Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YU Xiaoping(余晓平), GUO Yafei(郭亚飞), DENG Tianlong(邓天龙)
- Antimony speciation at the sediment-water interface of the Poyang Lake: response to seasonal variation
- Chinese Journal of Oceanology and Limnology, 36(6): 1941-1949
- http://dx.doi.org/10.1007/s00343-018-7314-3
Article History
- Received Nov. 9, 2017
- accepted in principle Jan. 15, 2018
- accepted for publication Feb. 22, 2018
2 College of Chemistry and Materials Science, Northwest University, Xi'an 710127, China
Antimony (Sb) is ubiquitous throughout the environment as a result of natural processes and human activities. Large quantities of antimony released into the environment through mining and smelting processes result in serious antimony contamination. Antimony can exist in many oxidation states (-Ⅲ, 0, Ⅲ, Ⅴ) in the environment, but it is mainly found in two oxidation states as antimonite [Sb(Ⅲ)] and antimonate [Sb(Ⅴ)] in environmental, biological and geochemical samples (Filella et al., 2002a). However, it can be converted into organic species such as dimethylantimony (DMSb) and trimethylantimony (TMSb) as a result of microbial activity (Andrewes et al., 2000; Bentley and Chasteen, 2002). Antimony and its compounds are considered to be hazardous to human health or even carcinogenic (Gebel et al., 1997; Hammel et al., 2000). Sb(Ⅲ) is more toxic than the pentavalent form (He and Wan, 2004).
Aquatic environments are the ultimate sink of the emitted antimony. However, the biogeochemical behavior of Sb in aquatic environments is not well understood due to its many existing forms and low content (Filella et al., 2002a, b ). At present, studies about the migration and transformation of antimony at the sediment-water interface (SWI) of aquatic environments, especially in lakes, are scarce. Usually, the sequential extraction procedures proposed by Tessier et al. (1979) or the European Community Bureau of Reference (BCR) (Cappuyns et al., 2007) are adopted to investigate the geochemical behavior of trace elements in soil or sediment. However, in order to facilitate the comparison of trace elements such as antimony, arsenic and mercury in lake sediments, a modified version of the Tessier method (Chen et al., 2003; Belzile et al., 2000) was also used to determine the proportion of total antimony associated to oxidized [mainly bound to iron and manganese oxyhydroxides, identified as Sb(oxal)] and organic fractions [bound to organic matters and amorphous sulfides, identified as Sb(H2O2)] in sediment.
Poyang Lake is the largest freshwater lake in China, and the geochemical behavior of antimony in this environment is still unknown (Deng et al., 2014). Studies of freshwater antimony geochemistry are crucial to understand its geochemical behavior, particularly in such a fragile and dynamic environment. Therefore, a simulation study based on speciation analysis was carried out in laboratory. The purpose of this research is to reveal the specific influence of temperature on the geochemical cycling of antimony in the sediment-water interface of Poyang Lake.
2 MATERIAL AND METHOD 2.1 Site descriptionPoyang Lake, located in the north of Jiangxi Province and to the south of mid-to-low reaches of the Changjiang (Yangtze) River (28°24′–29°46′N, 115°49′–116°46′E) (Fig. 1), is the largest freshwater lake in China with an area of about 3 500 km2 at high level. The average depth is approximately 8 m, the maximum depth being around 23 m. Poyang Lake is surrounded by eleven counties, and is connected to five main rivers named Xiushui, Ganjiang, Fuhe, Xinjiang, and Raohe. The water from the five rivers runs through the lake and then discharges into the Changjiang River through a 1-km long channel at Hukou. Poyang Lake belongs to a subtropical monsoon climate with an annual average temperature of 16.5–17.8℃. The lowest and highest monthly average temperatures are 4.4℃ and 30℃ appeared in January and July, respectively.
