Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Evgeniya MATYUGINA, Natalia BELKOVA, Svetlana BORZENKO, Pavel LUKYANOV, Marsel KABILOV, Olga BATURINA, Alexandra MARTYNOVA-VAN KLEY, Armen NALIAN, Aleksei PTITSYN
- Structure and diversity dynamics of microbial communities at day and night: investigation of meromictic Lake Doroninskoe, Transbaikalia, Russia
- Chinese Journal of Oceanology and Limnology, 36(6): 1978-1992
- http://dx.doi.org/10.1007/s00343-018-7332-1
Article History
- Received Jan. 27, 2018
- accepted in principle Mar. 7, 2018
- accepted for publication Mar. 27, 2018
2 Limnological Institute SB RAS, Irkutsk, Russia;
3 Institute of Chemical Biology and Fundamental Medicine, Novosibirsk, Russia;
4 Stephen F. Austin State University, TX, USA
Meromictic soda and saline lakes are unique ecosystems found globally (Sorokin et al., 2002, 2007; Koizumi et al., 2004a, b; Dimitriu et al., 2008; Lauro et al., 2011; Comeau et al., 2012; Andrei et al., 2015; Leboulanger et al., 2017). These reservoirs are excellent model systems for limnological studies. A stable stratification of a wide range of physical, chemical and biological parameters is maintained through-out the year (Overmann et al., 1991). Today, biogeographical studies of meromictic ecosystems show that in each geographic area, stratified conditions are defined by the particular combination of ecological parameters. This is expressed by the spatial and temporal diversity and specific structure of microbial communities of meromictic water bodies (Bosshard et al., 2000; Lehours et al., 2005; Gregersen et al., 2009; Habicht et al., 2011; Comeau et al., 2012; Garcia et al., 2013; Hamilton et al., 2016; Zhong et al., 2016).
Thus, studies of microbial communities, with respect to spatial distribution, have shown that some meromictic lakes exhibit a higher level of microbial variety in the monimolimnion than the mixolimnion layer. Researchers have associated these findings with the peculiarities of the multistep process of mineralization, that involves a diverse and complex bacterial community, abundant diversity promoting nutrients in the anoxic layer (Lauro et al., 2011; Baatar et al., 2016) and a relative lack of anoxic layer mixing and downward metabolic fluxes (Andrei et al., 2015). Analysis of dominant bacteria in the chemocline zone of meromictic lakes confirmed that key biogeochemical transformations of sulfur compounds in this layer are carried out by sulfuroxidizing chemoautotrophs (Sorokin et al., 2002, 2007; Gorlenko et al., 2010), anoxygenic photoautotrophic purple sulfur bacteria (Overmann et al., 1991; Dimitriu et al., 2008; Meuser et al., 2013), anoxygenic photoautotrophic green sulfur bacteria (Garcia-Gil et al., 1996; Koizumi et al., 2004a, b; Tonolla et al., 2005), anoxygenic photoheterotrophic purple non-sulfur bacteria (Meuser et al., 2013; Matyugina and Belkova, 2015), iron-reducing, ironoxidizing and sulfate-reducing bacteria (Johnson and Hallberg, 2009; Falagán et al., 2014). The dominance of bacteria with certain metabolic strategies may be due to a combination of functional characteristics, the physiology of these organisms (Frigaard and Dahl, 2008) and favorable environmental conditions for the realization of their basic ecological role.
Nowadays, improved access to modern highthroughput sequencing and bioinformatics tools has greatly enhanced our capacity to interrogate the complexity of microbial community dynamics in meromictic ecosystems (Lauro et al., 2011; Comeau et al., 2012; Lanzén et al., 2013; Andrei et al., 2015; Leboulanger et al., 2017). Large-scale changes in space and time in meromictic ecosystems, such as inter-seasonal and interannual dynamics of microbial communities (Bosshard et al., 2000; Lehours et al., 2005; Gregersen et al., 2009; Habicht et al., 2011; Comeau et al., 2012; Garcia et al., 2013; Hamilton et al., 2016; Zhong et al., 2016) or their biogeographical diversity have been well studied (Lindström and Langenheder, 2012; Baatar et al., 2016).
Comparatively little is known about small-scale temporal changes, such as circadian dynamics, in the structure and function of microbial communities during day and night. Previous research was mainly concerned with the study of circadian systems in cyanobacteria, for example Synechococcus elongatus (Hut and Beersma, 2011). Studies of microbial communities have revealed diurnal changes in composition and structure, both in soil and aquatic ecosystems (Dvornyk et al., 2003; Tagkopoulos et al., 2008; Poretsky et al., 2009; Gilbert et al., 2010; Hewson et al., 2010; Hut and Beersma, 2011; Vila-Costa et al., 2013; Gifford et al., 2014; Ottesen et al., 2014; Zhang et al., 2014; Andrade et al., 2015; Shilova et al., 2016; Gunnigle et al., 2017; Silveira et al., 2017). Investigations of Namib Desert soil communities clearly demonstrated that significant changes in the active fraction of bacteria can occur within a single day. Furthermore, these changes were not primarily governed by abiotic factors (i. e., physicochemical variables and climatic regime), and distinct microbial interactions may be important in recorded community dynamics (Gunnigle et al., 2017). In the bacterial planktonic assemblage from the oligotrophic Lake Llebreta, studies using metatranscriptome sequencing for the analysis of day and night gene expression profiles revealed an overabundance of energy acquisition gene transcripts in the daytime compared with the night. Comparison of metatranscriptomes from a freshwater lake with pelagic marine systems led to the identification of common differences between day and night gene expression patterns related to energy production (Vila-Costa et al., 2013). Studies of the microbial communities of coastal marine (Gifford et al., 2014) and oceanic (Ottesen et al., 2014) ecosystems, revealed that a significant part of the community, the heterotrophic part, had strong day-night activity dynamics. Day-night differences in gene expression of the coastal marine ecosystem microbial communities were determined. Interestingly, heterotrophic taxa activities cycled daily. Specifically, the processes associated with the synthesis of transport and metabolic genes were activated at night, while the processes associated with growth, energy conservation and repair were started at daytime. Among these studies, only 55 of 200 taxa had no detectable temporal pattern of potential activity, indicating a wide variety of microbial responses and environmental interactions within this coastal marine ecosystem (Gifford et al., 2014). A three-day series experiment on hypersaline microbial communities of Lake Tyrrell, Australia revealed that microbial community functioning cycled daily. The ecological parameters of the lake may be linked with microbial day-night fluctuations (Andrade et al., 2015). These studies suggest that small-scale temporal changes, in particular the daily dynamics of microbial communities, are important and are a promising topic for research. Meromictic ecosystems have stable physicochemical conditions with simplified and limited biodiversity. Because of these peculiarities, they are advantageous model systems.
