Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Longle(王龙乐), ZHANG Xin(张鑫), ZOU Dinghui(邹定辉), CHEN Weizhou(陈伟洲), JIANG Heng(姜恒)
- Growth and Fv/Fm in embryos of Hizikia fusiformis (Harvey) Okamura (Sargassaceae, Phaeophyta) cultured under different temperature and irradiance conditions
- Chinese Journal of Oceanology and Limnology, 36(5): 1798-1805
- http://dx.doi.org/10.1007/s00343-018-7055-3
Article History
- Received Mar. 2, 2017
- accepted in principle Apr. 17, 2017
- accepted for publication Jul. 25, 2017
2 The Science and Technology Division, South China University of Technology, Guangzhou 510006, China;
3 The Key Lab of Pollution Control and Ecosystem Restoration in Industry Clusters, Ministry of Education, Guangzhou 510006, China;
4 Marine Biology Institute, Science Center, Shantou University, Shantou 515063, China
Hizikia fusiformis (Harvey) Okamura, a brown macroalgal species, is endemic to the northwest coasts of the Pacific Ocean, commonly occurring on lower intertidal and upper subtidal rocks, gravel or shells (Zeng, 2000). This macroalga is traditionally used as healthy foodstuff, medicinal herb and marine vegetable in Southeast Asian countries, such as China, Japan and Korea. In China, H. fusiformis has been under experimental pilot cultivation since the early 1980s in Shandong and Zhejiang provinces, using the rope technique (Zhang et al., 2002). Hizikia fusiformis is now becoming one of the most important species for mariculture in China—cultivated H. fusiformis produced 9.3% of the total farmed macroalgae in China in 2011 (Yan et al., 2011)—owing to increasing market demand for it as a food delicacy and industrial material (Luo and Li, 2002).
Traditionally, at the beginning of the artificial cultivation period (November to March) every year, a large number of young fronds of Hizikia (3–10 cm in length) are collected from natural beds for cultivation. Consequently, natural populations of Hizikia have been severely depleted by over-exploitation (Zhang et al., 2002; Zou and Gao, 2004). Hizikia, previously one of the most common brown seaweeds along the coast of China, is now difficult to find in many places (Luo and Li, 2002).
The culture of seedlings through sexual reproduction is an alternative approach for artificial Hizikia seed production, which is essential for both conserving natural resources and further promoting cultivation development of this economic important species (Luo and Li, 2002; Pang et al., 2001, 2006, 2007; Yan et al., 2011). This approach has the advantages of requiring small amounts of mature fronds and producing large numbers of seedlings at a high density on a substrate (Li, 2001). During the sexual maturity season, female and male receptacles of H. fusiformis release eggs and spermatozoids, and the fertilized eggs develop into embryos and then into young seedlings.
Previous studies have focused on the synchronous and mass release of gametes by Hizikia receptacles for fertilization success, seedling culture in a landbased system (Pang et al., 2000, 2001, 2005, 2006), embryo culture at different adhesion matrix attachment rates (Luo, 2001; Zhang et al., 2012), and the effects of environmental conditions, such as temperature and light conditions, on the growth of young seedlings (Pang et al., 2007; Liu et al., 2010; Zou et al., 2012). Additionally, previous research has indicated that the sensitivity of brown algae, including H. fusiformis (Liu et al., 2008, 2010; Gao et al., 2009; Zhao et al., 2015), to changing temperature and light conditions depends on the algal life history stage (Dring et al., 1996; Altamirano et al., 2003b; Roleda et al., 2004; Véliz et al., 2006). All of the above studies have been useful for increasing the efficiency and effectiveness of artificial seedling culture (Gao, 2014).
However, biological information is lacking regarding the influence of environmental factors on embryos (young seedlings) in H. fusiformis. In this study, we investigated the effects of environmental factors on embryo physiology under laboratory and field conditions, to promote the development of artificial seedlings. Embryos of H. fusiformis were cultured under different temperatures, sunlight intensities, and ultraviolet radiation (UVR) conditions, and then the growth and optimal photochemical yield of photosystem Ⅱ (Fv/Fm) of the embryos was measured to examine their physiology with regard to temperature and irradiance conditions.
