Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YANG Xiaoqian(杨小倩), WEN Xin(文欣), ZHOU Chengxu(周成旭), ZHU Xiaojuan(朱小娟), MENG Ran(孟冉), LUO Qijun(骆其君), YAN Xiaojun(严小军)
- Comparative study of brine shrimp bioassay-based toxic activities of three harmful microalgal species that frequently blooming in aquaculture ponds
- Chinese Journal of Oceanology and Limnology, 36(5): 1697-1706
- http://dx.doi.org/10.1007/s00343-018-7140-7
Article History
- Received May. 10, 2017
- accepted in principle Jul. 25, 2017
- accepted for publication Aug. 28, 2017
2 Key Laboratory of Marine Biotechnology of Zhejiang Province, Ningbo University, Ningbo 315211, China;
3 Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Research Center, Ningbo University, Ningbo 315211, China;
4 Senior High School of Yudu, Ganzhou 343200, China
With the problems such as high eutrophication, poor water exchange, low biodiversity, marine aquaculture ponds become areas where some specific notorious harmful microalgal species blooms were most likely triggered (Stanley et al., 2005; Martínez-Porchas et al., 2010; Granéli et al., 2012; Place et al., 2012). In return, the blooms caused tremendous damages to the cultured organisms and economical losses (Deeds et al., 2002; Houdan et al., 2004; Roelke et al., 2011; Martinez-Porchas and Martinez-Cordova, 2012).
Harmful microalgae in aquaculture ponds face complicated interactions with different cultured organisms. During the interactions, toxic materials may be released from the algal cell or just keep being content inside the cell. On account of the toxins, harmful effects may be caused by definite algal toxins or normal metabolites that are not generally thought as toxins, such as predation detergents DMS (P) (Kiene et al., 2000). It is difficult to answer the question that how toxic microalgae produce toxin or anti-predation detergent when predator present. Simulation of these processes is necessary to analyze the instant moment when the toxic species are physically disturbed or damaged because of the activities of cultured animals.
Harmful microalgae, such as Prymnesium parvum, Pleurochrysis elongata, Karlodinium veneficum, were usually found in some aquaculture ponds in Sanmen Bay, Zhejiang province, blooming or coblooming. Cell densities ranged 102–107 cells/mL occasionally. Prymnesium parvum is a wide-spread species and can cause mortality to many cultured animals such as fish, shellfish, shrimp and crab (Edvardsen and Paasche, 1998; Igarashi et al., 1998; Sasaki et al., 2001; Edvardsen and Imai, 2006; Baker et al., 2007). Many substances were thought to be responsible to its toxic potency (Henrikson et al., 2010). However, toxic effect of P. parvum was highly labile from case to case (Granéli and Salomon, 2010). Modes of action, potency of toxins or toxic effects may change in response to the ambient physical, chemical and biological factors such as salinity, light and temperature (Larsen et al., 1998; Brooks et al., 2010), pH (Valenti et al., 2010), metal ion availability and photolysis (James et al., 2011). Quantification of prymnesins is not analytically feasible because of different geographical strain of different toxic potency existed (Schug et al., 2010; Manning and La Claire, 2010).
As P. parvum, calcified coccolithophorid specie P. elongata belong to Prymnesiophyceae. Genus Pleurochrysis is drawing much attention because that some species of this Genus typically bloom in aquaculture pools in a way of long lasting notorious foam bloom. Pleurochrysis elongata is lethal to aquatic organisms such as Artemia salina (Houdan et al., 2004). However, few studies are done to clarify its toxic mechanism. Unlike the former one with some identified toxins, Pleurochrysis has no definite chemical compounds that could be classified as toxin(s). Mechanism of the lethal effect of Pleurochrysis is still unknown (Not et al., 2012).
Small dinoflagellate K. veneficum contains toxins (karlotoxins) of multiple toxic characteristic, such as hemolytic, ichthyotoxic, and cytotoxic activities (Deeds et al., 2002; Kempton et al., 2002; Zhou et al., 2011). Feature and activity model of the toxins differ from different strains and have not been completely characterized (Bachvaroff et al., 2009; Calbet et al., 2011). The content and structure of karlotoxins vary significantly with different strains, locations, environmental conditions (Place et al., 2012).
It is difficult to detect instant interaction between prey and predator in situ. In order to simulate the possible origination of the harmful effects, in this study, different algal samples were prepared to analysis the toxic potency based on brine shrimp toxicological bioassay. Toxic assessment based on crustaceans Artemia nauplii has been widely used for long time (Michael et al., 1956; Vanhaecke et al., 1981; Nunes et al., 2005; Hameed et al., 2009). When simultaneous assessment of multiple ecotoxicological significance of harmful species with multiple toxic characteristics is needed, Artemia nauplii are perfect model candidates (Nunes et al., 2006; Waggett et al., 2008; Faimali et al., 2012).