The soda lake, Lake Doroninskoe, is one of three known meromictic reservoirs of Siberia. Microorganisms in the lake are active under specific environmental conditions (Gorlenko et al., 2010; Borzenko et al., 2014; Matyugina et al., 2014; Matyugina and Belkova, 2015), including the continental climate of Transbaikalia, the permafrost zone and specific physical and chemical conditions, such as alkaline pH, relatively high salinity of water and sediments up to 35.0 g/L (Zamana and Borzenko, 2007; Borzenko et al., 2015). Lake Doroninskoe differs from other meromictic reservoirs of the world by certain parameters (Overmann et al., 1991; Humayoun et al., 2003). There is a low level of chemocline illumination (0.001%) (Matyugina et al., 2014), and a rare type of alkaline water is formed by evaporative concentration in the sedimentation zone (Borzenko et al., 2015; Matyugina and Belkova, 2015). Stable in space and time, the meromictic conditions in the lake are characterized, not only by high microbial diversity (Matyugina and Belkova, 2015), but also in the chemocline zone, by the dominance of the metabolically flexible, anoxygenic, photoheterotrophic, non-sulfur purple bacteria family Rhodospirillaceae and Rhodobacteraceae (class Alphaproteobacteria), which have the ability to switch from anoxic photosynthesis to aerobic chemotrophic metabolism and develop into dense microbial populations. A specific feature of the lake was the finding of bacteria with alternative photosynthesis strategies (for example, actinorhodopsin photosynthesis) in a minor part of the microbial community, with a maximum detected in the mixolimnion (Matyugina and Belkova, 2015).
The main purpose of this study was to study the dynamics of the structure and diversity of the microbial community in the water column of Lake Doroninskoe during the day and at night.
2 MATERIAL AND METHOD 2.1 Water samplingThe dynamics of the microbial community of the meromictic Lake Doroninskoe were studied during natural stratification according to the illumination period—day and night—in September 2013.
Water samples were collected September 5 and 6, 2013 at the central station of the lake (51°14′N; 112°14′E, water depth 6.2 m) from 2.5, 3.0, 3.15, 3.4, 3.6, 3.75, 4.0, 4.25, 4.5, 5.0 and 6.0 m depth. Samples were collected from 13:00 to 15:00 (GMT+8) during the day, and at night, from 23:00 to 01:00. Air temperature during the day was 21.0℃, at night 7.0℃.
Values of temperature, conductivity, oxygen, pH and salinity were measured in the field station Miltu-340, Germany.
2.2 Chemical analysisHydrogen sulfide H2S (hydrosulfide HS-) and elemental sulfur S0 were precipitated from 100 mL of water with zinc acetate and collected on 0.45 μm filters. Thiosulfates S0S4+ and sulfites SO32- were isolated from the remaining filtrate with AgNO3 (Zerkle et al., 2010). All forms of sulfur were determined by a photometric method described previously (Volkov and Zhabina, 1990). This method relied on successive transition of sulfur compounds into H2S with subsequent photometric determination. The advantage of this method consists of concentrating solution during analysis up to required volumes. This step significantly increases the detection limit of all sulfur forms up to 0.001 mg/L for S.
2.3 Molecular analysisFor metagenomic analysis, water samples were filtered sequentially through 0.65 and 0.22 μm poresized polycarbonate filters (Millipore) and stored at -20℃. The volume of 200 mL was filtered from 2.5– 4.5 m, whereas 100 mL was filtered from 4.75–6.2 m.
Genomic DNA was extracted from both filters in two replicates and pooled to construct a single library from each water sample using commercial kits: Bacterial Genomic DNA kit (Axygen, USA) and DNA-sorb B (AmpliSens, Moscow) according to the manufacturer's protocols. In total, 22 libraries were analyzed.
The V3 and V4 hypervariable regions of the 16S rRNA gene were amplified with the primer pair 343F and 806R, combined with Illumina adapter sequences (Fadrosh et al., 2014). PCR amplification was performed in 50 μL reactions containing 0.7 U Phusion Hоt Start Ⅱ High-Fidelity and 1× Phusion GC buffer (Thermo Fisher Scientific), 0.2 μmol/L of each fоrward and reverse primers, 10 ng template DNA, 2.3 mmol/L MgCl2 (Sigma-Aldrich) and 0.2 mmol/L of each dNTP (Life Technologies). All reactions were performed using the Bio-Rad CFX96 real-time PCR system with the following thermal cycle protocol: initial denaturation at 98℃ for 1 min, followed by 30 cycles of 98℃ for 15 s, 62℃ for 15 s, and 72℃ for 15 s, with final extension at 72℃ for 10 min. Equimolar mixed PCR products were purified using MinElute Gel Extraction Kits (Qiagen). Amplicons were sequenced (2×300 bp) using the MiSeq Reagent Kit v3 (Illumina) at the SB RAS Genomics Core Facility (ICBFM SB RAS, Novosibirsk, Russia). Data obtained in this study have been deposited in the NCBI database with accession no. PRJNA420191.
2.4 Bioinformatic and statistical analysisRaw paired reads were analyzed with the UPARSE pipeline (Edgar, 2013). The UPARSE pipeline included merging; read quality filtering; length trimming; dereplication; discarding singletons; removing chimeras and OTU clustering using the UPARSE-OTU algorithm.
The ОTU sequences were assigned a taxonоmy using the RDP classifier 2.11 (Wang et al., 2007). Community structure analyses were based on the phylum and genus taxonomy levels. Microbial community diversity values (Shannon index, Chao1) were calculated using Explicet 2.10.5 (Robertson et al., 2013) at the rarefaction point with 500 bootstrap re-samplings.