2 MATERIAL AND METHOD 2.1 Plant materialHealthy fertile fronds of Hizikia fusiformis were gathered at low tide during the sexual reproduction season (from April to June) from a cultivation field at Dongtou, Wenzhou, Zhejiang, China (27°29ʹ35″N, 121°05ʹ24″E) in April 2015, and were immediately transported in plastic barrels filled with seawater to the laboratory. The fertile fronds were incubated indoors in a glass aquarium containing filtered natural seawater (salinity of 33) with approximately 10 μmol/L NaNO3 and 3 μmol/L NaH2PO4. Eggs and spermatozoids were released from ripe receptacles on the reproductive branches of the fronds. A high rate of fertilization success was achieved by tumbling the receptaclebearing male and female branchlets as described by Pang et al. (2005). The embryos from fertilized eggs were then gently washed down with seawater and introduced into incubation dishes. The embryos were maintained at room temperature (ca. 20±1℃) under 120 μmol photons/(m2·s) (photosynthetically active radiation, PAR; 400–700 nm) illumination supplied by a bank of cool-white fluorescent tubes with a 12 h:12 h (light:darkness) photoperiod for two weeks before further experiments. For practical reasons, the 12 h light cycle was set from 6:00 to 18:00 (local time).
2.2 Temperature experimentsTo examine the effects of different temperatures on the growth and maximal quantum yield of photosystem Ⅱ (Fv/Fm) of the embryos, a temperature experiment was carried out. The embryos were cultured at three different temperatures: 15℃, 20℃, and 25℃. Five hundred Hizikia embryos were inoculated into each of nine culture dishes containing 4 L filtered seawater. The dishes were then placed into three illumination incubators (GXZ-300D, Jiangnan Instrument Factory, Ningbo, China). Each treatment included three replicates. The light conditions (light intensity and light period) were the same as described above for embryo maintenance in the laboratory. The water motion resulting from the gentle aeration by the filterpump with ambient atmospheric air did not allow the embryos to move, and half of the seawater medium was changed every day. The embryos were cultured under the different temperature regimes for 20 days, and then harvested to determine their growth and Fv/Fm values.
The lengths of the embryos were measured in each culture to estimate growth. The embryos were placed under a camera with an anatomical lens, and then pictures were taken at the same magnification for the camera eyepiece micrometer. Using Adobe Photoshop 7.0, 30 embryos were randomly selected within the horizon of each picture and their lengths were measured, not including the rhizoid lengths. According to length measurements and the specific ratio of the micrometer eyepiece, the actual lengths of the embryos were calculated.
The Fv/Fm of the embryos, a widely used indicator of the effect of stress on PSII, was determined with a Plant Efficiency Analyzer (PEA, MK2, Hansatech Instruments, Norfolk, UK). Fv/Fm was calculated using the formula: Fv/Fm=(Fm–Fo)/Fm, where Fm is the maximum fluorescence and Fo is the initial fluorescence value measured after 15 min darkadaptation (Kitajima and Butler, 1975; Hiriart-Baer et al., 2008; Beer et al., 2014).
2.3 Sunlight level experimentsThe embryos were cultured under outdoor sunlight conditions. The experiments were started by placing culture dishes containing embryos and 4 L filtered seawater under three levels of sunlight: 100%, 50%, and 25% of the solar light intensity. Each treatment included three replicates. The different sunlight levels were achieved by filtering the light with layers of fine iron wire mesh. The culture temperature was kept at 20±1℃ by putting the dishes into a water tank with circulating water to control the temperature. After 20 days of culture, the lengths, Fv/Fm values and survival ratios of the embryos were determined.
Solar radiation was continuously monitored using a Terrestrial Spectro-Radiometer (ELDONET, Frankfurt, Germany) during the culture period (Fig. 1). The average intensities of the three bands PAR (400– 700 nm), UV-A (315–400 nm) and UV-B (280– 315 nm) during the culture period were 468, 91 and 2.8 W/m2, respectively, which was equal to about 70% of the full sunlight intensity.

|
| Figure 1 Changes of the intensities of solar irradiance components (PAR, UV-A and UV-B) during the culture period in the sunlight level experiments |

|
| Figure 2 The different solar radiation levels that Hizikia fusiformis embryos were exposed to during different ultraviolet radiation treatment experiments |
About 200 embryos were randomly harvested and placed on glass slides. Then, pictures were taken under a dissecting microscope. The total number and the number of surviving embryos were counted. The survival percentage of the embryos was calculated using the formula: survival percentage=number of surviving embryos/total number of embryos×100%.