2 MATERIAL AND METHOD 2.1 Microalgal cultures and culture conditionsThree harmful microalgal species, namely P. parvum, P. elongata, K. veneficum, were isolated from aquaculture pond in Sanmen Bay, typical aquaculture area in Zhejiang Province, in the coastal area of the East China Sea. Identification was done through analyses of morphology and Nucleic acid sequencing and SEM. Pure isolates were kept in Microalgae Collection Center in Ningbo University, China. Accession code in NCBI for P. parvum is MF039887. Strain number of P. elongate and K. veneficum are NMBjih026-1 and NMBjah047-1 respectively in the Collection Center. Isochrysis galbana (strain NMBjih021-1) was used as non-toxic species control.
The cultures were grown in sterile natural seawater (salinity 25) enriched with NMB3 medium (Xu et al., 2012). Conditions of culture room were 20℃, 40 μmol photons/(m2·s) illumination with 12 h:12 h L:D cycle.
2.2 Artemia nauplii preparationArtemia cysts preserved in 4℃ were warmed up to room temperature before use. The cysts were incubated at 30℃ in an incubator with continuous light of 50 μmol photons/(m2·s). The cysts hatched gradually after 24 h. Active nauplii were picked and placed into fresh seawater to keep for an additional 24 h to make sure all the nauplii moulted into instar Ⅱ–Ⅲ stages. These nauplii were prepared in the study.
2.3 Mortalities of Artemia as a function of microalgal cell densitiesExperiment was performed using 24-well plate. Cell densities of the pre-cultures were 1.1×106, 2.8×106 and 9.8×105 cells/mL for P. parvum, P. elongata and K. veneficum respectively at their early stationary growth phase. The cultures were 10 fold serially diluted with fresh culture medium. Five groups of different densities were prepared for each species. Ten nauplii together with 10 μL seawater were added to each well with micropipette (Eppendorf, Germany). Two milliliter algal cultures were put into each well. Nauplii in fresh culture medium were used as starving control. All treatments were replicated four times. The plates were placed in the culture room of 20℃ with continuous illumination of 15 μmol photons/(m2·s). Conditions of nauplii were documented and the dead ones were counted under inverted microscope (Nikon Ti-U Microscope Type 108) at 24 and 48 h. Still nauplii on the bottom without any kind of moving were considered dead.
2.4 Lethal effects of different algal samples on ArtemiaAlgal samples preparation. Various samples were prepared after the cultures grew to late exponential phase. For each microalgae strain, totally 5 sorts of samples from 4 aliquots of each culture were prepared for the bioassay:
Cell free mediums (CFM). There were two ways to get CFM: cell free supernatants of the cultures centrifuged at 4℃ by 4 700×g for 10 min (CR11GIII, Hitachi, Japan), CFMcf; cell free filtrates by natural gravity filtration through 0.3 μm GF/C film (Whatman, USA), CFMgf.
Cell extracts (CEcf). Cell sediments by centrifugation were re-suspended in fresh culture medium to original volume. The cells were ultrasonic ruptured for 10–30 min (20 kHz, 400 W, with 5 s interval at 4℃). Cells totally ruptured were confirmed by observing under microscope. The suspensions were centrifuged at 42 300×g (4℃) for 10 min. The supernatants were used as cell extracts, CEcf.
Cell debris (CD). Ultrasonic ruptured cell debris was got from sediments while CEcf was prepared. The debris was re-suspended to original volume with fresh culture medium before use.
Original cultures of live cells (LC) were used to compare the effects of the algal samples. Original cell densities of the cultures were: 3.3×106, 5.3×105, 7.5×105, 6.4×106 cells/mL for P. parvum, P. caterae, K. veneficum and I. galbana respectively.
Toxicity detection process was the same as described in Section 2.3. Effects on Artemia were observed at time point of 0, 5, 12, 24, 36, 48 and 72 h. Dead nauplii were counted at each time.
Artemia in fresh culture medium (DSW) was set as starving control in all the tests.
2.5 Data handling, statistical analyses, LD50 and LT50Data are present as means ±SD.
Mortality of Artemia nauplii was calculated as:
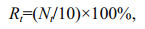
where Nt represents dead nauplii at time t.
Median lethal density (LD50) or median lethal time (LT50) was obtained by applying a linear regression between cell concentration (or time) and mortality of Artemia nauplii according the method of Hewlett and Plackett (1979). LD50 in 24 h and 48 h were analyzed in Section 2.3. LT50, the time when 50% mortality of Artemia nauplii occurred, was analyzed in 2.4.
Statistical analyses were conducted with Statistical Package for the Social Sciences (SPSS). Significant differences were determined by one-way ANOVA test.