Principal component analysis (PCA), non-metric multidimensional scaling (NMDS) and redundancy analysis (RDA) ordinations on Bray-Curtis dissimilarities were performed in R-3.2.3 (R Development Core Team, 2016, Oksanen et al., 2018). All permutation tests were performed with 10 000 permutations. Constrained ordination was performed to test for correlation between bacterial community structure and physicochemical characteristics of water.
3 RESULT 3.1 Analysis of physicochemical characteristics of water at day and nightThe physicochemical parameters of the meromictic soda Lake Doroninskoe at day and night were characterized by strong stratification and clear separation of the water layers into:
a) an upper-oxic layer with vertical seasonal mixing (mixolimnion), which extended to a depth from the surface to 3.75 m during the day and up to 4.0 m at night;
b) a narrow redox zone, which had sharp gradients of O2/S2- and was located between the depths of 3.75– 4.0 m at daytime and 4.0–4.25 m at night;
c) a deep hydrogen sulfide layer that is immiscible with the upper oxidized waters (monimolimnion), which covered depths from 4.0 to 6.2 m at daytime and was below 4.25–6.2 m at night (Table 1).
|
The water temperature in the mixolimnion during the day fluctuated from 16.28–13.17℃, to 16.23– 11.50℃ at night, with a more gradual decrease in depth (Table 1). The thermocline was detected in the redox zone, both during the day and at night. The temperature in the monimolimnion at night was slightly lower than during the day.
The chemical composition of the water, on average, was composed of 70% Na2CO3+NaHCO3; 29% NaCl, 1% Na2SO4. These results are consistent with data obtained previously (Zamana and Borzenko, 2007). Chloride, sodium carbonate and hydrogen carbonate dominated the stratified layers of the mixolimnion, monimolimnion and redox zones, while sulfates were present in lake water in insignificant amounts.
During the studied period, the salinity of the top layers of the mixolimnion was 22.23 g/L both at day and night. At a depth of 3.0 m, it increased sharply to 33.63 g/L at night and to 32.59 g/L during the day, reaching the maximum values in the anaerobic monimolimnion, where it was 35.82 (night) and 36.01 (in the day) g/L (Table 1). Such water stratification according to salinity is typical for seasonal and annual cycles of Lake Doroninskoe, but higher values have been reported (Borzenko et al., 2015).
The day-night stratification of the water column was verified by the heterogeneous distribution of hydrosulfide (HS-), thiosulfate (S0S4+) and elemental (S0) sulfur, dissolved oxygen O2 and values of pH and Eh (Table 1).
The daytime concentration of oxygen in the mixolimnion was determined at a depth of 4.0 m and ranged from 6.08–0.46 mg/L, whereas the night values of oxygen varied from 5.94–0.09 mg/L at a depth of 4.25 m. At depths between 3.75–4.0 m at daytime and 4.0–4.25 m at night, a transition of Eh from positive to negative values was observed. At night it was higher and changed from +18 to -300 mV (Table 1).
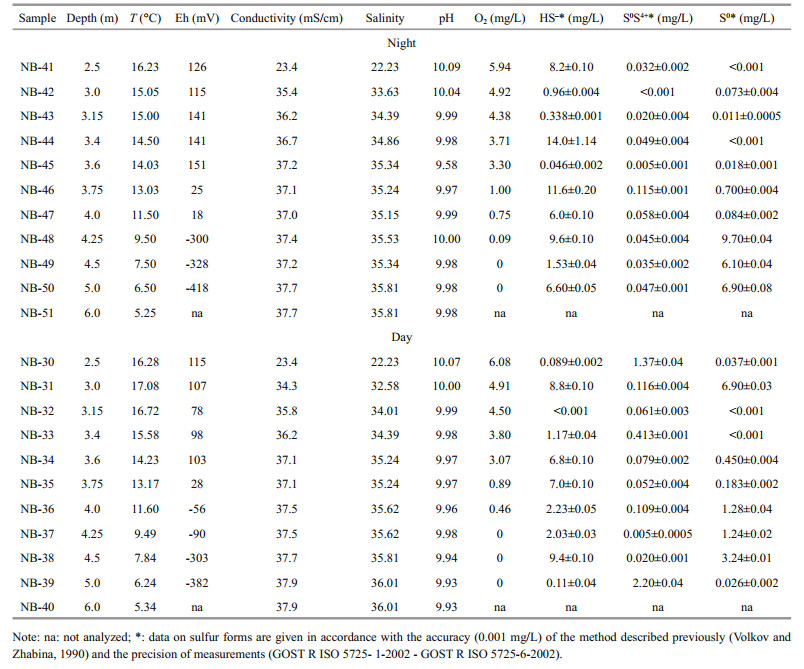
The content of hydrosulfide sulfur (HS-), both maximum and average, was higher at night. This was confirmed by lower values of Eh. The elemental sulfur content (S0), on average, was 2.45 mg/L in the water column during the day and 3.27 mg/L at night. During the day, the average concentration of thiosulfate sulfur (S0S4+) was one order of magnitude higher than at night, and amounted to 0.47 mg/L. At night, a consistent water column distribution of the hydrosulfide and elemental sulfur forms was determined. At day the distribution was reversed (Fig. 1). A significant decrease in the concentration of hydrosulfide sulfur in the direction S2-→S0S4+→S0 to the surface water layer was not detected for Lake Doroninskoe, even though it is characteristic of other meromictic lakes, such as Mono Lake, USA (Gulati et al., 2017). The day-night depth distribution of the hydrosulfide, thiosulfate and elemental sulfur forms (Fig. 1) was consistent with previously identified seasonal peculiarities (Borzenko and Zamana, 2011). This indicates the possibility of simultaneous oxidation processes in the anoxic reducing environment and reduction processes in the oxidized layers. The day-night values of pH varied slightly, except at a depth of 3.6 m, where the lowest value of 9.58 was noted.
