Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Chunhui(吴春辉), JIANG Peng(姜鹏), ZHAO Jin(赵瑾), FU Huihui(付慧慧)
- High efficiency of protoplast preparation for artificially cultured Ulva prolifera (Ulvophyceae, Chlorophyta)
- Chinese Journal of Oceanology and Limnology, 36(5): 1806-1811
- http://dx.doi.org/10.1007/s00343-018-7058-0
Article History
- Received Mar. 1, 2017
- accepted in principle May. 16, 2017
- accepted for publication Sep. 13, 2017
2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China
Protoplast is live plant cell devoid of cell wall. Artificial removal of cell wall would lead to cell dedifferentiation and further development into a new individual. Due to the cell totipotency of protoplast, there has been continued interest in exploiting protoplast for breeding, somatic hybridization and genetic transformation in higher plant (Davey et al., 2005), as well as in algae especially for red seaweed Pyropia spp. and green seaweed Ulva spp. (Reddy et al., 2008).
At present, enzymatic digestion is the most effective method for producing a large number of viable protoplasts. For Ulva, which were in forms of either distromatic blade or monostromatic tubular thalli, the attempt with U. intestinalis gained the first report of enzymatic protoplast preparation in seaweed (Millner et al., 1979), followed by extensive optimization on a series of enzymatic hydrolysis formulas among various Ulva species (Saga, 1984; Reddy and Fujita, 1991; Huang et al., 1996; Chen and Shih, 2000; Uppalapati and Fujita, 2002). Although complicated composition including varieties of polysaccharides were involved in Ulva cell wall (Lahaye and Robic, 2007), a universal protocol using single cellulase with low osmotic strength was developed and proved effective to a wide range of Ulva species (Reddy et al., 2006).
By far, almost all Ulva thalli used for protoplast isolation were wildly collected. However, only artificially cultured thalli with clonal genetic background could meet the need for genetic transformation. For this reason, reproducibility of protoplast production with high efficiency from artificially cultured thalli was top-ranked in the case of protoplast-mediated genetic manipulation. Compared with seaweeds lived in natural habitat, unialgal cultures in lab were at diversified physiological states and natural or artificial developmental stages according to culture conditions (Chen and Shih, 2000; Wang et al., 2011). Due to the fact that the polysaccharides composition in Ulva cell wall varied with environmental factors (Lahaye and Robic, 2007), it could be suspected that the protoplast yield may be dependent on the characteristics of artificially cultured seaweed, which might challenge the reliability of the subsequent genetic transformation.
Tank-based cultivation model has been established with varieties of Ulva species including U. prolifera (Hiraoka and Oka, 2008; Carl et al., 2014; Kerrison et al., 2016) and artificially cultured Ulva had potential for producing high value-added products (Jiang et al., 2016). In this study, the effects of physiological states and developmental stages on protoplast yield from artificially cultured U. prolifera were investigated, with a purpose to set up a reliable protocol to meet the need for protoplast-mediated transformation with clonal cultures.
2 MATERIAL AND METHOD 2.1 Seaweed and culture conditionsIn July 2007, U. prolifera (Müller) J. Agardh was collected from Qingdao coast, China (35°54′N, 120°11′E). Thalli were cleaned with a brush to remove any epiphytes using autoclaved seawater, then healthy individuals were selected for unialgal culture in Von Stosch's Enriched (VSE) medium (Von Stosch, 1964), and proliferated by continuous vegetative growth for several years. Just before protoplast isolation, wild U. pertusa and U. linza were collected from Huiquan Bay (36°3′N, 120°20′E), Qingdao, as the positive controls to test effects of ready enzyme formulas. Species identification for Ulva spp. was performed using ribosomal DNA internal transcribed spacer (ITS) as a molecular marker (Leskinen and Pamilo, 1997).
Ulva prolifera was maintained with vegetative growth at 20℃ with a 12 h:12 h L:D light cycle under 100 μmol photons/(m2·s) irradiance in a 15-L tank. Ambient air provided by a pump was sterilized through air filter membrane with 0.22 μm pore size, and the medium was renewed once a week.