2.4 Ultraviolet radiation experimentsThe embryos were cultured under different UVR treatments. The embryos were placed into quartz tubes (diameter of 6 cm, length of 50 cm), which were completely transparent to UVR. The tubes were all exposed to solar irradiation (the average intensities of the three bands PAR, UV-A, and UV-B were about 327, 60, and 2.2 W/m2, respectively). Each treatment included three replicates. The outsides of the tubes were covered with different filters for the different radiation treatments (Fig. 1): (1) PAB, the outsides of the quartz tubes were wrapped with Ultraphan 295 film (Ultraphan, Digefra, Munich, Germany). The embryos received PAR+UV-A+UV-B (PAB 280– 700 nm) radiation; (2) PA, the outsides of the quartz tubes were wrapped with Folex 320 film (Montagefolie No. I OI 55099, Folex, Dreieich, Germany). The UV-B was filtered out, and the embryos received UVA+PAR (PA, 320–700 nm); and (3) P treatment, the outsides of the quartz tubes were wrapped with Ultraphan 395 film (UV Opak Digefra, Munich, Germany), which filtered out UV-A and UV-B. The embryos only received PAR (400–700 nm). All quartz tubes were placed into a water tank with circulating water to control the temperature.
The above treatments were applied for 4 h. After the treatment period, the same number of embryos was harvested from each treatment and the living cells were induced to generate blue crystals with the addition of methyl thiazolyl tetrazolium (MTT) (Bruggisser et al., 2002; James and Davey, 2007; Smith et al., 2009; Al-Talib, 2014). The amount of crystallization was positively proportional to the relative viability of the embryos. The generated crystals were dissolved with dimethyl sulfoxide (DMSO), and the optical density (OD) value at 490 nm was determined with a microplate reader. Therefore, the OD values were positively related to the viabilities of the embryos. The degree of injury was the reciprocal of the viability.
To examine the recovery capability of the embryos after UVR treatment, recovery experiments were carried out. The embryos were treated with three treatments, PAR, UV-A, and UV-B, as described above for 30 min. After the treatment, the embryos were moved indoors under weak light for recovery. The Fv/Fm values of the embryos were then determined at different times.
2.5 Statistical analysesThe data were expressed as means±standard deviation (SD, n≥3). The statistical significance of the data was tested with one-way analysis of variance (ANOVA) or t-tests using SPSS for Windows version 19.0. The significance level was set at P < 0.05.
3 RESULT 3.1 Effects of temperature on embryosThere lengths of the embryos increased during the temperature experiments. The average length of H. fusiformis embryos was greatest at 20℃ among the different temperature treatments (P < 0.05; Fig. 3). The Fv /Fm values (0.43±0.03) of the embryos were also highest at 20℃, followed by 15℃ with 0.37±0.02, and lowest at 25℃ with 0.29±0.02 (P < 0.05; Fig. 3).
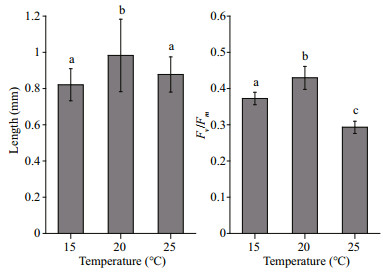
|
| Figure 3 The length and maximal quantum yield of photosystem Ⅱ (Fv/Fm) of Hizikia fusiformis embryos grown at different temperatures (15℃, 20℃, or 25℃) Different letters indicate significant difference (P < 0.05). |
The average length of H. fusiformis embryos was greater when grown under 25% solar radiation than 100% or 50% solar radiation. There were no significant differences (P > 0.05) in the lengths of embryos grown under 100% and 50% solar radiation. Both the Fv/Fm values and survival ratios of the embryos increased markedly (P < 0.05) with decreasing sunlight intensity in the culture (Fig. 4).