|
| Fig.1 Day-night distribution of hydrosulfide (HS-), thiosulfate (S0S4+) and elemental (S0) sulfur forms at different depths of Lake Doroninskoe (September, 2013) |
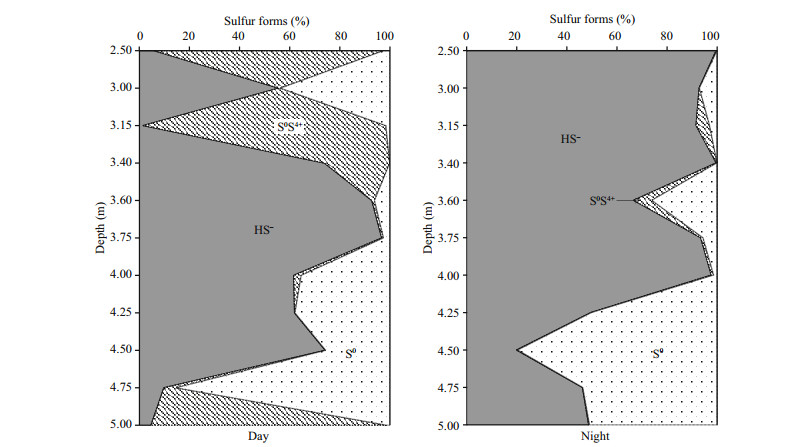
The Principal Component Analysis (PCA) established a correlation between some of the physicochemical parameters of the water (Fig. 2). The first component was most closely related to Eh and oxygen, as well as salinity and depth. The second component showed a positive correlation with the hydrosulfide and elemental sulfur forms and a negative correlation with thiosulfate.
|
| Fig.2 PCA analysis of environmental variables in Lake Doroninskoe (September, 2013) |
A total of 676 343 sequences were obtained (average length of 300 bp), which were grouped into 2 254 OTUs.
Analysis of the microbial community alpha diversity showed high microbial diversity in the water column during the day (Table 2, Fig. 3). The diversity indices were maximal and varied in a wide range of magnitudes (Table 2). At daytime, maximum values were obtained in the chemocline zone (ACE 261.4; Chao1 256.6; Shannon 3.46). According to the reverse Simpson index, the species richness at daytime was most pronounced in the mixolimnion of the oxic zone: 5.70. At night, the indices of species richness were higher in the monimolimnion of the anoxic zone (ACE 155.2; Chao1 170.0), while the diversity indices in the mixolimnion of the oxic zone of the lake lower (Shannon 2.61; Simpson 3.61) (Table 2).
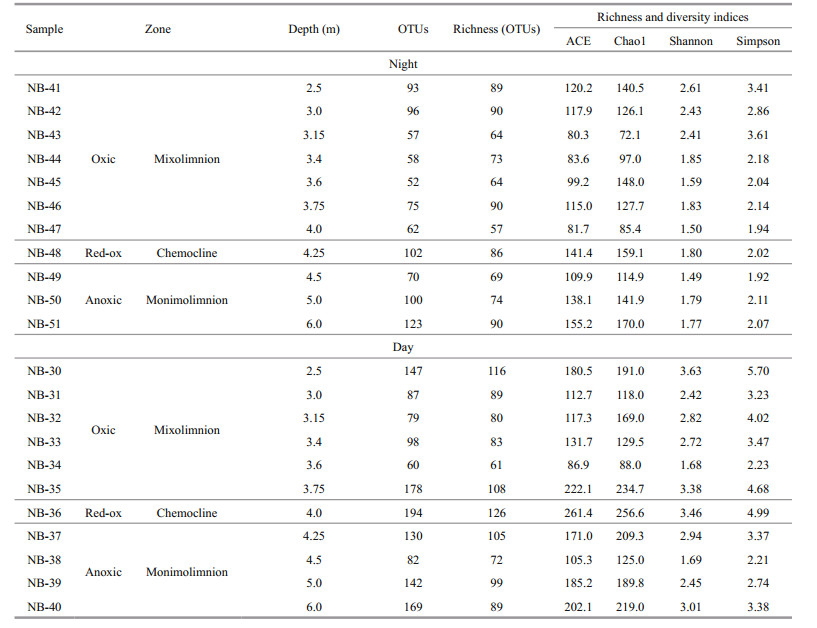
|
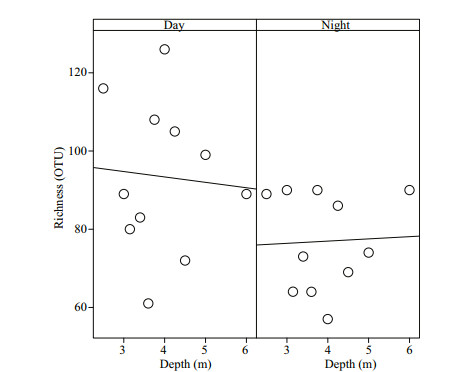
|
| Fig.3 Day-night observed OTU richness at the different depths in Lake Doroninskoe (September, 2013) Individual points represent the samples at different depths through the water column. |
The day-night species richness at the different depths of the lake did not significantly differ (Fig. 3). It was higher at daytime than at night; while at lower depths, opposite trends are observed: in the daytime, the richness of the chemocline was higher and decreased to the bottom, and at night, vice versa. Thus, in the day, the microbial community was more diverse, stratified and differentiated in depth, unlike the microbial community at night.
3.3 Comparison of microbial communities at day and night. Beta diversityStatistical and non-statistical methods (RDA, NMDS and heatmap) were used to compare microbial communities at day and night in Lake Doroninskoe.
RDA analysis established reliable differences between microbial communities formed during the day and night at all depths in the lake (Fig. 4). The phylotypes of Pseudomonas, Enterobacteriaceae, Bacteroidetes were dominant and correlated with the night microbial community, whereas the dominant phylotypes of Achromobacter, Firmicutes, Nitriliruptor, Microbacteriaceae and cyanobacteria group GpIIa correlated with the daytime microbial community. It is interesting to note that the phylotype Serratia had the highest positive correlation for the main component of RDA1. Moreover, we observed a correlation between microbial community structure and depth. The night bacterial community of analyzed layers correlated and formed a dense cluster, whereas the daytime bacterial community did not have an explicit correlation.