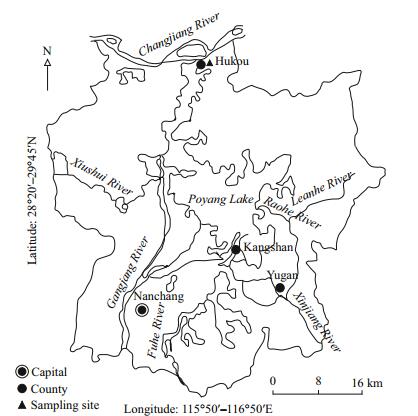
|
| Fig.1 Map of Poyang Lake and the sampling site (modified from Deng et al., 2014) |
A sampling campaign was performed in Huhou (29°44′089″N, 116°11′71.4″E) with a water depth of about 6–7 m. Overlying water at the surface was collected using a pre-cleaned polyethylene container. Three sediment cores of approximately 30 cm depth were collected by a diver using light plexiglas corers at the same site of Hukou where overlying water was collected. These cores were immediately transported to laboratory, and were subsequently placed at 5℃, 15℃ and 25℃ constant temperature and humidity chambers respectively to simulate different seasonal conditions (i.e., winter, spring/autumn and summer). These chambers were filled with high-pure nitrogen and placed in a dark environment during the simulation. About 10 cm depth of overlying water was added on the top of the sediment cores. The temperature, conductivity, pH, DO and ORP of the overlying water were regularly measured by an YSI handheld multiparameter meter (556MPS, YSI Inc.). A certain volume of overlying water on the top of each core was collected at regular intervals, and then acidified to pH < 2 with superpure HNO3 after filtration through a microfiltration membrane. All samples were preserved in pre-cleaned Teflon containers and stored at -80℃ to keep the stability of element species. An identical volume of overlying water collected at the sediment cores' sampling site was subsequently added to the cores to replenish the loss by periodical sampling. The overlying water on the sediment cores was kept un-stirred during the simulation period. When the physicochemical parameters measured by the YSI multiparameter meter remained unchanged, then the simulation experiment was finished.
2.3 Sample preparation and analysisAfter the simulation, each sediment core was extruded in a glove-box bag filled with nitrogen gas to prevent oxidation of Sb(Ⅲ). Sediment slices from each 1 cm depth were placed in pre-cleaned Teflon bottles and kept frozen at -80℃ until analysis. Porewaters in sediments were obtained by centrifugation at 4℃ in an oxygen-free environment. Different bound antimony forms in sediments at different temperatures were obtained by the sequential extraction technique described by Chen et al. (2003).
Different antimony species in overlying water and porewaters were measured by hydride generation atomic fluorescence spectrometry (HG-AFS) according to the procedures previously developed by Deng et al. (2001). The detection limit was 0.022 μg/L for each antimony species with a relative standard deviation less than 5%. Briefly, Sb(Ⅲ) was determined by HG-AFS after Sb(Ⅴ) was masked by 8-hydroxyquinoline, then Sb(Ⅴ) was obtained by the difference between the total inorganic antimony and Sb(Ⅲ). A third sample was digested for the total antimony (TSb) analysis. The concentration of organic antimony (Sb-org) was obtained by subtracting the sum of [Sb(Ⅴ) + Sb(Ⅲ)] from the concentration of TSb.
For the determination of total antimony (Tot-Sb), associated to oxidized [Sb(oxal)] and organic fractions [Sb(H2O2)] in sediments, it was described in detail by Chen et al. (2003). The difference among the Tot-Sb, Sb(oxal) and Sb(H2O2) in sediment was identified as sulfide minerals [M(pyrite)].
3 RESULT 3.1 Variation of antimony species in overlying water at different temperaturesThe profiles of dissolved Sb(Ⅲ), Sb(Ⅴ), Sb-org and TSb in overlying water as a function of time during simulation period at different temperatures are plotted in Fig. 2. The concentrations of Sb(Ⅲ), Sb(Ⅴ) and Sb-org in the overlying water when the sample was collected were 0.020, 0.56, and 0.24 μg/L, respectively. The simulation temperatures were different from the temperature when the samples were collected (about 20℃), thus the equilibrium of antimony at SWI will be disturbed and a new balance will be reached during the simulation process. The results showed that the new equilibrium will be achieved after about 20, 23 and 45 d for 5℃, 15℃ and 25℃, respectively. The average concentrations of different antimony species at different temperatures after equilibration are given in Table 1.