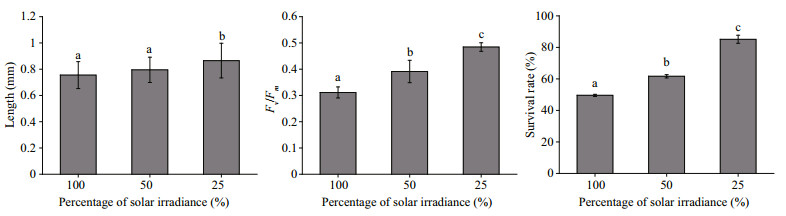
|
| Figure 4 The length, maximal quantum yield of photosystem Ⅱ (Fv/Fm) and survival rate of Hizikia fusiformis embryos grown under different solar irradiance levels (100%, 50%, or 25%) Different letters indicate significant difference (P < 0.05). |
During the UV radiation treatments involving exposure to solar radiation, the viabilities of the embryos decreased, as reflected by decreasing OD values (Fig. 5), in all three radiation treatments. This indicated that the damage to the H. fusiformis embryos increased gradually. With increasing UV radiation exposure duration up to 4 h, the relative degree of damage to the embryos in the PA and PAB treatments was markedly higher (P < 0.05) than that in the PAR treatment (Fig. 6). Moreover, there were no significant differences in the relative degree of damage under the PA and PAB treatments (P > 0.05).
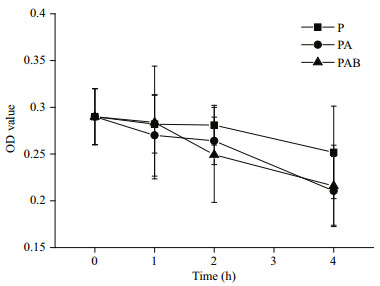
|
| Figure 5 OD values (related to the viability of the cells) during UVR treatments Hizikia fusiformis embryos were exposed to different irradiance treatments. P: PAR; PA: PAR+UV-A; PAB: PAR+UV-A+UV-B. |
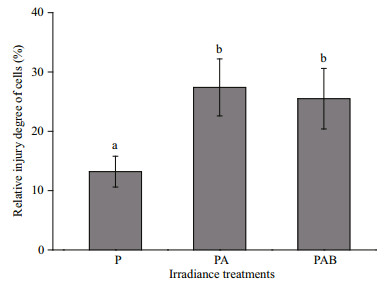
|
| Figure 6 The degree of cell injury of Hizikia fusiformis embryos after 4 h exposure to different irradiance treatments P: PAR; PA: PAR+UV-A; PAB: PAR+UV-A+UV-B. Different letters indicate significant difference (P < 0.05). |
After 30 min exposure to the UV radiation treatments, the Fv/Fm values of all embryos were significantly decreased (P < 0.05), with 28.3%, 32.8% and 37.3% declines compared with the initial levels for the P, PA, and PAB treatments, respectively (Fig. 7). In the recovery experiment, the Fv/Fm values of the embryos increased with time, reaching as high as 90% of the control levels after 2 h of recovery for all radiation treatments (Fig. 8). Thereafter, the recovery percentages leveled off.

|
| Figure 7 Percentage decline of Fv/Fm values of Hizikia fusiformis embryos after exposure to different irradiance treatments P: PAR; PA: PAR+UV-A; PAB: PAR+UV-A+UV-B for 30 min. Different letters indicate significant difference (P < 0.05). |

|
| Figure 8 Recovery of Fv/Fm of Hizikia fusiformis embryos indoors under weak light after 30 min exposure to different irradiance treatments P: PAR; PA: PAR+UV-A; PAB: PAR+UV-A+UV-B. |
In this study, the Hizikia fusiformis embryos cultivated under different temperature conditions all displayed relatively high Fv/Fm values, and almost no embryo death occurred. Nevertheless, the results showed that the lengths and Fv/Fm values of the embryos were highest under a culture temperature of 20℃, and decreased with increasing or decreasing temperature. This indicated that 20℃ was the optimum temperature for the development and physiology of Hizikia embryos, which is in agreement with earlier reports for thallus (Zhu and Chen, 1997; Liu et al., 2008; Zhao et al., 2015), germlings, and young sporophytes (Luo and Li, 2002) of H. fusiformis. According to these studies, the optimum temperature for the development and physiology of H. fusiformis is the same in all life cycle phases.