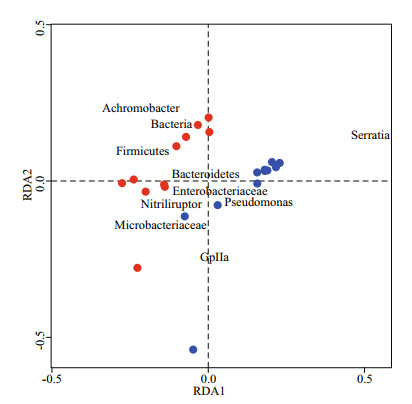
|
| Fig.4 RDA analysis of microbial comminutes at day (red dots) and night (blue dots) and dominant phylotypes in Lake Doroninskoe (September, 2013) |
Based on our statistical analysis, a reliable correlation of dominant phylotypes with environmental factors affecting the differentiation of the night and day microbial communities was revealed. A clear spatial differentiation demonstrated higher heterogeneity in the microbial communities from the day, than those in the night, which were grouped closer to each other. NMDS analysis showed that bacterial communities at the different water layers reliably correlated with depth, salinity, oxygen and Eh (Fig. 5). In addition, some bacterial communities were closely correlated with specific environmental variables: e.g., night microbial community of upper oxic mixolimnion (2.5 m for NB-41) associated with Eh and oxygen (Fig. 5), while the daytime microbial community of oxic (3.6 m for NB-34) and hydrogen sulfide layers (4.5 m for NB-38) were associated with salinity and depth. Additionally, NMDS analysis showed that the night microbial communities of oxic layers from 3.4–4.0 m, including the chemocline, and the oxygen-free hydrogen sulfide layers from 3.4–6.0 m were grouped together. Exceptions were found for night microbial communities of the oxic layer from 2.5–3.15 m and daytime microbial communities from 2.5–6.0 m: which showed weak correlation, clear differentiation and high spatial heterogeneity.

|
| Fig.5 NMDS analysis of environmental factors and microbial comminutes at day (red dots) and night (blue dots) at different depths in Lake Doroninskoe (September, 2013) Day-night difference is significant (P=0.000 1), while depth and oxygen (O2) correlate to microbial community structure with high significance P=0.004 and P=0.003 respectively. |
Metagenomic analysis of 16S rRNA gene amplicons revealed that the microbial community of the lake's water, both at day and night, was characterized by high taxonomic diversity (Fig. 6). Representatives of sixteen bacterial and three archaeal phyla were identified. Among candidate phyla, only representatives of SR1 (Candidatus Absconditabacteria) and Candidatus Saccharibacteria (TM7) were detected. Proteobacteria were dominant. They comprised 75% of the species at day and increased up to 90% at night. The share of such phyla as Acidobacteria, Cyanobacteria, Firmicutes, Bacteroidetes, Spirochaetes, Tenericutes and Verrucomicrobia ranged from 0.1%–7.3% at day and from 0.1%–4.4% at night. Chloroflexi were detected only at night, whereas SR1 (Candidatus Absconditabacteria), Euryarchaeota, Candidatus Saccharibacteria were detected only during the day. Pacearchaeota, Woesearchaeota, Parcubacteria were detected only in separate layers of the lake during day or night.
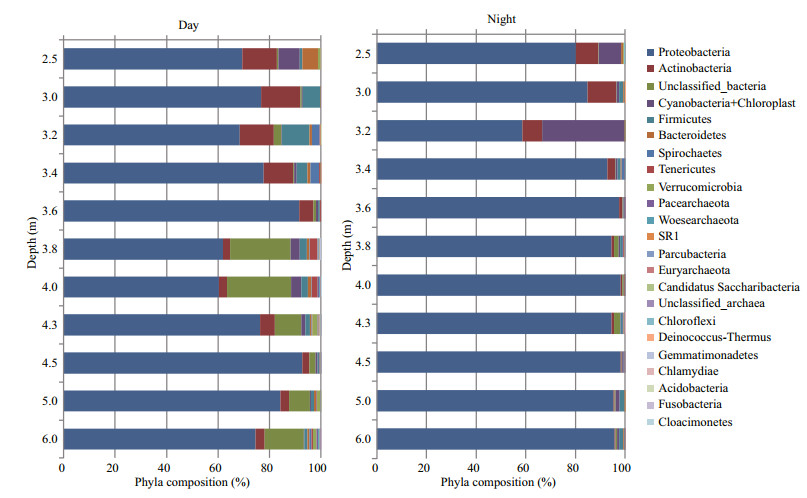
|
| Fig.6 Phyla composition of microbial communities at day and night at the different depths in Lake Doroninskoe (September, 2013) |
Heat map analysis of the microbial communities helped to establish the presence of phylotypes and their compact clustering at day and night (Fig. 7). At the genus level, Desulfurivibrio and Desulfuromonadaceae were more prevalent in the lower oxygen zone at a depth of 3.75-4.0 m during the day, while Clostridia were observed in the hydrogen sulfide zone at a depth of 5.0 m and 6.0 m at night. The microbial community, at day and night, was clearly clustered in the contact zone of oxygen and hydrogen sulfide layers, including the chemocline. Phylotypes of Alcaligenaceae, Aliidiomarina and Spirochaetaceae were reliably located in this zone at depths of 2.5 m at night; and 3.4 m at day. During the day in the upper oxygen zone at a depth of 2.5 m, Rhodobaca, Alishewanella, Cyclobacteriaceae and Rhodobacteraceae were present, while at night in the oxygen zone at a depth of 3.15 m, Acidimicrobiales, Ilumatobacter, and cyanobacteria were identified.

|
| Fig.7 Heatmap showing the relative abundance of bacterial phylotypes representing > 1% in microbial communities at day and night at different depths in Lake Doroninskoe (September, 2013) |
Among top 15 phylotypes, Serratia was dominant in microbial communities at both day and night in the water column of the lake (Table 3). The Serratia fraction in the communities was on average, 60%. These bacteria are known as chemoorganoheterotrophic facultative anaerobes. They reduce nitrate and possess both respiratory and fermentative types of metabolism. The maximum fraction of Serratia bacteria was recorded in the anoxic zone for day and night. The proportion of the bacteria increased at night from 58.8%–69.3%. Microorganisms of the genus Achromobacter were also dominant in the lake microbial community. These chemoheterotrophic microorganisms reduce nitrate to nitrogen. On average they comprised about 23.5% of the microbial communities. The maximum fraction was 34% at day in the anoxic zone and 24.8% at night in the upper oxygen zone. Anoxygenic phototrophic bacteria of genus Rhodobaca represented 0.1%–6.2% of the lake microbial community. The 2.5-meter layer was the only one, where Rhodobaca was dominant and its maximum was 6.2% during daytime, decreasing to 4.1% at night. Interestingly, these bacteria were dominant in the winter, in the chemocline of the lake (Matyugina and Belkova, 2015). Oxygenic phototrophic chlorophyll a— containing cyanobacteria consisted from 0.1%–9.1% in the microbial community. These bacteria showed a maximum of 32.7% at night in the oxic zone. Minor phylotypes accounted for up to 14.7% of the microbial community of the lake. These bacteria were represented by 67 genera.