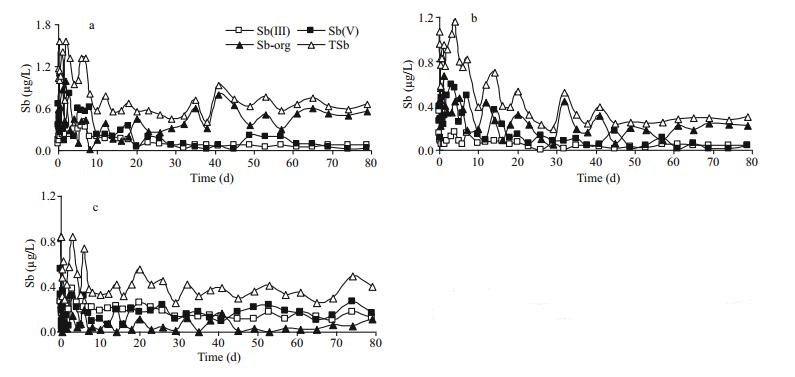
|
| Fig.2 Profiles of antimony species in overlying water at 5℃ (a), 15℃ (b) and 25℃ (c) |
As we expected that the antimony concentration in the overlying water presented obvious changes during this simulation process, especially at the beginning of the simulation (Fig. 2). Probably resulting from the larger temperature difference (the sediment cores were collected at about 20℃), the concentration variation of each antimony species at 5℃ was particularly drastic (Fig. 2a). It is worth mentioning that the concentration variation of antimony in the overlying water mainly resulted from the temperature variation as the simulation conditions were strictly controlled.
The variation trend and numerical size relationship of the different antimony species were similar between 5℃ and 15℃ as shown in Table 1. Specifically, inorganic antimony [Sb(Ⅲ) and Sb(Ⅴ)] gradually increased at the beginning and subsequently decreased before reaching a new equilibrium, while it was just opposite for Sb-org. Inorganic species predominate over organic species in most environmental systems (Andreae et al., 1981; Ellwood and Maher, 2002), but the concentration of Sb-org was the largest in this study both at 5℃ and 15℃. Sb(Ⅲ) can be detected in the overlying water at each temperature. It is noted that Sb(Ⅴ) should be the dominant and only detectable species in oxic waters, and the concentration ratio between Sb(Ⅴ) and Sb(Ⅲ) is 1018.4 in well aerated oxic water at pH=7 and pE=13.6 (Turner et al., 1981). However, a chemical equilibrium may not be attained in real natural dynamic systems where energy and matter can be exchanged, thus thermodynamically unstable chemical species may exist (Byrd, 1990; Takayanagi and Cossa, 1997). The continued existence of Sb(Ⅲ) in overlying water may be explained by slow reaction rates (Filella et al., 2002b) or biotic production (Cutter, 1992). The concentration of Sb(Ⅴ) was higher than Sb(Ⅲ) at each temperature, demonstrating that Sb(Ⅴ) is more stable than Sb(Ⅲ) at oxidizing conditions.
Based on the comparison of antimony species at different temperatures as well as the average concentrations in Table 1, we found that both Sb(Ⅴ) and Sb(Ⅲ) were higher at 25℃ than that at 5℃ and 15℃. A high temperature may be more favorable for antimony existing in inorganic forms. This conclusion was supported by the finding that the higher the temperature, the lower the Sb-org in overlying water. Meanwhile, TSb in overlying water at 5℃ was significantly higher than at 15℃ and 25℃. We also observed an apparent antimony migration from porewaters to the overlying water when the temperature is decreased. Furthermore, if the concentration variation of antimony species in the overlying water only results from the migration of antimony at the SWI, only a trend of "increase → equilibrium" or "decrease → equilibrium" will be expected. However, for both Sb(Ⅴ) and Sb(Ⅲ) at 5℃ and 15℃ the result was different. Therefore, obvious transformation of antimony species also occurred at the SWI when the temperature is changed.