Embryos are capable of photosynthesis soon after the eggs are fertilized to provide energy for cell development (Zou et al., 2011). The present study showed that light intensity markedly affected the photosynthetic activity. The maximal quantum yield of PSII increased with decreasing sunlight intensity, exhibiting the highest values when the embryos were cultured under 25% sunlight intensity. Similarly, the survival percentage of the embryos was strongly affected by the sunlight intensity (PAR and UVR). Under 100% sunlight intensity, half of the embryos died, while the survival percentage was as high as 84% when the embryos were cultured at 25% sunlight intensity. This suggests that low light conditions are suitable for the growth and physiological activity of embryos, while excessive solar radiation causes damage to the photosynthetic apparatus and the loss of embryo vitality (Häder et al., 2007). Zhao et al. (2013) also found that cell division rates in the Sargassum cinereum embryos decreased with increasing light intensity. Note that Pang et al. (2006) did not observe photoinhibition in Hizikia embryos (24 h after fertilization) exposed to PAR of 540 μmol photons/(m2·s) (about 419–675 W/m2). This light level was close to the intensity of sunlight used in the present work, in which embryos showed a decrease in Fv /Fm (i.e. photoinhibition). The reason for the two contradictory results might be that sunlight contains not only PAR, but also UVR.
In this study, the viabilities of the embryos were markedly reduced with exposure to solar radiation for 4 h. Compared with PAR exposure, PA and PAB caused more damage to the embryos, with the degree of damage being similar between PA and PAB exposure. These results suggested that additional UV-B had no further effect on H. fusiformis embryo viability. Similar results were also reported by Van De Poll et al.(2001, 2002) in Coccotylus truncatus, Phycodrys rubens and Polyneura hilliae, in which the decrease of PSII activities was caused mainly by UV-A, not UV-B. Additionally, UV-A had a stronger effect on photosynthesis during the bloom period than UV-B in Patagonian oceanic plankton assemblages and on hardbottom shallow marine communities (Häder et al., 2007). However, Altamirano et al. (2003a) showed that UV-B, rather than UV-A, mainly inhibited seedling growth in three brown macroalgal species, Fucus spiralis, F. vesiculosus and F. serratus. Thus, the relative sensitivity of macroalgae to UV-A and UV-B may depend on the species and environmental factors.
The Fv/Fm values of H. fusiformis embryos all decreased with exposure to different radiation treatments for 30 min (PAR, PA and PAB), with the degree of Fv/Fm decline being highest with PAB exposure, followed by PA exposure. This indicated that a high intensity of PAR and UVR could inhibit PSII activity and photochemical efficiency, and that UVR exacerbated the damage to PSII. Similar results have been reported in Lessonia nigrescens and Durvillaea antarctica by Cruces et al. (2013), in which Fv/Fm values were seriously inhibited under full solar radiation (PAR+UVR) at noon. It has been shown that high irradiance and UVR can cause damage to the PSII D1 protein, as the high energy quanta of the UVR band affect aromatic residues and protein bonds, and thereby negatively affect the electron transfer rate and lead to photoinhibition (Pattison et al., 2012; Raven and Hurd, 2012; Cruces et al., 2013).
The subsequent recovery experiments, which were done after 30 min radiation exposure, showed that Hizikia embryos had considerable ability to repair photochemical damage resulting from not only high PAR, but also from UVR or UV-A radiation, in that the Fv/Fm values reached 90% of the level of the control with 2 h of recovery. Moreover, the results showed that the embryos exposed to UVR had similar recovery capacity to those exposed to high PAR. Our results suggest that the photosystems of Hizikia embryos are capable of resisting short-term exposure to UVR and high PAR. The physiological mechanisms might include an efficient photorepair system induced by UVR or high PAR. The xanthophylls cycle, which relies on the thermal dissipation of excess excitation energy to reduce the formation of singlet oxygen in the chloroplasts, is one mechanism of photoprotection against high solar radiation in brown algae (Häder et al., 2007). UVR exposure decreases Fv/Fm values, with increased non-photochemical quenching based on the migration of electronic excitation energy from the PSII chlorophyll to nearby carotenoids. According to Liu et al. (2010), H. fusiformis embryos exhibited a higher concentration of carotenoids under UVR treatments (Liu et al., 2010). Subsequently, the removal of UV-A or total UVR improved the photosynthetic activity and the Fv/Fm values increased rapidly. Similar results have been reported in Posidonia oceanica from southern Spain (Figueroa et al., 2002). Another effective UV screen is UVR-absorbing compounds (UVAC) (Liu et al., 2010), such as phlorotannins (1, 3, 5-trihydroxybenzen; Ragan and Glombitza, 1986) and mycosporine-like amino acids (MAAs; Pavia and Brock, 2000), which can effectively protect algae against UVR exposure, as described in many macroalgae inhabiting tidal zones (Peinado et al., 2004; Han and Han, 2005; Häder et al., 2007). Phlorotannins are phenolic compounds found exclusively in brown algae and are related with increased tolerance to UV radiation, as demonstrated in young and adult sporophytes of Fucus gardneri (Henry and Van Alstyne, 2004), thallus of Saccharina latissima and Nereocystis luetkeana (Swanson and Fox, 2007). Liu et al.(2008, 2010) previously reported that both young and adult sporophytes of H. fusiformis showed the capacity to resist UVR, and suggested that MAAs were the fundamental materials for UVR resistance. Here, Hizikia embryos were capable of rapid recovery from the damage resulting from short-term exposure to excessive solar radiation (PAR, PA and PAB).