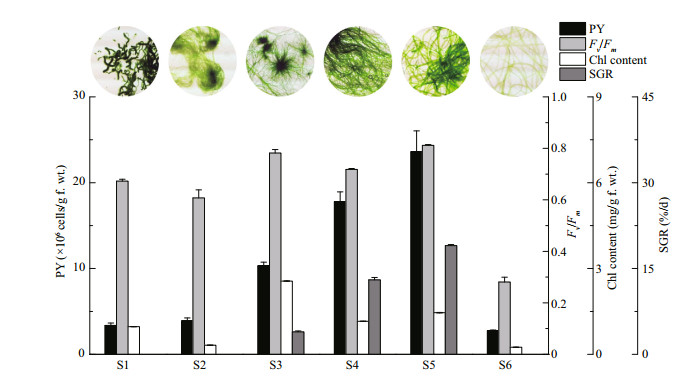
To investigate the effects of different physiological states and developmental stages on protoplast yield for U. prolifera, six typical samples cultivated in laboratory were chosen. Sample 1 (S1) was uniseriate germlings developed from vegetative cells by in situ germination which was used for seed conservation. S2 was non-tubular and multiseriate germlings under nutrition stress (no nutrients were added during cultivation). S3 was filamentous thalli cultured in petri dish with adequate nutrition (the VSE medium was replaced weekly). S4 was filamentous thalli cultured in tank supplied with adequate nutrition and filtered air. S5 was tubular thalli cultured in tank supplied with adequate nutrition and filtered air. S6 was tubular thalli under nutrition stress.
2.2 Measurement of physical and biochemical indexesTo characterize different physiology states of U. prolifera thalli, three indexes were chosen. The maximal PSII quantum yield Fv/Fm was measured using Multi-Function Plant Efficiency Analyser (M-PEA-2, Hansatech, UK). The measurement of chlorophyll content was carried out following the method described by Lin et al. (2009). Specific growth rate (SGR) was calculated using the equation below:
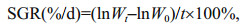
where Wt and W0 represent the ultimate or initial fresh weight (g) respectively, and t represents the growth period (d).
For each treatment, three independent experiments (biological replicates) were carried out. Each sample was detected in triplicate and then the data was analyzed by t-test and one-way ANOVA. Differences were considered statistically significant at P < 0.05. Statistical analysis was performed using the statistical software SPSS 18.0.
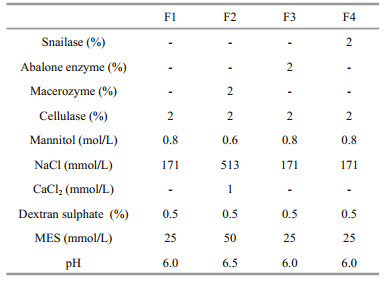
2.3 Protoplast preparation and regenerationFour enzyme formulas were tested to isolate protoplasts from Ulva spp. (Table 1). Formula 1 (F1) containing 2% Cellulase Onozuka R-10 (Yakult, Tokyo, Japan) were in accordance with that described by Reddy et al. (2006). Formula 2 (F2) containing 2% Cellulase Onozuka R-10 and 2% Macerozyme R-10 (Yakult, Tokyo, Japan) followed that described by Uppalapati and Fujita (2002). Formula 3 (F3) and 4 (F4) were prepared by adding 2% abalone acetone powder (Sigma, St. Louis, MO, USA) and 2% snailase (Solarbio, Beijing, China) into F1 respectively.