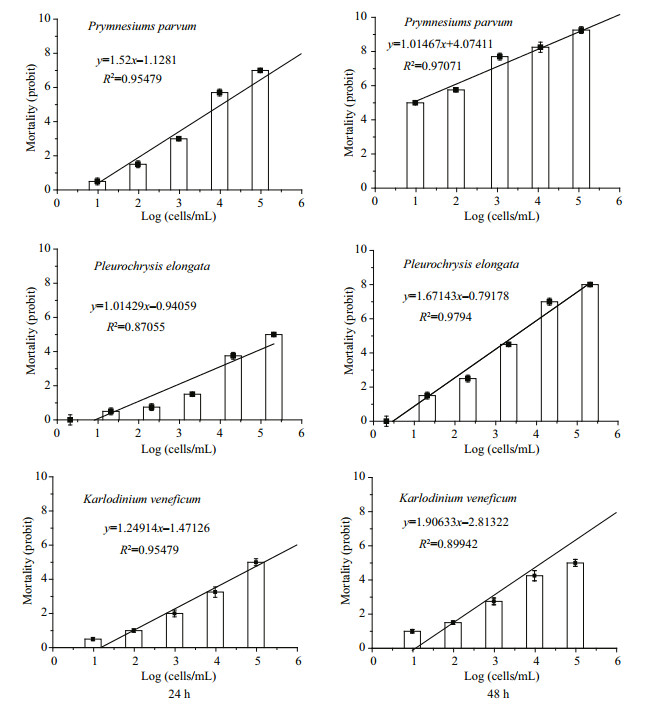
3 RESULT 3.1 Mortality of Artemia nauplii as a function of microalgal cell densityAll the three cultures had density dependent lethal effects on Artemia nauplii. Linear regression relationship existed in 24 h and 48 h (Fig. 1). For P. parvm, linear relationship between cell density and mortality of Artemia nauplii was the most significant (R2=0.954 79 and 0.970 71 in 24 h and 48 h respectively). LD50 were 1.1×104 cells/mL in 24 h and 8 cells/mL in 48 h. For P. elongate, linear relationship was more significant in 48 h (R2=0.979 4) than in 24 h (R2=0.870 55). LD50 was 7.2×105 and 2.9×103 cells/mL in 24 h and 48 h respectively. At the early time of exposure, there was no significant difference among the mortalities in groups of cell density of 102–104 cells/mL (P > 0.05, ANOVA). Not until the cell density rise to magnitude of 105 did the mortality increase significantly. In 48 h, however, mortality increased with rising culture density in a way of significant linear relationship. For K. veneficum, LD50 in 24 h was 1.5×105 cells/mL. It was 1.3×104 cells/mL in 48 h. Linear regression relationship in both 24 h and 48 h were significant.
|
| Figure 1 Mortality of Artemia nauplii as a function of microalgal cell density in 24 h and 48 h Error bars: standard deviation. |
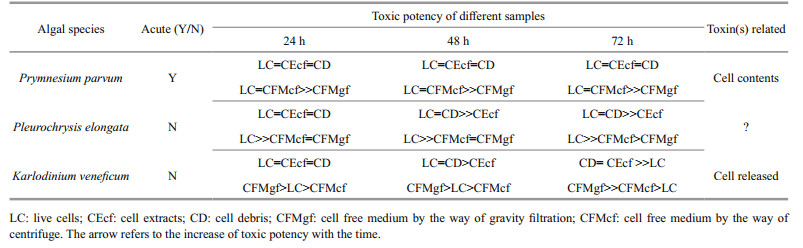
Table 1 compared toxic potency of the three harmful species based on simultaneously assessment of mortalities of Artemia in 24 h and 48 h. P. parvm had the highest lethal activities than the other two. In 24 h, K. veneficum showed higher toxic activity than P. elongata. In 48 h, P. elongata showed higher toxic activity than K. veneficum. No dead nauplii were observed in feed or starving controls.
|
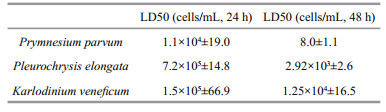
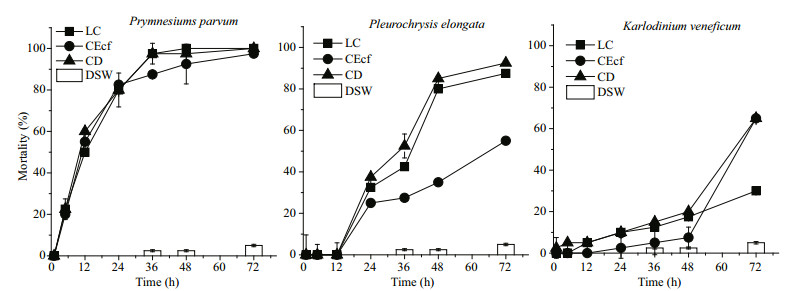
By comparing with the effects of live cells (LC) of each culture, mortality of Artermia as function of cell extracts (CEcf) and cell debris (CD) of the three species were studied (Fig. 2).
|
| Figure 2 Lethal effects of living cells (LC), cellular extracts (CEcf) and cell debris (CD) of the three microalgal species on Artemia nauplii DSW: fresh culture medium. Error bars: standard deviation. |
For P. parvm, acute effects were observed in 5 h from live cells, cell extracts and cell debris groups, mortality rates were 20%–22% without significant difference. In 36 h, the mortality reached 100%.
For P. elongata, no lethal effect was observed in 5 h or 12 h. Lethal effects showed no significant difference in 24 h. From 36 to 72 h, cell debris and live cells groups had similar lethal activities without significant differences. Artemia mortality was 2 times higher than that of the cells extracts in 48 h. It reached 90% in 72 h.
For K. veneficum, no acute effect occurred in 5 h. Mortalities, as low as 2% to 10%, were no significant differences in 24 h. Similar mortality in live cell and cell debris groups ranged 15%–18%. That of cell extracts was the lowest (5%). However, from 48 to 72 h, mortalities in cell extracts and cell debris groups increased significantly, averaged 60%, which was 2 times higher than that in live cells.
