botany, 36(3): 169-177.
DOI:10.1007/s40415-013-0021-6 |
Nivolianitou Z, Synodinou B. 2012. Environmental management of big riverine floods: the case of evros river in greece. In: Advances in Environmental Science and Sustainability. WSEAS Press, Sliema, Malta. p.15-20.
|
Nõges N, Luup H, Feldmann T. 2010. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquatic Ecology, 44(1): 83-92.
DOI:10.1007/s10452-009-9249-4 |
Nusch E A. 1980. Comparison of different methods for chlorophyll and phaeopigment determination. Archiv für Hydrobiologie, 14: 14-36.
|
Öterler B, Albay M, Çamur-Elipek B, Güher H, Kırgız T. 2015. Spatial and temporal distribution of phytoplankton in Lake Gala (Edirne/TURKEY). Trakya University Journal of Natural Sciences, 16(2): 71-80.
|
Padial A A, Thomaz S M, Agostinho A A. 2009. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia, 624(1): 161-170.
DOI:10.1007/s10750-008-9690-8 |
Pelicice F M, Thomaz S M, Agostinho A A. 2008. Simple relationships to predict attributes of fish assemblages in patches of submerged macrophytes. Neotropical Ichthyology, 6(4): 543-550.
DOI:10.1590/S1679-62252008000400001 |
Pestalozzi H G. 1982. Das phytoplankton des susswasser Teil: 8 E. Schweizerbart'sche Verlagsbuchhandlund (Nagele U. Obermiller). Stuttgart. p.1-539.
|
Prescott G W. 1973. Algae of Western Great Lake Area. Fifth printing. William C. Brown Publishers, Dubaque. p.1-977.
|
Ros J. 1979. Práticas de ecologia. Editora Omega, Barcelona. p.181.
|
Schallenberg M, Burns C W. 2004. Effects of sediment resuspension on phytoplankton production: teasing apart the influences of light, nutrients and algal entrainment. Freshwater Biology, 49(2): 143-159.
DOI:10.1046/j.1365-2426.2003.01172.x |
Schippers P, van de Weerd H, de Klein J, de Jong B, Scheffer M. 2006. Impacts of agricultural phosphorus use in catchments on shallow lake water quality: about buffers, time delays and equilibria. Sci. Total Environ., 369(1-3): 280-294.
DOI:10.1016/j.scitotenv.2006.04.028 |
Steinman A, Abdimalik M, Ogdahl M E, Oudsema M. 2016. Understanding planktonic vs. benthic algal response to manipulation of nutrients and light in a eutrophic lake. Lake and Reservoir Management, 32(4): 402-409.
DOI:10.1080/10402381.2016.1235065 |
Stevenson R J, Singer R, Roberts D A, Boylen C W. 1985. Patterns of epipelic algal abundance with depth, trophic status, and acidity in poorly buffered New Hamshire lakes. Canadian Journal of Fisheries and Aquatic Sciences, 42(9): 1501-1512.
DOI:10.1139/f85-188 |
Sun J, Liu D Y. 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research, 25(11): 1331-1346.
DOI:10.1093/plankt/fbg096 |
Tokatli C. 2014. Drinking water quality of a rice land in Turkey by statistical and GIS perspectives. Polish Journal of Environmental Studies, 23(6): 2247-2258.
DOI:10.15244/pjoes/26967 |
Tokatli C. 2015. Assessment of water quality in the Meriç River as an ecosystem element in Turkey's Thrace region. Polish Journal of Environmental Studies, 24(5): 2205-2211.
DOI:10.15244/pjoes/58780 |
Tunca H, Ongun-Sevindik T, Bal D N, Arabacı S. 2014. Community structure of epiphytic algae on three different macrophytes at Acarlar floodplain forest (Northern Turkey). Chinese Journal of Oceanology and Limnology, 32(4): 845-857.
DOI:10.1007/s00343-014-3205-4 |
Utermöhl H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitt. Int. Ver. Theor. Angew. Limnol., 9: 1-38.
|
Vadeboncoeur Y, Kalff J, Christoffersen K, Jeppesen E. 2006. Substratum as a driver of variation in periphyton chlorophyll and productivity in lakes. Journal of the North American Benthological Society, 25(2): 379-392.
DOI:10.1899/0887-3593(2006)25[379:SAADOV]2.0.CO;2 |
Vadeboncoeur Y, Peterson G, Vander Zanden M J, Kalff J. 2008. Benthic algal production across lake size gradients: interactions among morphometry, nutrients, and light. Ecology, 89(9): 2542-2552.
DOI:10.1890/07-1058.1 |
Vadeboncoeur Y, Steinman A D. 2002. Periphyton function in lake ecosystems. The Scientific World Journal, 2: 1449-1468.
DOI:10.1100/tsw.2002.294 |
Vadeboncoeur Y, Devlin S P, McIntyre P B, Vander Zanden M J. 2014. Is there light after depth? Distribution of periphyton chlorophyll and productivity in lake littoral zones. Freshwater Science, 33(2): 524-536.
DOI:10.1086/676315 |
Vinebrooke R D, Leavitt P R. 1999. Phytobenthos and phytoplankton as potential indicators of climate change in mountain lakes and ponds: a HPLC-based pigment approach. J. North Am. Benth. Soc., 18(1): 15-33.
DOI:10.2307/1468006 |
Vis C, Hudon C, Carignan R, Gagnon P. 2007. Spatial analysis of production by macrophytes, phytoplankton and epiphyton in a large river system under different waterlevel conditions. Ecosystems, 10(2): 293-310.
DOI:10.1007/s10021-007-9021-3 |
 2018, Vol. 36
2018, Vol. 36