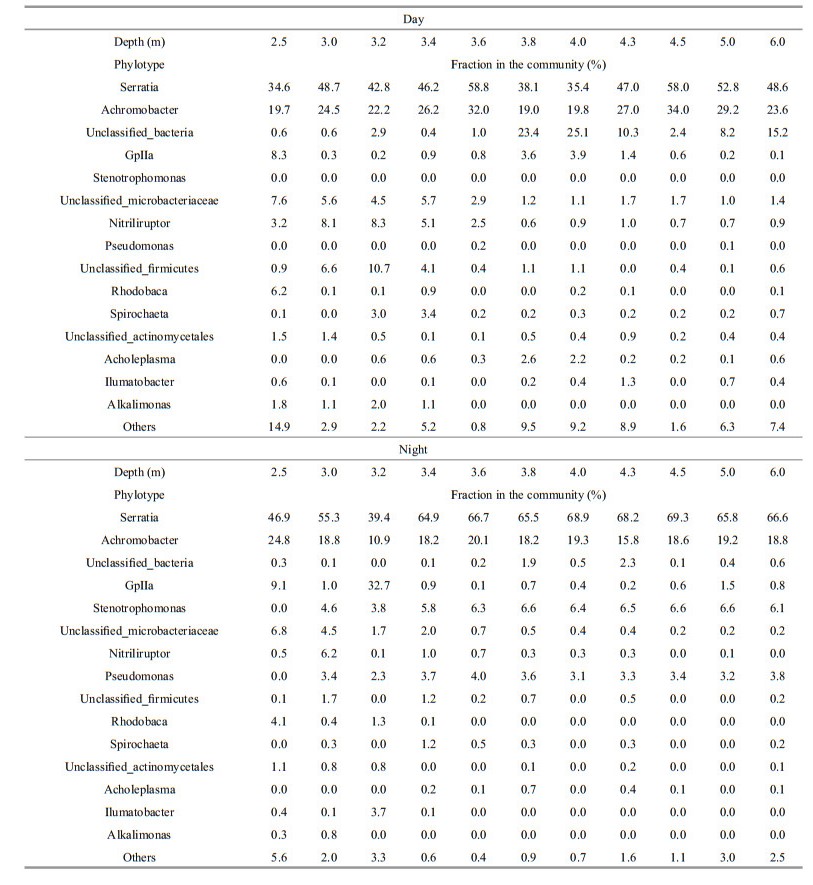
|
The species and sub-species level dynamics underpin a vast array of microbial population diversity (Shilova et al., 2016). To better understand the factors affecting the composition and structure of the microbial community, it is extremely important to investigate the macro and micro-changes in the community in space and time.
During the studied period, the water column of the lake was characterized by a stable stratification of physical and chemical characteristics, as well as in other long-term periods, including interseasonal and interannual (Zamana and Borzenko, 2007; Borzenko et al., 2015). A distinctive peculiarity of night stratification from the day was the depth of the lower boundary of the mixolimnion. During the day the boundary was at a depth of 4.0 m, and at night it deepened to 4.25 m. A similar pattern was revealed earlier, in August 2009 (unpublished data). Variations of the basic physicochemical parameters of stratified water layers were mainly related to depth. These trends persisted both during the day and at night. The day-night dynamics of the basic physicochemical parameters are also characteristic of other ecosystems (deserts, oceans, and seas) (Ottesen et al., 2014; Andrade et al., 2015; Shilova et al., 2016; Gunnigle et al., 2017).
It is interesting to note that the stratified conditions in Lake Doroninskoe were characterized by a different direction of processes associated with sulfur behavior at day compared to night. In the daytime, processes of sulfur reduction (S0→S0S4+→S2-) were the most pronounced, while at night, its oxidation (S2-→S0→S0S4+). During the day, there was a pronounced reverse relationship between the elemental (S0) and thiosulfate (S0S4+) forms of sulfur and oxygen, the oxidation-reduction potential and a direct correlation with depth and salinity. At night, the hydrosulfide (HS-) and elemental (S0) forms of sulfur weakly correlated with salinity, while thiosulfate (S0S4+) sulfur correlated with depth.
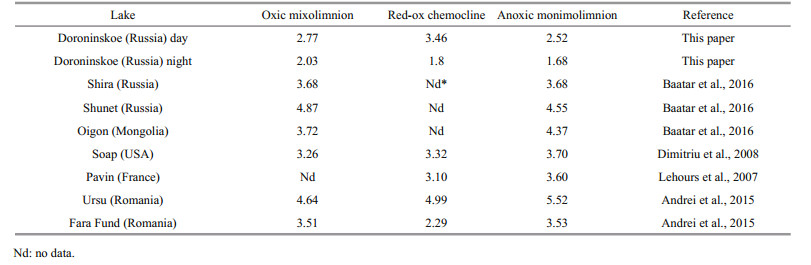
The values of Shannon indices from the stratified mixolimnion and monimolimnion layers revealed that they were higher in the oxic zone then anoxic ones (Table 4). A significant part of the study of meromictic ecosystems pertains to daytime studies. In some ecosystems of previously studied meromictic lakes, such as Pavin (Lehours et al., 2007), Soap Lake (Dimitriu et al., 2008), Ursu (Andrei et al., 2015), and Oigon (Baatar et al., 2016), a higher bacterial diversity in the anoxic layer was noted, while in the lakes Shunet, Shira (Baatar et al., 2016) and Fara Fund (Andrei et al., 2015), the Shannon index was either higher in the oxic zone or similar in the both zones.