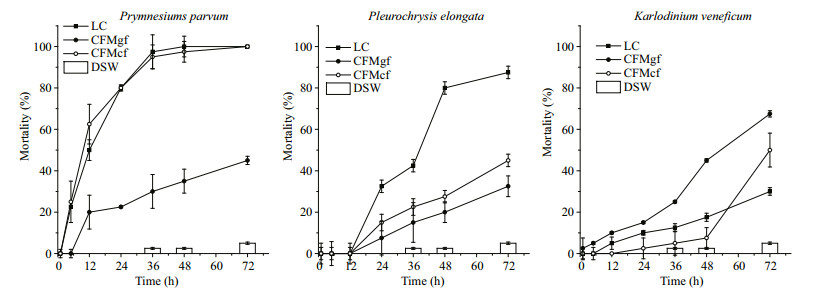
3.2.2 Toxic activities of cell free medium of the harmful microalgaeBy comparing with effects of live cells (LC), mortalities of Artermia as function of cell free mediums were studied. Cell free process changed the toxic activities with species specific characteristics as showed in Fig. 3.
|
| Figure 3 Lethal effects of living cells (LC) and cell free mediums of the three harmful species on Artermia nauplii CFMgf: cell free culture medium prepared by way of gravity filtration; CFMcf: cell free medium prepared by way of centrifuge; DSW: fresh culture medium. Error bars: standard deviation. |
Cell free process caused significant different results of toxic activities of the medium. For P. parvum, cell free medium prepared by the way of gravity filtration, CFMgf, had significant lower lethal effects on Artermia. Mortalities of Artermia in CFMgf decreased 60%–80%. And no acute mortality occurred in 5 h. However, cell free medium prepared by way of centrifuge, CFMcf, presented the same characteristics of toxic activities exactly as that of the live cells.
For P. elongata, mortality in cell free mediums decreased compared with LC group. However, there was no significant differences between CFMgf and CFMcf in 24 h–48 h (P > 0.05). In 72 h, toxic activity of CFMcf was higher than that of CFMgf (P < 0.05).
For K. veneficum, toxic activity of CFMgf significantly increased compared with that of live cells and CFMcf. In 72 h, both cell free culture mediums reached higher toxic activities than live cells. Still, mortality in the CFMgf was the highest (70% at 72 h).
Tables 2 and 3 summarized the different median lethal times (LT50) and different toxic potency of the algal samples. Cell debris, cellular extracts and live cells of P. parvum had the same toxic activity, LT50 averaged 10.8 h. Cell debris and cell extracts of K. veneficum showed higher toxicity than that of living cells. Similar higher toxicities were observed both in debris and live cells of P. elongate.

|
|
On account of the cell free culture medium, toxic activities were affected by the process of cell free especially when P. parvum and K. veneficum were compared. Process of gravity filtration significantly decreased the toxic activities of cell free medium of P. parvum. However, for K. veneficum, LT50 of gravity filtrates was 55.7 h, which showed significantly higher toxic activity than that of live cells (LT50=125.6 h) and that of supernatants by centrifugation (90.9 h).
Microalgae feed I. galbana was control in all the algal sampling process. The results showed that no lethal effects ever occurred in any of the algal sampling treatment in I. galbana.
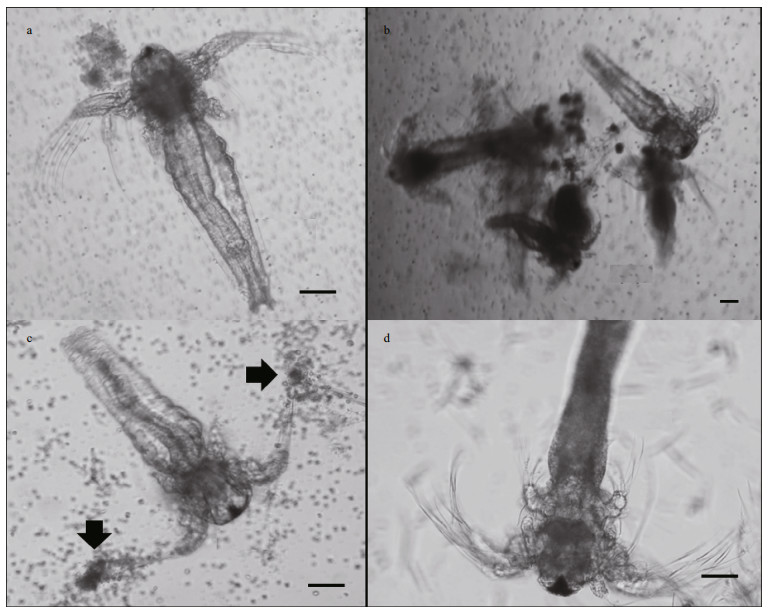
3.3 Different conditions of dead Artermia nauplii showed different damage originationConditions of dead Artermia nauplii showed different characteristics in cultures of the three harmful species (Fig. 4). Dead nauplii in P. parvum culture showed no abnormal structure (Fig. 4a). Dead nauplii in P. elongata culture entwined in small groups with some broken appendages littered in water. The dying individuals were observed struggling with their limb that looked like been stuck (Fig. 4b). In K. veneficum culture, the algal cells were actively aggregating around the tips of the appendages (Fig. 4c). This phenomenon was mostly found in K. veneficum cultures but never found in the other two. This phenomenon suggested different active interaction between harmful microalgae and victim.