Protoplasts isolation from three Ulva species were following the previously described methods with necessary improvements (Reddy and Fujita, 1991). Cleaned thalli were cut into small pieces (< 1 mm2) and washed three times with VSE medium, then incubated in 6 mL of filtered sterilized enzyme solution at 20℃ in the dark on a rotary shaker (30 r/min) for about 2 h. Following digestion, enzyme solution was gently sieved through a nylon mesh (50 μm pore diameter) to remove any debris. The suspension was centrifuged at 1 500 r/min for 5 min and half of the supernatant was replaced with the same volume of VSE medium. This procedure was repeated three times within 30 min to remove most enzyme solution. The residues of cell wall were visualized by staining with 0.01% fluorescent brightener 28 (FB28) (Sigma, St. Louis, MO, USA) (Cui et al., 2010). The number of protoplasts were counted with a hemocytometer. For protoplast regeneration, 1.0×105 cells was dispensed in 30 mL sterilized VSE medium and maintained at 20±1℃ under white fluorescent lamps at 30 μmol photons/(m2·s) irradiance with a 12 h:12 h L:D photoperiod. The culture medium was refreshed weekly.
To improve protoplast yields, further optimizations on enzyme concentration (2% and 5%), incubation period (1, 2, 3, and 4 h), starting biomass (50, 100, and 200 mg) and pre-treatment with KI (0.7% KI for 5 min) were performed on the basis of F1 using the S5 type of samples.
3 RESULT AND DISCUSSIONThe prepared protoplasts of U. prolifera were green, spherical, and 15–20 μm in diameter (Fig. 1a). Under excitation by blue light (450–520 nm), protoplasts stained with FB28 only showed red auto fluorescence without bright white fluorescence, indicating the complete elimination of cell wall (Fig. 1b, c). After enzyme digestion and protoplast isolation, most thalli residues were found have sheathlike tubes at ends, implying the protoplasts were released in order from the incision (Fig. 1d). The overall regeneration rate of protoplasts isolated from all Ulva species using the F1 ranged from 85% to 90%. The developmental pattern of U. prolifera protoplasts including cell division order, and occasional abnormal morphology were similar to those reported for other Ulva species (Fig. 1e, f, g) (Reddy and Fujita, 1991; Chen and Shih, 2000).

|
| Figure 1 Isolation and regeneration of U. prolifera protoplasts a. protoplasts; b. FB28 stained protoplast in visible light field; c. FB28 stained protoplast in fluorescent light field; d. protoplast release from incision; e. protoplast regeneration in two-cell stage; f. protoplast regeneration in multicellular stage; g. regenerated germling in abnormal shape. Scale bar=10 μm. |
As a universal enzyme formula, F1 was first used to isolate protoplasts from wild U. pertusa and U. linza. The results showed that the protoplast yield in U. pertusa ((85.5±1.6)×106 cells/g f. wt.) was slightly higher than that reported by Reddy et al. (2006), and the yield in U. linza ((11.2±1.1)×106 cells/g f. wt.) was almost three times higher than ever (Uppalapati and Fujita, 2002). It was confirmed that F1 was applicable to wild Ulva samples quite well.
To investigate the effects of physiological state and developmental stage on protoplast yield for artificially cultured Ulva, six samples of U. prolifera (S1–S6) in different characteristics were compared using F1 (Fig. 2). It was showed that the protoplast yields varied significantly, and the highest value (S5, (23.6±2.44)×106 cells/g f. wt.) was about 8.6-fold higher than the lowest one (S6, (2.75±0.08)×106 cells/g f. wt.), proving that they were strongly dependent on the characteristics of samples. Based on the calculated Pearson correlation coefficiency and P value between the protoplast yield and each tested index, it was concluded that neither Fv/Fm (0.661, P > 0.05) nor chlorophyll content (0.473, P > 0.05) exhibited an ideal correlation with the protoplast yield. Alternatively, SGR (0.991, P < 0.01), coupled with developmental stage, could roughly serve as an effective combined index to determine the right time for protoplast isolation.