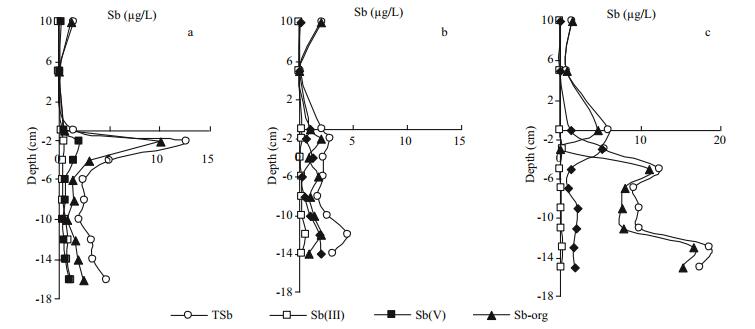
3.2 Vertical distribution of antimony species in porewaters at different temperaturesThe profiles of dissolved antimony species in porewaters at different temperatures are plotted in Fig. 3, and the average concentrations of different antimony species are also given in Table 2.
|
| Fig.3 Profiles of antimony species in porewaters at 5℃ (a), 15℃ (b) and 25℃ (c) |
Sharp concentration variations were found for Sborg in porewaters at 5℃ and 25℃. It was far higher at 25℃ (8.93 μg/L) than that at 5℃ (2.59 μg/L) and 15℃ (1.37 μg/L). An obvious concentration peak (10.1 μg/L) of Sb-org was observed in porewater of subsurface sediment at 5℃ (Fig. 3a), while it was low in middle and lower porewaters below 6 cm depth. The high concentration of Sb-org in top porewaters at 5℃ probably resulted from microbial action or release from sediments, in which organic antimony existed in a bound state in organic matter or adsorbed on Fe and Mn oxides/hydroxides. However, Sb(H2O2) in sediment (Fig. 4, to be discussed later) didn't present great vertical differences at any temperature; thus it was confirmed that the high concentration of Sb-org in top porewaters at 5℃ is mainly derived from microbial action (Bentley and Chasteen, 2002). Meanwhile, the high concentration of Sb-org in overlying water at 5℃ was explained by the diffusion of Sb-org from porewaters due to the sharp concentration gradient at the SWI. The concentration of Sb-org in bottom porewaters at 25℃ were extraordinarily high, while it was only 0.040 1 μg/L in the overlying water. Although temperature variation may promote the mobility of antimony at SWI (Frohne et al., 2011), it seems more likely that the low Sb-org concentration in the overlying water at 25℃ mainly resulted from the degradation of Sb-org to inorganic forms. Both Sb(Ⅲ) and Sb(Ⅴ) in the overlying water were higher at 25℃ than that at 5℃ and 15℃ can adequately support this conclusion. Based on the analysis of antimony species in the overlying water we knew that Sb-org was sensitive to temperature. Distributions of Sb-org in porewaters at different temperatures also indicated the temperature sensitivity of Sb-org.
|
| Fig.4 Profiles of different bound antimony species in sediments at 5℃ (a), 15℃ (b) and 25℃ (c) |
Sb(Ⅴ) will diffuse from porewaters toward the overlying water at 25℃ due to its concentration gradient. Thus, the concentration peak of Sb(Ⅴ) in subsurface porewater at 25℃ (Fig. 3c) cannot be derived from the overlying water. Meanwhile, it is impossible for Sb(Ⅲ) in porewaters to be largely oxidized into Sb(Ⅴ) under reducing conditions in sediments. Therefore we have adequate reasons to believe that this concentration peak mainly resulted from the desorption of antimony from sediment. It is noted that desorption reaction belongs to an endothermic process, thus a high temperature will favor this process.