|
Daytime microbial communities revealed higher diversity, were differentiated and layer-specific. Moreover, microbial communities of the upper oxygenated layers of the mixolimnion had the most pronounced horizon-specificity. The core of the microbial community in the daytime was made up of bacteria with various types of metabolism, from photo- and heterotrophy to nitrate and metal reduction, with a high proportion of bacteria possessing alternative photosynthetic strategies or unidentified ones. The night microbial community was characterized by both lower diversity and insignificant depth stratification. In this community, the proportion of heterotrophic bacteria with denitrification as a key function was increased. Interestingly, a high content of cyanobacteria was detected at night. A similar result was previously noted in day-night studies of marine microbial communities (Gilbert et al., 2010), and might be associated with a nightly increase in cyanobacteria cell division. Thus, it can be assumed that an increase in the diversity of microbial communities during the day may be associated with metabolite production that promotes a variety of niches through the maintenance of various sources of nutrients, not only in the illuminated zone, but also deeper, as well as the existence of multiple parallel metabolic pathways that continue to act over time. For each trophic step, a functionally diverse microbial community represented a set of parallel pathways and systems with a more reliable function over time (Hashsham et al., 2000). Moreover, a balance in the structure of microbial diversity ensures that the community has more opportunities to use its diverse set of metabolic pathways and that the microbial community has a more robust function with greater evenness (Wittebolle et al., 2009). An oxygen-free environment has the ability to support a wider range of energy generation pathways than oxygen-rich ones, leading to a higher ecological diversity, combined with lower interspecific competition (Humayoun et al., 2003). It is interesting to note that the night microbial community from the oxygen-free environment of the Lake Doroninskoe monimolimnion was characterized by higher bacterial richness compared with the oxic mixolimnion.
We assumed that the day-night changes in both taxonomic and functional diversity microbial communities of the lake might be related to environmental factors. It was found that during day in mixolimnion in the zone of a sharp decrease of salinity, the reduction of richness and diversity indices was observed. However, in the studies of other authors (Andrei et al., 2015), a smooth, rather than an abrupt decrease in bacterial diversity with increasing salinity and depth was found. The day-night link of phylogenetic community structure with environmental gradients showed that such ecological factors as salinity and depth do not form a cluster with night and daytime microbial communities from oxic layers of mixolimnion and redox zones. At the same time, these microbial communities possessed the highest diversity indicators, despite the fact that according to the research of other authors (Lozupone and Knight, 2007; Wang et al., 2011), salinity is the major factor relating microbial communities. In the community there may be additional regulation or compensation mechanisms that can lead to acceleration or deceleration of the biological response to an external factor. However, the data obtained do not allow us to draw any meaningful conclusions about the influence of environmental factors on day-night changes in bacterial diversity. Our findings are in accordance with the discussion of Gunnigle and coauthors, who studied desert ecosystems (Gunnigle et al., 2017). They did not associate variability in richness and diversity of microbial communities with environmental factors.
The analysis revealed day-night shifts in dominant taxa. It was found that bacteria of the families Enterobacteriaceae (class Gammaproteobacteria) and Alcaligenaceae (class Betaproteobacteria) were dominant in Lake Doroninskoe during both day and night. These bacteria are known as denitrifying microorganisms (Imhoff, 2016). They are facultative aerobic bacteria, which switch to denitrification in the absence of oxygen. Their denitrification ability is associated with the use of oxygen as the terminal electron acceptor. Under anaerobic conditions, these bacteria used nitrate as a terminal electron acceptor. They display different reactions to different environmental conditions and occupy a variety of ecological niches (Imhoff, 2016). Some representatives of denitrifying bacteria, such as Serratia (Thorpe et al., 2012), might be dominant in the microbial community under conditions of a sharp change in environmental parameters and the high availability of electron donors and acceptors. Some denitrifying bacteria are capable of anaerobic oxidation of thiosulfate and sulfide during denitrification. Clarification of the functional role of denitrification in the lake is a task for future studies.
5 CONCLUSIONThis is the first study of short-term dynamics of the structure and diversity of microbial communities of the meromictic soda Lake Doroninskoe. High taxonomic diversity of day-night microbial communities formed under extreme environmental conditions and in stable water stratification was shown. The study confirmed that, both in the largescale (inter-annual and inter-seasonal) and in shortterm periods, Lake Doroninskoe is a dynamic extreme system with a wide variety of niches, and is supported by high phylogenetic and functional diversity of microbial communities. The microbial community of the lake is dominated by bacteria that use a variety of alternative acceptors and electron donors under extreme conditions, which is indicative of a complex and heterogeneous environment in the lake. These results may indicate the relationship between environmental parameters and the lake microbial community as well as the complex system of response of the microbial community in time.
6 ACKNOWLEDGEMENTThis research study was conducted according to the project Ⅸ.137.1.1 "Biodiversity of natural and natural-technogenic ecosystems of Transbaikalia (Cenrtal Asia) as indicators of regional climate changes" (AAAA-A17-117011210078-9).
Andrade K, Logemann J, Heidelberg K B, Emerson J B, Comolli L R, Hug LA, Probst A J, Keillar A, Thomas B C, Miller C S, Allen E E, Moreau J W, Brocks J J, Banfield J F. 2015. Metagenomic and lipid analyses reveal a diel cycle in a hypersaline microbial ecosystem. ISME Journal, 9(12): 2 697-2 711.
DOI:10.1038/ismej.2015.66 |
Andrei A Ş, Robeson II M S, Baricz A, Coman C, Muntean V, Ionescu A, Etiope G, Alexe M, Sicora C I, Podar M, Banciu H L. 2015. Contrasting taxonomic stratification of microbial communities in two hypersaline meromictic lakes. ISME Journal, 9(12): 2 642-2 656.
DOI:10.1038/ismej.2015.60 |
Baatar B, Chiang P W, Rogozin D Y, Wu Y T, Tseng C H, Yang C Y, Chiu H H, Oyuntsetseg B, Degermendzhy A G, Tang S L. 2016. Bacterial communities of three saline meromictic lakes in Central Asia. PLoS One, 11(3): e0150847.