|
| Figure 2 Effects of physiological state and developmental stage on protoplasts yield from U. prolifera PY: protoplast yield. |
Cell wall of Ulva species consisted of skeletal components and amorphous matrix (Reddy et al., 2008). The former were glucose-containing polysaccharides mainly as cellulose which made up packed inner layers surrounding the cells, and the latter including ulvan and glucuronan formed the outer layer of cell wall and faced the outside of thalli (Bobin-Dubigeon et al., 1997; Lahaye and Robic, 2007). We hypothesized that the synthesis of skeletal components was prior to amorphous matrix during cell division, thus when SRG was high (S3, S4, S5), fast cell division seemed to facilitate cellulase to get access to cellulose. Otherwise, the access would be hindered by fully filled amorphous matrix in stunted growth state (S1, S2 and S6). In brown seaweed, Fisher and Gibor (1987) also suggested that protoplasts were more readily released from meristematic areas in which the newly formed cell walls of fast-dividing cells were less developed than those of older cells. In addition, the existence of a layer of cuticle outside thalli may prevent cellulase attacking cellulose from surface (Bobin-Dubigeon et al., 1997). We found that enzyme degradation only happened at incision sites as described by Millner et al. (1979), and tubular thallus seemed to generate much more incisions than uniseriate or multiseriate germling. Therefore, we suggested that the thallus in tubular stage and exponential growth phase was optimum material to obtain high protoplast yield for artificially cultured U. prolifera.
The Fv/Fm and chlorophyll content were usually used to reflect the potential maximal photosynthetic rate in plant. For U. prolifera, the thalli with Fv/Fm value between 0.6 and 0.8 were fresh and in darkgreen, whereas those less than 0.6 were old and in yellow-green (Zhang et al., 2013). However, such photosynthetic physiological indexes were found very sensitive to indicate environmental changes on salinity, illumination or desiccation (Wang et al., 2012), or they might be almost stable under a constant culture condition in spite of developmental stages (Fig. 2). We thought that they had no correlation with variations on cell wall composition, thus could not be employed as indexes to direct protoplast preparation.
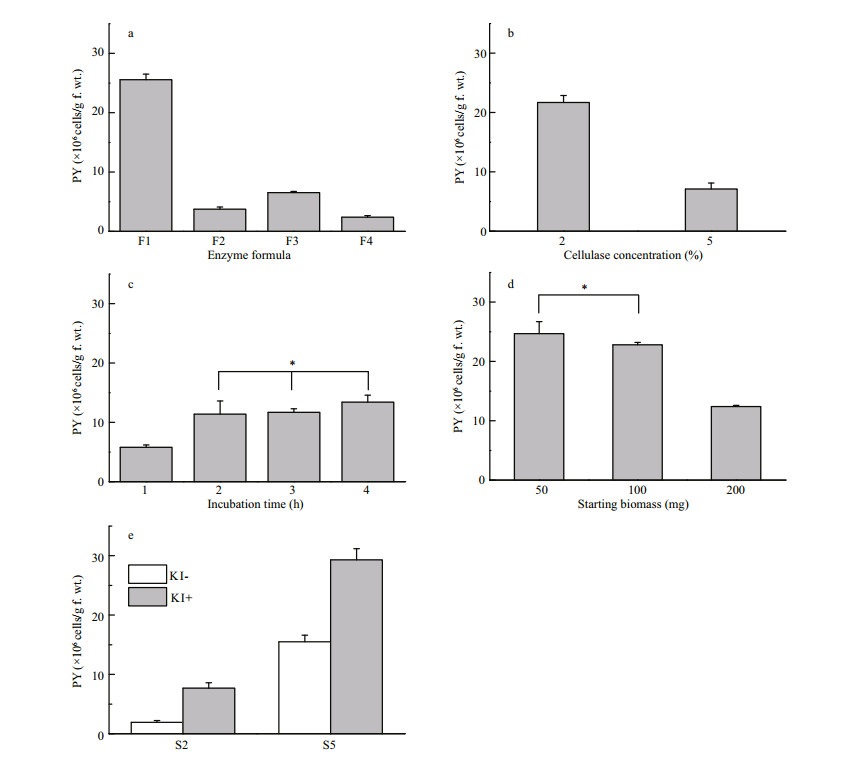
Considering that the significant difference of protoplast yields may be attributed to the amount of amorphous matrix, other three enzyme formulas (F2, F3 and F4) with enzyme combination were compared with F1 using S5 type of samples. However, all three formulas were found to result in significant decreases of protoplast yield (Fig. 3a). In particular, serious protoplast burst was detected in F3 and F4, in which crude enzymes extracted from animal digestive gland were supplied. The yield decrease must be mainly due to the presence of proteases in the crude enzyme extract degrading enzymes as cellulase.