The concentration relationship among different antimony species in porewaters was Sborg > Sb(Ⅴ) > Sb(Ⅲ) for each temperature (vertical comparison in Table 2). According to the horizontal comparison we also knew that the higher the temperature the lower the Sb(Ⅲ) concentration, while it was just on the contrary for Sb(Ⅴ). The ratios between Sb(Ⅴ) and Sb(Ⅲ) in porewaters were 1.46, 3.79 and 17.3 at 5℃, 15℃ and 25℃, respectively. Therefore, we can infer that it is beneficial to the equilibrium shift toward Sb(Ⅴ) when the temperature was increased. Amorphous Fe and Mn oxyhydroxides existing in sediments may play important roles in this process (Belzile et al., 2001).
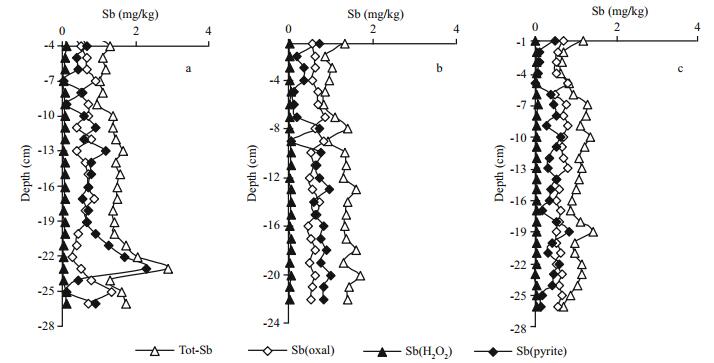
3.3 Vertical distribution of bound antimony species in sediments at different temperaturesThe profiles of different bound antimony species in sediments at different temperatures are plotted in Fig. 4, and the average contents are also given in Table 3.
The contents of Sb(oxal), Sb(H2O2) and M(pyrite) in the surface sediment when the samples were collected were 0.611, 0.070 6, 0.263 mg/kg, respectively. After the simulation, the average contents of Tot-Sb in sediments at different temperatures (1.02–1.43 mg/kg) were in the range reported for other stream sediments (Reimann et al., 2010). The profiles of antimony in sediments (Fig. 4) did not present great variation for different bound antimony species except for Sb(pyrite) at 5℃, for which an obvious peak was present at a depth of -23 cm. Table 3 shows that the content of antimony bound to organic matter and amorphous sulfides, i.e., Sb(H2O2), was especially lower (< 0.10 mg/kg) than the other species at each temperature. This situation was similar to the behavior of antimony in another freshwater lake (Chen et al., 2003). Meanwhile, it is interesting that the ratio between Sb(pyrite) and Sb(oxal) gradually increased with the decrease of temperature: 0.588, 0.954 and 1.11 at 25℃, 15℃ and 5℃, respectively. Sb(pyrite) contents were lower than Sb(oxal) both at 15℃ and 25℃; the opposite was true at 5℃. Therefore, we speculate that antimony in sediment easier forms the more stable Sb(pyrite) state at lower temperature.
The vertical distribution of the same antimony species in sediments at different temperatures did not present large differences, but the average content of each bound antimony species presented a slight increase with decreasing temperature as shown in Table 3. It is inferred that a decrease in temperature will be beneficial for the enrichment of antimony in sediment.
4 DISCUSSIONAntimony and arsenic (As) are in the same main group in the periodic table of elements, thus antimony is assumed to exhibit a similar behavior to arsenic (Hindmarsh, 2000). Some comparative studies on the physicochemical behavior of antimony and arsenic in soil or sediment have been carried out (Mitsunobu et al., 2006; Wilson et al., 2010; Asaoka et al., 2012). Like for arsenic, migration and transformation of antimony at the SWI are closely correlated with its existing species and the external conditions at the SWI. Because the external conditions were strictly controlled during the simulation period, differences such as microbial actions in sediments, DO in overlying water and physicochemical behavior of antimony at SWI mainly resulted from the variation in temperature. Based on our experimental results in Tables 1–3, the behavior of antimony species at the SWI in response to seasonal alternation can be summarized as shown in Fig. 5. The response to the other two seasonal alternations, i.e., winter to spring, spring to summer, is the reverse processes presented in Fig. 5b and Fig. 5a, respectively.