5 CONCLUSIONIn conclusion, our results showed that the growth and photochemical activity of Hizikia embryos was promoted by culture under 20℃ and low-sunlight conditions. Full solar radiation severely reduced the growth and survival ratios of the embryos, which develop on the ground with much lower irradiance in nature. Hizikia embryos were capable of tolerating short-term (30 min) exposure to UVR; however, the viabilities of the embryos were significantly decreased with long-term (4 h) exposure to UVR. We propose that culture conditions including indoor natural light and room temperature would favor the growth, development and physiology of Hizikia embryos.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Al-Talib S M, Al-Ghabsha T S, Al-Sabha T N. 2014. Spectrophotometric determination of adrenaline using methyl thiazolyl diphenyl-tetrazolium-bromide (MTT). AL-Taqani, 27(2): 46-53.
|
Altamirano M, Flores-Moya A, Figueroa F L. 2003a. Effects of UV radiation and temperature on growth of germlings of three species of Fucus (Phaeophyceae). Aquatic Botany, 75(1): 9-20.
DOI:10.1016/S0304-3770(02)00149-3 |
Altamirano M, Flores-Moya A, Kuhlenkamp R, Figueroa F L. 2003b. Stage-dependent sensitivity to ultraviolet radiation in zygotes of the brown alga Fucus serratus. Zygote, 11(2): 101-106.
DOI:10.1017/S0967199403002132 |
Beer S, Björk M, Beardall J. 2014. Photosynthesis in the Marine Environment. Wiley Blackwell, Oxford.
|
Bruggisser R, Von Daeniken K, Jundt G, Schaffner W, Tullberg-Reinert H. 2002. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Medica, 68(5): 445-448.
DOI:10.1055/s-2002-32073 |
Cruces E, Huovinen P, Gómez I. 2013. Interactive effects of UV radiation and enhanced temperature on photosynthesis, phlorotannin induction and antioxidant activities of two Sub-Antarctic brown algae. Marine Biology, 160(1): 1-13.
DOI:10.1007/s00227-012-2049-8 |
Dring M J, Makarov V, Schoschina E, Lorenz M, Lüning K. 1996. Influence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta). Marine Biology, 126(2): 183-191.
DOI:10.1007/BF00347443 |
Figueroa F L, Jiménez C, Viéegla B, Péter-Rodígueze E, Aguilera J, Flores-Moya A, Altamirano M, Lebert M, Häder D P. 2002. Effects of solar UV radiation on photosynthesis of the marine angiosperm Posidonia oceanica from southern Spain. Marine Ecology Progress Series, 230: 59-70.
DOI:10.3354/meps230059 |
Gao G, Wu H Y, Gao K S. 2009. Effects of solar ultraviolet radiation on growth and photosynthesis of Hizikia fusiformis. Acta Hydrobiologica Sinica, 33(2): 284-288.
(in Chinese with English abstract) DOI:10.3724/SP.J.1035.2009.00284 |
Gao K S. 2014. Algal Carbon Fixation. Science Press, Beijing. 405p.
(in Chinese)
|
Häder D P, Kumar H D, Smith R C, Worrest R C. 2007. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochemical & Photobiological Sciences: Official Journal of the European Photochemistry Association and the European Society for Photobiology, 6(3): 267-285.
|
Han Y S, Han T. 2005. UV-B induction of UV-B protection in Ulva pertusa (Chlorophyta). Journal of Phycology, 41(3): 523-530.