|
| Figure 4 Condition of dead Artemia nauplii affected by harmful microalgal species a. in Prymnesium parvum culture. The nauplii showed normal structure; b. in Pleurochrysis elongata culture. The nauplii aggregated in small groups, and some broken appendages littered in water; c. in Karlodinium veneficum culture. Karlodinium veneficum actively aggregated around the tips of the appendages (arrow); d. dead nauplii in filtrates of the harmful species showed common condition as that of live individuals. Bar=50 μm. |
Prymnesins, toxins of P. parvum, was hemolytic, ichthyotoxic and cytotoxic (Igarashi et al., 1998). In the present study, toxic activities of P. parvum medium significantly decreased when cell integrity was maintained by gentle gravity filtration. Cell debris had the same toxic characteristic as that of live cells. This phenomenon agreed with the study of Remmel and Hambright (2012) that direct contact between predator and prey contributed a lot to the toxic activity. By separating algal cell and tested animals in a system that medium could exchange freely and algal cell integrity maintained, the authors demonstrated that toxic effects of P. parvum on micropredators occurred only if the microalgae and the predator contacted with each other, rather than exotoxicity.
Toxicity potency of P. parvum may not be dependent on prymnesins only, but on ambient nutrient conditions (Granéli and Johansson, 2003), growth conditions (Fresnel et al., 2001), laboratory-grown or natural field samples (Henrikson et al., 2010; James et al., 2011), and the activity and stages of tested animals. Different geographical strains may also show different toxic activities. In the study of Houdan et al. (2004), algal growth phase affected the toxicity of P. parvum. The highest toxicity came from the cultures of stationary phase. In their study, P. parvum was not the most toxic one to A. salina nauplii. LD50 in 24 h and 48 h were at the order of magnitude of 105 cells/mL, which was much larger than that in our study. Simonsen and Moestrup (1997) found that toxicity of P. parvum on A. salina was not significantly. According the supplier of Artemia nauplii in our study, the tested animals were originally collected from Qinghai Lake, a big salt lake in China. Differences of Artemia intoxication might be due to different strain of tested animals as well. So, even multiple toxicity characteristics can be obtained, the quantitative parameters of toxic activity based on Artemia lethality was not supposed to be compared among different studies, especially for those whose toxicity potency is dependent on various conditions.
Mechanism of lethal effects of Pleurochrysis is still a mystery. No toxin was found in Pleurochrysis genus even though obvious lethal effects on Artemia had been demonstrated (Houdan et al., 2004). Reifel et al. (2001) found that P. pseudoroscoffensis had a moderately toxic effect on A. salina, but was not toxic to vertebrates using mouse bioassay. In the present study, exposure time related mortalities were found and linear relationship depended on different time period. No acute toxic effects in 5 h or 12 h were observed in P. elongata. Linearity between mortality and algal cell density was less significant in 24 h than in 48 h. Mortality in control of the culture medium increased from 0 at 24 h to detectable at 48 h. At this time, probably the nauplii began to feed. Algae culture and cell debris of Pleurochrysis caterea were lethal to Artermia nauplii only when nauplii begin to feed (Jiang et al., 2009). The reason that entwined dead nauplii and broken appendages existed in P. elongata culture probably because of direct contact between prey and predator. In study of Houdan (2004), not all the calcified coccolithophorids was lethal to A. salina. Obviously, both the algae and the predator need further studies.
Compared with the former two species, toxic activities of K. veneficum of different samples were relatively lower in 72 h. However, species specific character was observed in K. veneficum. Firstly, it actively aggregated at the tip of the appendages of Artermia nauplii. Secondly, cell free filtrate of gentle gravity filtration was significantly higher than that of live cells and of cell free supernatant by centrifugation. To some extent, these two features are correlated because of one important fact that, K. veneficum is an active predator armed with specific toxin, karlotoxins. K. veneficum is mixotrophic dinoflagellate with ability of phagotrophy. It actively feed on various microalgae such as cryptophytes, diatoms, dinoflagellates and zooplankton such as Acartia tonsa (Place et al., 2012; Zhou et al., 2015). Karlotoxins help K. veneficum in predation (Deeds et al., 2002; Van Wagoner et al., 2010). Not only it can penetrate through cell membrane and makes prey's cell leaking, it can immobilize smaller prey for K. venefivum to catch the prey during predation (Sheng et al., 2010). Sterol composition have been demonstrated playing a role in sensitivity to the effects of karlotoxin (Deeds and Place, 2006; Place et al., 2006). Cholesterol is an important sterol component of Artermia (Payne and Kuwahara, 1972). Respiration apparatus of Artermia nauplii locates on the fibrous, feather like plates (Hickman, 1967). The relationship needs further demonstration.