|
| Fig.5 Behaviors of antimony at the SWI in response to seasonal variation a. summer → autumn (25℃ → 15℃); b. autumn → winter (15℃ → 5℃). |
The ratio between Sb(Ⅲ) and Sb(Ⅴ) in the overlying water was slightly higher than generally found for As(Ⅲ) and As(Ⅴ) (Deng et al., 2014); the opposite was true in porewaters. As(Ⅲ) mainly exists in reducing environments for thermodynamic reasons (Sadiq, 1997). However, Sb(Ⅲ) can be detected in the overlying water in Poyang Lake in different seasons, and it was especially high in summer, suggesting that Sb(Ⅲ) may be more soluble than arsenic over a wide range of pE–pH. This conclusion was supported by observations of dissolved Sb(Ⅲ) in aqueous systems (Filella et al., 2002b). Usually, Sb(Ⅲ) is the main antimony species in anoxic waters and porewaters (Cutter, 1991; Cutter et al., 2001) except in highly contaminated areas. Therefore the reasons for high ratio between Sb(Ⅲ) and Sb(Ⅴ) in overlying water are still unknown. Organic arsenic compounds usually cannot be detected in overlying water by common analytical methods. However, Sb-org can be measured both in overlying water and porewaters. Meanwhile, it was sensitive to variations of temperature during seasonal alternations. The Sb-org concentration in the overlying water was distinctly higher at low temperature (winter) than that at high temperature (summer). Thus, low temperature will favor existence of organic forms of antimony in the overlying water.
The vertical distribution of antimony species in porewaters presents large differences in different seasons, thus seasonal variation has important effects on the behavior of antimony species in porewaters, especially for Sb-org. Usually, Eh is the dominating factor to affect the fate of metals (Du Laing et al., 2009), but the concentration peak of Sb-org in the subsurface porewater (Fig. 3a) (5℃) may derive from microbial action, while the high concentration of Sborg in porewaters of the middle and lower sediments (Fig. 3c) (25℃) more likely resulted from upward migration of Sb-org from deep sediments. TSb increases both in overlying water and porewaters when season alternates from autumn to winter (Fig. 5b). A high temperature in summer will contribute to the release of Sb(Ⅴ) from top sediments into porewaters by desorption, leading to the appearance of a concentration peak of Sb(Ⅴ) (Fig. 3c). The ratios between Sb(Ⅴ) and Sb(Ⅲ) in porewaters gradually increase with the increase of temperature, thus the equilibrium between Sb(Ⅲ) and Sb(Ⅴ) in porewaters will shift toward Sb(Ⅴ) when the temperature increases during seasonal alternation.
The average content of each antimony species in sediments increased with the decrease of temperature. Therefore a decrease in temperature will result in the enrichment of antimony in the sediment. Especially, it leads to the generation of stable Sb(pyrite). Antimony is thus easily transferred into the sediment by adsorption/coprecipitation or reacting to form Sb(pyrite) in winter when the temperature is decreased. The latter seems to be the main reason.
In summary, the behavior of antimony at the SWI (Fig. 5) demonstrates that seasonal variations in Poyang Lake will result in strong effects on the migration and transformation of antimony species at the SWI. Of course, the physicochemical behavior of antimony at the SWI in real environments will be affected by many factors such as aquatic organisms, flow disturbance, human activities, adsorption/ desorption processes, organic matter, etc. (Du Laing et al., 2009). Therefore, further research is needed, including more experimental conditions, development of new analytical methodology, and establishment of a theoretical model.