DOI:10.1111/(ISSN)1529-8817 |
Henry B E, Van Alstyne K L. 2004. Effects of UV radiation on growth and phlorotannins in Fucus gardneri (Phaeophyceae) juveniles and embryos. Journal of Phycology, 40(3): 527-533.
DOI:10.1111/jpy.2004.40.issue-3 |
Hiriart-Baer V P, Arciszewski T J, Malkin S Y, Guildford S J, Hecky R E. 2008. Use of pulse-amplitude-modulated fluorescence to assess the physiological status of Cladophora sp. along a water quality gradient. Journal of Phycology, 44(6): 1 604-1 613.
|
James C E, Davey M W. 2007. A rapid colorimetric assay for the quantitation of the viability of free-living larvae of nematodes in vitro. Parasitology Research, 101(4): 975-980.
DOI:10.1007/s00436-007-0572-1 |
Kitajima M, Butler W L. 1975. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 376(1): 105-115.
DOI:10.1016/0005-2728(75)90209-1 |
Li S Y. 2001. Studies on techneque of industrial raising seedlings of Sargasstem fusiforme (Harvey) setch.. Journal of Zhejiang Ocean University (Natural Science), 20(3): 521-255, 265.
(in Chinese with English abstract) |
Liu S X, Zou D H, Xu J T, Gao K S. 2008. Responses of the brown seaweed Hizikia fusiformis cultured at different N levels to the solar radiation. Marine Science Bulletin, 27(6): 44-51.
(in Chinese with English abstract) |
Liu S X, Zou D H, Xu J T. 2010. Response of the young sporophytes of Hizikia fusiformis to different N growth conditions and the solar radiation. Acta Ecologica Sinica, 30(20): 5 562-5 568.
(in Chinese with English abstract) |
Luo Q J, Li W B. 2002. Breed seedling in door of Hizikia fusiforme from cultivation. Journal of Ningbo University (NSEE), 15(4): 34-36.
(in Chinese with English abstract) |
Luo Q J. 2001. An experiment on the culturing seedling at sea of Sargassum fusiforme. setch. Transactions of Oceanology and Limnology, (3): 17-21.
(in Chinese with English abstract) |
Pang S J, Chen L T, Zhuang D G, Fei X G, Sun J Z. 2005. Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: enhanced seedling production in tumbled culture. Aquaculture, 245(1-4): 321-329.
DOI:10.1016/j.aquaculture.2004.12.011 |
Pang S J, Fei X G, Xiao T, Wang J C. 2000. Suspended culture of the receptacles of Hizikia fusiformis. Marine Sciences, 24(3): 1-3.
(in Chinese with English abstract) |
Pang S J, Fei X G, Xiao T, Wang J G. 2001. Mass production of the artificial seeds of Hizikia fusiformis by controling the laying out of the ovules and semens. Marine Sciences, 25(4): 53-54.
(in Chinese with English abstract) |
Pang S J, Gao S Q, Sun J Z. 2006. Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: controlled fertilization and early development of seedlings in raceway tanks in ambient light and temperature. Journal of Applied Phycology, 18(6): 723-731.
DOI:10.1007/s10811-006-9078-y |
Pang S J, Zhang Z H, Zhao H J, Sun J Z. 2007. Cultivation of the brown alga Hizikia fusiformis (Harvey) Okamura: stress resistance of artificially raised young seedlings revealed by chlorophyll fluorescence measurement. Journal of Applied Phycology, 19(5): 557-565.
DOI:10.1007/s10811-007-9170-y |
Pattison D I, Rahmanto A S, Davies M J. 2012. Photo-oxidation of proteins. Photochemical & Photobiological Sciences: Official Journal of the European Photochemistry Association and the European Society for Photobiology, 11(1): 38-53.
|
Pavia H, Brock E. 2000. Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Marine Ecology Progress Series, 193: 285-294.
DOI:10.3354/meps193285 |
Peinado N K, Díaz R T A, Figueroa F L, Helbling E W. 2004. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina1. Journal of Phycology, 40(2): 248-259.
DOI:10.1046/j.1529-8817.2004.03013.x |
Ragen M A, Glombitza K W. 1986. Phlorotannins, brown algal polyphenols. Progress in Phycological Research, 4: 129-241.
|
Raven J A, Hurd C L. 2012. Ecophysiology of photosynthesis in macroalgae. Photosynthesis Research, 113(1-3): 105-125.