Karlodinium venificum cells actively release karlotoxins either by filtration or by centrifugation (Deeds et al., 2002). This present study demonstrated that different toxic activity occurred during different cell free processes and K. venificum actively enhance toxin releasing when the cell experiences external force disturbance. For K. veneficum cell, both gravity filtration and centrifugation are stresses. Gentle gravity filtration process might mimic a kind of mechanical stimulation to the mciroalgae. Possibly karlotoxin release in respond to the mechanical stimulation such as shear stress, or to some kind of water current stirred by interaction between prey and predator in a micro scale (Adolf et al., 2007). Compared gravity filtration, centrifugation process gave K. venificum cells much stronger force. However, it is also a much rapid process. During this process, K. venificum cells had been captured before they were able to release any further toxins. It is reasonable that toxic activity was lower than gravity filtration. This phenomenon strongly demonstrated that K. venificum is an active toxin releaser. Gravity filtration may be applied in further studies on how and why K. venificum enhances toxin releasing.
5 CONCLUSIONAmong the three harmful species that frequently occurred in aquaculture ponds, P. parvum is the most toxic one and acute toxic. Prymnesins, toxin of P. parvum, is possibly not exotoxic active. Lethal effects of P. elongata on Artermia mainly originated from cell's structural materials, suggesting harmful effects occurred when predator directly contact with the algal cells. Physical turbulence will significantly trigger K. veneficum to release more toxins into medium and increase its toxic activity. Karlotoxin is exotoxic active.
6 DATA AVAILABILITY STATEMENTThe authors declare that the data supporting the findings of this study are available within the article.
Adolf J E, Krupatkina D, Bachvaroff T, Place A R. 2007. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae, 6(3): 400-412.
DOI:10.1016/j.hal.2006.12.003 |
Bachvaroff T R, Adolf J E, Place A R. 2009. Strain variation in Karlodinium veneficum (Dinophyceae):toxin profiles, pigments, and growth characteristics. Journal of Phycology, 45(1): 137-153.
DOI:10.1111/jpy.2009.45.issue-1 |
Baker J W, Grover J P, Brooks B W, Ureña-Boeck F, Roelke D L, Errera R, Kiesling R L. 2007. Growth and toxicity of Prymnesium parvum (Haptophyta) as a function of salinity, light, and temperature. Journal of Phycology, 43(2): 219-227.
DOI:10.1111/jpy.2007.43.issue-2 |
Brooks B W, James S V, Valenti T W Jr, Urena-Boeck F, Serrano C, Berninger J P, Schwierzke L, Mydlarz L D, Grover J P, Roelk D L. 2010. Comparative toxicity of Prymnesium parvum in inland waters. JAWRA Journal of the American Water Resources Association, 46(1): 45-62.
DOI:10.1111/j.1752-1688.2009.00390.x |
Calbet A, Bertos M, Fuentes-Grünewald C, Alacid E, Figueroa R, Renom B, Garcés E. 2011. Intraspecific variability in Karlodinium veneficum:growth rates, mixotrophy, and lipid composition. Harmful Algae, 10(6): 654-667.
DOI:10.1016/j.hal.2011.05.001 |
Deeds J R, Place A R. 2006. Sterol specific membrane interactions with the toxins from Karlodinium micrum(Dinophyceae)-a strategy for self-protection. African Journal of Marine Science, 28(2): 421-425.
DOI:10.2989/18142320609504190 |
Deeds J R, Terlizzi D E, Adolf J E, Stoecker D K, Place A R. 2002. Toxic activity from cultures of Karlodinium micrum(=Gyrodinium galatheanum) (Dinophyceae)-a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae, 1(2): 169-189.
DOI:10.1016/S1568-9883(02)00027-6 |
Edvardsen B, Imai I. 2006. The ecology of harmful flagellates within Prymnesiophyceae and Raphidophyceae. In: Granéli E, Turner J T eds. Ecology of Harmful Algae.Springer-Verlag, New York, p.67-79.
|
Edvardsen B, Paasche E. 1998. Bloom dynamics and physiology of Prymnesium and Chrysochromulina. In: Anderson D M, Cembella A D, Hallegraeff G M eds.Physiological Ecology of Harmful Algal Blooms.Springer-Verlag, Heidelberg. p.193-208.
|
Faimali M, Giussani V, Piazza V, Garaventa F, Corrà C, Asnaghi V, Privitera D, Gallus L, Cattaneo-Vietti R, Mangialajo L, Chiantore M. 2012. Toxic effects of harmful benthic dinoflagellate Ostreopsis ovata on invertebrate and vertebrate marine organisms. Marine Environmental Research, 76: 97-107.
DOI:10.1016/j.marenvres.2011.09.010 |
Fresnel J, Probert I, Billard C. 2001. Pyrmnesium faveolatum sp. nov. (Prymnesiopyceae), a new toxicspecies from the Mediterranean Sea. Vie et Milieu, 51(1): 89-97.
|
Granéli E, Edvardsen B, Roelkec D L, Hagström J A. 2012. The ecophysiology and bloom dynamics of Prymnesium spp. Harmful Algae, 14: 260-270.