5 CONCLUSIONA simulation study was carried out in the laboratory to reveal the migration and transformation behavior of antimony at the SWI of Poyang Lake showing the action of seasonal variation. Concentrations of different antimony species in the overlying water presented major changes, especially at the beginning of the simulation. The concentration of Sb(Ⅴ) was higher than Sb(Ⅲ) in the overlying water at each temperature, and high temperature is more favorable for antimony to exist in inorganic forms. The ratio between Sb(Ⅴ) and Sb(Ⅲ) in porewaters gradually increased with the increase of temperature, suggesting that the equilibrium between Sb(Ⅲ) and Sb(Ⅴ) in porewaters will shift toward Sb(Ⅴ) when the temperature increases during seasonal alternation. TSb increases both in overlying water and in porewaters when the season alternates from autumn to winter, and the high temperature in summer will contribute to the release of Sb(Ⅴ) from top sediments into porewaters. Transformation of antimony species was occurred at the SWI besides the migration process when temperature changes. The average content of each antimony species in sediments increased with the decrease of temperature, thus decrease in temperature will result in the enrichment of antimony in the sediment. Seasonal variation has strong effects on the migration and transformation of antimony species at the SWI of Poyang Lake.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Andreae M O, Asmode J F, Foster P, Van't Dack L. 1981. Determination of antimony(Ⅲ), antimony(Ⅴ), and methylantimony species in natural waters by atomic absorption spectrometry with hydride generation. Anal. Chem., 53(12): 1 766-1 771.
DOI:10.1021/ac00235a012 |
Andrewes P, Cullen W R, Polishchuk E. 2000. Antimony biomethylation by Scopulariopsis brevicaulis: characterization of intermediates and the methyl donor. Chemosphere, 41(11): 1 717-1 725.
DOI:10.1016/S0045-6535(00)00063-1 |
Asaoka S, Takahashi Y, Araki Y, Tanimizu M. 2012. Comparison of antimony and arsenic behavior in an Ichinokawa River water–sediment system. Chem. Geol., 334: 1-8.
DOI:10.1016/j.chemgeo.2012.09.047 |
Belzile N, Chen Y W, Wang Z J. 2001. Oxidation of antimony (Ⅲ) by amorphous iron and manganese oxyhydroxides. Chem. Geol., 174(4): 379-387.
DOI:10.1016/S0009-2541(00)00287-4 |
Belzile N, Chen Y W, Xu R R. 2000. Early diagenetic behaviour of selenium in freshwater sediments. Appl. Geochem., 15(10): 1 439-1 454.
DOI:10.1016/S0883-2927(00)00011-1 |
Bentley R, Chasteen T G. 2002. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev., 66(2): 250-271.
DOI:10.1128/MMBR.66.2.250-271.2002 |
Byrd J T. 1990. Comparative geochemistries of arsenic and antimony in rivers and estuaries. Sci. Total Environ., 97-98: 301-314.
DOI:10.1016/0048-9697(90)90247-R |
Cappuyns V, Swennen R, Niclaes M. 2007. Application of the BCR sequential extraction scheme to dredged pond sediments contaminated by Pb-Zn mining: a combined geochemical and mineralogical approach. J. Geochem. Explor., 93(2): 78-90.
DOI:10.1016/j.gexplo.2006.10.001 |
Chen Y W, Deng T L, Filella M, Belzile N. 2003. Distribution and early diagenesis of antimony species in sediments and porewaters of freshwater lakes. Environ. Sci. Technol., 37(6): 1 163-1 168.
DOI:10.1021/es025931k |
Cutter G A, Cutter L S, Featherstone A M, Lohrenz S E. 2001. Antimony and arsenic biogeochemistry in the western Atlantic Ocean. Deep Sea Res. Part II: Top. Stud. Oceanogr., 48(13): 2 895-2 915.
DOI:10.1016/S0967-0645(01)00023-6 |
Cutter G A. 1991. Dissolved arsenic and antimony in the Black Sea. Deep Sea Res. Part A. Oceanogr. Res. Pap, 38(2): S825-S843.
|
Cutter G A. 1992. Kinetic controls on metalloid speciation in seawater. Mar. Chem., 40(1-2): 65-80.
DOI:10.1016/0304-4203(92)90048-F |
Deng T L, Chen Y W, Belzile N. 2001. Antimony speciation at Ultra Trace levels using hydride generation atomic fluorescence spectrometry and 8-hydroxyquinoline as an efficient masking agent. Anal. Chim. Acta, 432(2): 293-302.