|
| Figure 3 Optimization of protoplast yield in U. prolifera a. enzyme formula; b. cellulase concentration; c. incubation time; d. starting biomass; e. KI pre-treatment; PY: protoplast yield. Significant differences are indicated (* P > 0.05). |
To improve protoplast yields further, optimizations on enzyme concentration, incubation period, starting biomass and pre-treatment were performed on the basis of F1 using the S5 type of samples. The results demonstrated that 2% Cellulase Onozuka R-10, 2 h incubation time, 50 mg starting biomass and pretreatment with 0.7% KI for 5 min could generate the highest protoplast yield as (31.5±1.9)×106 cells/g f. wt. (Fig. 3b, c, d, e), which was about 1.5-fold higher than that reported for U. prolifera (Reddy and Fujita, 1991). It should be noted that the sterilization process with KI could double the yield for S5, and even increase by three times for S2 which was in stunted growth due to nutrition stress (Fig. 3e). However, the mechanism of why KI pre-treatment could improve the yield was still unknown and it deserved to be further clarified.
4 CONCLUSIONOur results proved that for artificially cultured U. prolifera, the protoplast yields were strongly dependent on algal physiological states and developmental stages. We found SGR coupled with developmental stage could serve as an index to determine the right time for protoplast isolation. A defined protocol was proposed to produce the highest protoplast yields which could meet the need for protoplast-mediated genetic transformation in U. prolifera.
5 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author on reasonable request.
Bobin-Dubigeon C, Lahaye M, Guillon F, Barry J L, Gallant D J. 1997. Factors limiting the biodegradation of Ulva sp cell-wall polysaccharides. Journal of the Science of Food and Agriculture, 75(3): 341-351.
DOI:10.1002/(ISSN)1097-0010 |
Carl C, de Nys R, Paul N A. 2014. The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS One, 9(6): e98700.
DOI:10.1371/journal.pone.0098700 |
Chen Y C, Shih H C. 2000. Development of protoplasts of Ulva fasciata (Chlorophyta) for algal seed stock. Journal of Phycology, 36(3): 608-615.
DOI:10.1046/j.1529-8817.2000.99128.x |
Cui Y L, Wang J F, Jiang P, Bian S G, Qin S. 2010. Transformation of Platymonas (Tetraselmis) subcordiformis (Prasinophyceae, Chlorophyta) by agitation with glass beads. World Journal of Microbiology & Biotechnology, 26(9): 1 653-1 657.
|
Davey M R, Anthony P, Power J B, Lowe K C. 2005. Plant protoplasts: status and biotechnological perspectives. Biotechnology Advances, 23(2): 131-171.
DOI:10.1016/j.biotechadv.2004.09.008 |
Fisher D D, Gibor A. 1987. Production of protoplasts from the brown alga, Sargassum muticum (Yendo) Fensholt (Phaeophyta). Phycologia, 26(4): 488-495.
DOI:10.2216/i0031-8884-26-4-488.1 |
Hiraoka M, Oka N. 2008. Tank cultivation of Ulva prolifera in deep seawater using a new "germling cluster" method. Journal of Applied Phycology, 20(1): 97-102.
DOI:10.1007/s10811-007-9186-3 |
Huang X, Weber J C, Hinson T K, Mathieson A C, Minocha S C. 1996. Transient expression of the GUS reporter gene in the protoplasts and partially digested cells of Ulva lactuca L. (Chlorophyta). Botanica Marina, 39(1-6): 467-474.
|
Jiang R, Ingle K N, Golberg A. 2016. Macroalgae (seaweed) for liquid transportation biofuel production: what is next?. Algal Research, 14: 48-57.