DOI:10.1016/j.hal.2011.10.024 |
Granéli E, Johansson N. 2003. Effects of the toxic haptophyte Prymnesium parvum on the survival and feeding of a ciliate:the influence of different nutrient conditions. Marine Ecology Progress Series, 254: 49-56.
DOI:10.3354/meps254049 |
Granéli E, Salomon P S. 2010. Factors influencing allelopathy and toxicity in Prymnesium parvum. JAWRA Journal of the American Water Resources Association, 46(1): 108-120.
DOI:10.1111/j.1752-1688.2009.00395.x |
Hameed S, Sultana V, Ara J, Ehteshamul-Haque S, Athar M. 2009. Toxicity of Fusarium solani strains on brine shrimp(Artemia salina). Zoological Research, 30(4): 468-472.
DOI:10.3724/SP.J.1141.2009.04468 |
Henrikson J C, Gharfeh M S, Easton A C, Easton J D, Glenn K L, Shadfan M, Mooberry S L, Hambright K D, Cichewicz R H. 2010. Reassessing the ichthyotoxin profile of cultured Prymnesium parvum (golden algae) and comparing it to samples collected from recent freshwater bloom and fish kill events in North America. Toxicon, 55(7): 1 396-1 404.
DOI:10.1016/j.toxicon.2010.02.017 |
Hewlett P S, Plackett R L. 1979. An Introduction to the Interpretation of Quantal Responses in Biology. University Park Press, Baltimore. p.11-19.
|
Hickman C P. 1967. Biology of the Invertebrates. CV Mosby Company, St. Louis.
|
Houdan A, Bonnard A, Fresnel J, Fouchard S, Billard C, Probert I. 2004. Toxicity of coastal coccolithophores(Prymnesiophyceae, Haptophyta). Journal of Plankton Research, 26(8): 875-883.
DOI:10.1093/plankt/fbh079 |
Igarashi T, Aritake S, Yasumoto T. 1998. Biological activities of prymnesin-2 isolated from a red tide alga Prymnesium parvum. Natural Toxins, 6(1): 35-41.
DOI:10.1002/(ISSN)1522-7189 |
James S V, Valenti T W Jr, Prosser K N, Grover J P, Roelke D L, Brooks B W. 2011. Sunlight amelioration of Prymnesium parvum acute toxicity to fish. Journal of Plankton Research, 33(2): 265-272.
DOI:10.1093/plankt/fbq082 |
Jiang Y, Zhou C X, Luo Q J, Ma B. 2009. Lethal effects of different Pleurochrysis carterae cells on brine shrimp. Asian Journal of Ecotoxicology, 4(4): 561-568.
(in Chinese) |
Kempton J W, Lewitus A J, Deeds J R, Law J M, Place A R. 2002. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae, 1(2): 233-241.
DOI:10.1016/S1568-9883(02)00015-X |
Kiene R P, Linn L J, Bruton J A. 2000. New and important roles for DMSP in marine microbial communities. Journal of Sea Research, 43(3-4): 209-224.
DOI:10.1016/S1385-1101(00)00023-X |
Larsen A, Bryant S, Båmstedt U. 1998. Growth rate and toxicity of Prymnesium parvum and Prymnesium patelliferum (Haptophyta) in response to changes in salinity, light, and temperature. Sarsia, 83(5): 409-418.
DOI:10.1080/00364827.1998.10413700 |
Manning S R, La Claire J W Ⅱ. 2010. Prymnesins:toxic metabolites of the golden alga, Prymnesium parvum Carter (Haptophyta). Marine Drugs, 8(3): 678-704.
DOI:10.3390/md8030678 |
Martínez-Porchas M, Martínez-Córdova L R, Porchas-Cornejo M A, López-Elías J A. 2010. Shrimp polyculture:a potentially profitable, sustainable, but uncommon aquacultural practice. Reviews in Aquaculture, 2(2): 73-85.
DOI:10.1111/(ISSN)1753-5131 |
Martinez-Porchas M, Martinez-Cordova L R. 2012. World aquaculture:environmental impacts and troubleshooting alternatives. The Scientific World Journal, 2012: Article ID 389623.
|
Michael A S, Thompson C G, Abramovitz M. 1956. Artemia salina as a test organism for bioassay. Science, 123(3194): 464.
|
Not F, Siano R, Kooistra W H C F, Simon N, Vaulot D, Probert I. 2012. Diversity and ecology of eukaryotic marine phytoplankton. Advances in Botanical Research, 64: 1-53.
|
Nunes B S, Carvalho F D, Guilhermino L M, Van Stappen G. 2006. Use of the genus Artemia in ecotoxicity testing. Environmental Pollution, 144(2): 453-462.
DOI:10.1016/j.envpol.2005.12.037 |
Nunes B, Carvalho F, Guilhermin L. 2005. Acute toxicity of widely used pharmaceuticals in aquatic species:Gambusia holbrooki, Artemia parthenogenetica and Tetraselmis chuii. Ecotoxicology and Environmental Safety, 61(3): 413-419.
DOI:10.1016/j.ecoenv.2004.08.010 |
Payne T H, Kuwahara S S. 1972. Sterols of the brine shrimp, Artemia salina, from Mono Kake, California. Experientia, 28(9): 1 022-1 023.