DOI:10.1016/S0003-2670(00)01387-8 |
Deng T L, Wu Y, Yu X P, Guo Y F, Chen Y W, Belzile N. 2014. Seasonal variations of arsenic at the sediment-water interface of Poyang Lake, China. Appl. Geochem., 47: 170-176.
DOI:10.1016/j.apgeochem.2014.06.002 |
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack F M G. 2009. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci. Total Environ., 407(13): 3 972-3 985.
DOI:10.1016/j.scitotenv.2008.07.025 |
Ellwood M J, Maher W A. 2002. Arsenic and antimony species in surface transects and depth profiles across a frontal zone: the Chatham Rise, New Zealand. Deep Sea Res. Part I: Oceanogr. Res. Pap., 49(11): 1 971-1 981.
DOI:10.1016/S0967-0637(02)00115-2 |
Filella M, Belzile N, Chen Y W. 2002a. Antimony in the environment: a review focused on natural waters: I. Occurrence. Earth-Sci. Rev., 57(1-2): 125-176.
DOI:10.1016/S0012-8252(01)00070-8 |
Filella M, Belzile N, Chen Y W. 2002b. Antimony in the environment: a review focused on natural waters: II. Relevant solution chemistry. Earth-Sci. Rev., 59(1-4): 265-285.
|
Frohne T, Rinklebe J, Diaz-Bone R A, Du Laing G. 2011. Controlled variation of redox conditions in a floodplain soil: Impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma, 160(3-4): 414-424.
DOI:10.1016/j.geoderma.2010.10.012 |
Gebel T, Christensen S, Dunkelberg H. 1997. Comparative and environmental genotoxicity of antimony and arsenic. Anticancer Res., 17(4A): 2 603-2 607.
|
Hammel W, Debus R, Steubing L. 2000. Mobility of antimony in soil and its availability to plants. Chemosphere, 41(11): 1 791-1 798.
DOI:10.1016/S0045-6535(00)00037-0 |
He M C, Wan H Y. 2004. Distribution, speciation, toxicity and bioavailability of antimony in the environment. Prog.Chem., 16(1): 131-135.
(in Chinese with English abstract) |
Hindmarsh J T. 2000. Arsenic, its clinical and environmental significance. J. Trace Elem. Exp. Med., 13(1): 165-172.
DOI:10.1002/(ISSN)1520-670X |
Mitsunobu S, Harada T, Takahashi Y. 2006. Comparison of antimony behavior with that of arsenic under various soil redox conditions. Environ. Sci. Technol., 40(23): 7 270-7 276.
DOI:10.1021/es060694x |
Reimann C, Matschullat J, Birke M, Salminen R. 2010. Antimony in the environment: lessons from geochemical mapping. Appl. Geochem., 25(2): 175-198.
DOI:10.1016/j.apgeochem.2009.11.011 |
Sadiq M. 1997. Arsenic chemistry in soils: an overview of thermodynamic predictions and field observations. Water, Air, Soil Poll., 93(1-4): 117-136.
DOI:10.1007/BF02404751 |
Takayanagi K, Cossa D. 1997. Vertical distributions of Sb(Ⅲ) and Sb(Ⅴ) in Pavin Lake, France. Water Res., 31(3): 671-674.
DOI:10.1016/S0043-1354(96)00285-0 |
Tessier A, Campbell P G C, Bisson M. 1979. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem., 51(7): 844-851.
DOI:10.1021/ac50043a017 |
Turner D R, Whitfield M, Dickson A G. 1981. The equilibrium speciation of dissolved components in freshwater and sea water at 25℃ and 1 atm pressure. Geochim. Cosmochim. Acta, 45(6): 855-881.
DOI:10.1016/0016-7037(81)90115-0 |
Wilson S C, Lockwood P V, Ashley P M, Tighe M. 2010. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environ. Pollut., 158(5): 1 169-1 181.
DOI:10.1016/j.envpol.2009.10.045 |
 2018, Vol. 36
2018, Vol. 36