DOI:10.1007/s11120-012-9768-z |
Roleda M Y, Hanelt D, Kräbs G, Wiencke C. 2004. Morphology, growth, photosynthesis and pigments in Laminaria ochroleuca (Laminariales, Phaeophyta) under ultraviolet radiation. Phycologia, 43(5): 603-613.
DOI:10.2216/i0031-8884-43-5-603.1 |
Smith R A, Pontiggia L, Waterman C, Lichtenwalner M, Wasserman J. 2009. Comparison of motility, recovery, and methyl-thiazolyl-tetrazolium reduction assays for use in screening plant products for anthelmintic activity. Parasitology Research, 105(5): 1 339-1 343.
DOI:10.1007/s00436-009-1560-4 |
Swanson A K, Fox C H. 2007. Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs. Global Change Biology, 13(8): 1 696-1 709.
DOI:10.1111/gcb.2007.13.issue-8 |
Van De Poll W H, Eggert A, Buma A G J, Breeman A M. 2001. Effects of UV-B-induced DNA damage and photoinhibition on growth of temperate marine red macrophytes: habitatrelated differences in UV-B tolerance. Journal of Phycology, 37(1): 30-38.
DOI:10.1046/j.1529-8817.2001.037001030.x |
Van De Poll W H, Eggert A, Buma A G J, Breeman A M. 2002. Temperature dependence of UV radiation effects in arctic and temperate isolates of three red macrophytes. European Journal of Phycology, 37(1): 59-68.
DOI:10.1017/S0967026201003407 |
Véliz K, Edding M, Tala F, Gómez I. 2006. Effects of ultraviolet radiation on different life cycle stages of the South Pacific kelps, Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae). Marine Biology, 149(5): 1 015-1 024.
DOI:10.1007/s00227-006-0301-9 |
Yan L W, Huang H J, Chen J T, Yang X G. 2011. Estimation of carbon sink capacity of algal mariculture in the coastal areas of China. Advances in Marine Science, 29(4): 537-545.
(in Chinese with English abstract) |
Zeng C K. 2000. Flora Algarum Marinarum Sinicarum. Science Press, Beijing. 238p.
(in Chinese)
|
Zhang L N, Luo Q J, Lin S Z, Yan X J. 2012. Seedling culturing of Sargassum fusiforme setch in summer in North Sea. Journal of Ningbo University (NSEE), 25(4): 6-9.
(in Chinese with English abstract) |
Zhang Z, Liu J G, Liu J D. 2002. Study review of Hizikia fusiformis. Marine Fisheries Research, 23(3): 67-74.
(in Chinese with English abstract) |
Zhao S F, Li H J, Sun H Q, Li J P, Li G R. 2013. Effects of light intensity on sexual reproduction and early development of Sargassum cinereum (Fucales, Phaeophyta) germlings. Journal of Shanghai Ocean University, 22(4): 563-570.
(in Chinese with English abstract) |
Zhao S F, Yao W L, Guo X Z, He T T, Sun H Q, Guo S D, Huang G H. 2015. Combined effects of temperature and light on the growth rate of Hizikia fusiformis young sporophyte. Journal of Aquaculture, 36(10): 42-47.
(in Chinese with English abstract) |
Zhu Z J, Chen P M. 1997. The relationship between water temperature, light intensity and the photosynthetic rates of Sargassum fusiforme. Journal of Fisheries of China, 21(2): 165-170.
(in Chinese with English abstract) |
Zou D H, Gao K S, Chen W Z. 2011. Photosynthetic carbon acquisition in Sargassum henslowianum (Fucales, Phaeophyta), with special reference to the comparison between the vegetative and reproductive tissues. Photosynthesis Research, 107(2): 159-168.
DOI:10.1007/s11120-010-9612-2 |
Zou D H, Gao K S. 2004. Comparative mechanisms of photosynthetic carbon acquisition in Hizikia fusiforme under submersed and emersed conditions. Acta Botanica Sinica, 46(10): 1 178-1 185.
|
Zou D H, Liu S X, Du H, Xu J T. 2012. Growth and photosynthesis in seedlings of Hizikia fusiformis (Harvey) Okamura (Sargassaceae, Phaeophyta) cultured at two different temperatures. Journal of Applied Phycology, 24(5): 1 321-1 327.
DOI:10.1007/s10811-011-9783-z |
 2018, Vol. 36
2018, Vol. 36