DOI:10.1007/BF01918647 |
Place A R, Bowers H A, Bachvaroff T R, Adolf J E, Deeds J R, Sheng J. 2012. Karlodinium veneficum-The little dinoflagellate with a big bite. Harmful Algae, 14: 179-195.
DOI:10.1016/j.hal.2011.10.021 |
Place A R, Harvey H R, Bai X, Coats D W. 2006. Sneaking under the toxin surveillance radar:parasitism and sterol content. African Journal of Marine Science, 28(2): 347-353.
DOI:10.2989/18142320609504175 |
Reifel K M, McCoy M P, Tiffany M A, Rocke T E, Trees C C, Barlow S B, Faulkner D J, Hurlbert S H. 2001. Pleurochrysis pseudoroscoffensis (Prymnesiophyceae) blooms on the surface of the Salton Sea, California. Hydrobiologia, 466(1-3): 177-185.
|
Remmel E J, Hambright K D. 2012. Toxin-assisted micropredation:experimental evidence shows that contact micropredation rather than exotoxicity is the role of Prymnesium toxins. Ecology Letters, 15(2): 126-132.
DOI:10.1111/ele.2011.15.issue-2 |
Roelke D L, Grover J P, Brooks B W, Glass J, Buzan D, Southard G M, Fries L, Gable G M, Schwierzke-Wade L, Byrd M, Nelson J. 2011. A decade of fish-killing Prymnesium parvum blooms in Texas:roles of inflow and salinity. Journal of Plankton Research, 33(2): 243-253.
DOI:10.1093/plankt/fbq079 |
Sasaki M, Shida T, Tachibana K. 2001. Synthesis and stereochemical confirmation of the HI/JK ring system of prymnesins, potent hemolytic and ichthyotoxic glycoside toxins isolated from the red tide alga. Tetrahedron Letters, 42(33): 5 725-5 728.
DOI:10.1016/S0040-4039(01)01067-X |
Schug K A, Skingel T S, Spencer S E, Serrano C A, Le C Q, Schug C A, Valenti T W Jr, Brooks B W, Mydlarz L D, Grover J P. 2010. Hemolysis, fish mortality, and LC-ESIMS of cultured crude and fractionated golden alga(Prymnesium parvum). JAWRA Journal of the American Water Resources Association, 46(1): 33-44.
DOI:10.1111/j.1752-1688.2009.00389.x |
Sheng J, Malkiel E, Katz J, Adolf J E, Place A R. 2010. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proceedings of the National Academy of Sciences of the United States of America, 107(5): 2 082-2 087.
DOI:10.1073/pnas.0912254107 |
Simonsen S, Moestrup Ø. 1997. Toxicity tests in eight species of Chrysochromulina (Haptophyta). Canadian Journal of Botany, 75(1): 129-136.
DOI:10.1139/b97-015 |
Stanley S M, Ries J B, Hardie L A. 2005. Seawater chemistry, coccolithophore population growth, and the origin of Cretaceous chalk. Geology, 33(7): 593-596.
DOI:10.1130/G21405.1 |
Valenti T W Jr, James S V, Lahousse M J, Schug K A, Roelke D L, Grover J P, Brooks B W. 2010. A mechanistic explanation for pH-dependent ambient aquatic toxicity of Prymnesium parvum Carter. Toxicon, 55(5): 990-998.
DOI:10.1016/j.toxicon.2009.09.014 |
Van Wagoner R M, Deeds J R, Tatters A O, Place A R, Tomas C R, Wright J L C. 2010. Structure and relative potency of several karlotoxins from Karlodinium veneficum. Journal of Natural Products, 73(8): 1 360-1 365.
DOI:10.1021/np100158r |
Vanhaecke P, Persoone G, Claus C, Sorgeloos P. 1981. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicology and Environmental Safety, 5(3): 382-387.
DOI:10.1016/0147-6513(81)90012-9 |
Waggett R J, Tester P A, Place A R. 2008. Anti-grazing properties of the toxic dinoflagellate Karlodinium veneficum during predator-prey interactions with the copepod Acartia tonsa. Marine Ecology Progress Series, 366: 31-42.
DOI:10.3354/meps07518 |
Xu J L, Zhou H B, Yan X J, Zhou C X, Zhu P, Ma B. 2012. Effect of unialgal diets on the composition of fatty acids and sterols in juvenile ark shell Tegillarca granosa Linnaeus. Journal of Agricultural and Food Chemistry, 60(15): 3 973-3 980.
DOI:10.1021/jf300620e |
Zhou C X, Fernández N, Chen H M, You Y R, Yan X J. 2011. Toxicological studies of Karlodinium micrum(Dinophyceae) isolated from East China Sea. Toxicon, 57(1): 9-18.
DOI:10.1016/j.toxicon.2010.08.014 |
Zhou C X, Place A R, Yan X J, Xu J L, Luo Q J, William E, Jiang Y. 2015. Interactions between Karlodinium veneficum and Prorocentrum donghaiense from the East China Sea. Harmful Algae, 49: 50-57.
DOI:10.1016/j.hal.2015.08.004 |
 2018, Vol. 36
2018, Vol. 36