DOI:10.1016/j.algal.2016.01.001 |
Kerrison P D, Le H N, Twigg G C, Smallman D R, MacPhee R, Houston F A B, Hughes A D. 2016. Decontamination treatments to eliminate problem biota from macroalgal tank cultures of Osmundea pinnatifida, Palmaria palmata and Ulva lactuca. Journal of Applied Phycology, 28(6): 3 423-3 434.
DOI:10.1007/s10811-016-0873-9 |
Lahaye M, Robic A. 2007. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules, 8(6): 1 765-1 774.
DOI:10.1021/bm061185q |
Leskinen E, Pamilo P. 1997. Evolution of the ITS sequences of ribosomal DNA in Enteromorpha (Chlorophyceae). Hereditas, 126(1): 17-23.
|
Lin A P, Wang C, Qiao H J, Pan G H, Wang G C, Song L Y, Wang Z Y, Sun S, Zhou B C. 2009. Study on the photosynthetic performances of Enteromorpha prolifera collected from the surface and bottom of the sea of Qingdao sea area. Chinese Science Bulletin, 54(3): 399-404.
|
Millner P A, Callow M E, Evans L V. 1979. Preparation of protoplasts from the green alga Enteromorpha intestinalis (L.) Link. Planta, 147(2): 174-177.
DOI:10.1007/BF00389521 |
Reddy C R K, Dipakkore S, Kumar G R, Jha B, Cheney D P, Fujita Y. 2006. An improved enzyme preparation for rapid mass production of protoplasts as seed stock for aquaculture of macrophytic marine green algae. Aquaculture, 260(1-4): 290-297.
DOI:10.1016/j.aquaculture.2006.06.034 |
Reddy C R K, Fujita Y. 1991. Regeneration of plantlets from Enteromorpha (Ulvales, Chlorophyta) protoplasts in axenic culture. Journal of Applied Phycology, 3(3): 265-275.
DOI:10.1007/BF00003585 |
Reddy C R K, Gupta M K, Mantri V A, Jha B. 2008. Seaweed protoplasts: status, biotechnological perspectives and needs. Journal of Applied Phycology, 20(5): 619-632.
DOI:10.1007/s10811-007-9237-9 |
Saga N. 1984. Isolation of protoplasts from edible seaweeds. The Botanical Magazine, Tokyo, 97(3): 423-427.
DOI:10.1007/BF02488673 |
Uppalapati S R, Fujita Y. 2002. A simple method for mass isolation of protoplasts from species of Monostroma, Enteromorpha and Ulva (Chlorophyta, Ulvales). Journal of Applied Phycology, 14(3): 165-168.
DOI:10.1023/A:1019960125548 |
Von Stosch H A. 1964. Wirkungen von Jod und Arsenit auf Meersalgen in Kurtur. Proceedings of the 4th International Seaweed Symposium, 4: 142-150.
|
Wang J X, Li A F, Zhou B C. 2011. Studies on developments of microspheres from macroalgal clones and cultivations in airlift photobioreactor. Marine Science, 35(2): 17-21.
(in Chinese with English abstract) |
Wang Y, Wang Y, Zhu L, Zhou B, Tang X X. 2012. Comparative studies on the ecophysiological differences of two green tide macroalgae under controlled laboratory conditions. PLoS One, 7(8): e38245.
DOI:10.1371/journal.pone.0038245 |
Zhang J H, Huo Y Z, Zhang Z L, Yu K F, He Q, Zhang L H, Yang L L, Xu R, He P M. 2013. Variations of morphology and photosynthetic performances of Ulva prolifera during the whole green tide blooming process in the Yellow Sea. Marine Environmental Research, 92: 35-42.
DOI:10.1016/j.marenvres.2013.08.009 |
 2018, Vol. 36
2018, Vol. 36