DOI:10.1371/journal.pone.0150847 |
Borzenko S V, Zamana L V, Buryukhaev S P. 2014. The isotopic composition of dissolved carbonates as a reflection of abiogenic and biogenic processes in the water column of the Lake Doroninskoe. Razvitie zhizni v protsesse abioticheskikh izmenenij na Zamle, 3: 319-323.
|
Borzenko S V, Zamana L V, Noskova E V. 2015. Meromixis of the Lake Doroninskoye (Eastern Transbaikalia). Geological and Mineralogical sciences, 1: 420-425.
|
Borzenko S V, Zamana L V. 2011. Reduced forms of sulfur in the brine of saline-soda Lake Doroninskoe, eastern Transbaikal region. Geochemistry International, 49(3): 253-261.
DOI:10.1134/S0016702911030037 |
Bosshard P P, Santini Y, Grüter D, Stettler R, Bachofen R. 2000. Bacterial diversity and community composition in the chemocline of the meromictic alpine Lake Cadagno as revealed by 16S rDNA analysis. FEMS Microbiology Ecology, 31(2): 173-182.
DOI:10.1111/fem.2000.31.issue-2 |
Comeau A M, Harding T, Galand P E, Vincent W F, Lovejoy C. 2012. Vertical distribution of microbial communities in a perennially stratified Arctic lake with saline, anoxic bottom waters. Scientific Reports, 2: 604.
DOI:10.1038/srep00604 |
Dimitriu P A, Pinkart H C, Peyton B M, Mormile M R. 2008. Spatial and temporal patterns in the microbial diversity of a meromictic soda lake in Washington State. Applied and Environmental Microbiology, 74(15): 4 877-4 888.
DOI:10.1128/AEM.00455-08 |
Dvornyk V, Vinogradova O, Nevo E. 2003. Origin and evolution of circadian clock genes in prokaryotes. Proceedings of the National Academy of Sciences of the United States of America, 100(5): 2 495-2 500.
DOI:10.1073/pnas.0130099100 |
Edgar R C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10(10): 996-998.
DOI:10.1038/nmeth.2604 |
Fadrosh D W, Ma B, Gajer P, Sengamalay N, Ott S, Brotman R M, Ravel J. 2014. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome, 2: 6.
DOI:10.1186/2049-2618-2-6 |
Falagán C, Sánchez-España J, Johnson D B. 2014. New insights into the biogeochemistry of extremely acidic environments revealed by a combined cultivation-based and culture-independent study of two stratified pit lakes. FEMS Microbiology Ecology, 87(1): 231-243.
DOI:10.1111/1574-6941.12218 |
Frigaard N U, Dahl C. 2008. Sulfur metabolism in phototrophic sulfur bacteria. Advances in Microbial Physiology, 54: 103-200.
DOI:10.1016/S0065-2911(08)00002-7 |
Garcia S L, Salka I, Grossart H P, Warnecke F. 2013. Depthdiscrete profiles of bacterial communities reveal pronounced spatio-temporal dynamics related to lake stratification. Environmental Microbiology Reports, 5(4): 549-555.
DOI:10.1111/1758-2229.12044 |
Garcia-Gil L J, Casamitjana X, Abella C A. 1996. Comparative study of two meromictic basins of Lake Banyoles (Spain) with sulphur phototrophic bacteria. Hydrobiologia, 319(3): 203-211.
DOI:10.1007/BF00013733 |
Gifford S M, Sharma S, Moran M A. 2014. Linking activity and function to ecosystem dynamics in a coastal bacterioplankton community. Frontiers in Microbiology, 5: 185.
|
Gilbert J A, Field D, Swift P, Thomas S, Cummings D, Temperton B, Weynberg K, Huse S, Hughes M, Joint I, Somerfield P J, Mühling M. 2010. The taxonomic and functional diversity of microbes at a temperate coastal site: a 'Multi-Omic' study of seasonal and diel temporal variation. PLoS One, 5(11): e15545.
DOI:10.1371/journal.pone.0015545 |
Gorlenko V M, Buryukhaev S P, Matyugina E B, Borzenko S V, Namsaraev Z B, Bryantseva I A, Boldareva E N, Sorokin D Y, Namsaraev B B. 2010. Microbial communities of the stratified soda Lake Doroninskoe (Transbaikal region). Microbiology, 79(3): 390-401.
DOI:10.1134/S0026261710030161 |
Gregersen L H, Habicht K S, Peduzzi S, Tonolla M, Canfield D E, Miller M, Cox R P, Frigaard N U. 2009. Dominance of a clonal green sulfur bacterial population in a stratified lake. FEMS Microbiology Ecology, 70(1): 30-41.
|
Gulati R D, Zadereev E S, Degermendzhi A G. 2017. Ecology of Meromictic Lakes. Springer, Cham. 405p.
|
Gunnigle E, Frossard A, Ramond J B, Guerrero L, Seely M, Cowan D A. 2017. Diel-scale temporal dynamics recorded for bacterial groups in Namib Desert soil. Scientific Reports, 7: 40 189.
DOI:10.1038/srep40189 |
Habicht K S, Miller M, Cox R P, Frigaard N U, Tonolla M, Peduzzi S, Falkenby LG, Andersen J S. 2011. Comparative proteomics and activity of a green sulfur bacterium through the water column of Lake Cadagno, Switzerland. Environmental Microbiology, 13(1): 203-215.
DOI:10.1111/emi.2011.13.issue-1 |
Hamilton T L, Bovee R J, Sattin S R, Mohr W, Gilhooly III W P, Lyons T W, Pearson A, Macalady J L. 2016. Carbon and sulfur cycling below the chemocline in a meromictic lake and the identification of a novel taxonomic lineage in the FCB Superphylum, Candidatus Aegiribacteria. Frontiers in Microbiology, 7: 598.
|
Hashsham S A, Fernandez A S, Dollhopf S L, Dazzo F B, Hickey R F, Tiedje J M, Criddle C S. 2000. Parallel processing of substrate correlates with greater functional stability in methanogenic bioreactor communities perturbed by glucose. Applied and Environmental Microbiology, 66(9): 4 050-4 057.
DOI:10.1128/AEM.66.9.4050-4057.2000 |
Hewson I, Poretsky R S, Tripp H J, Montoya J P, Zehr J P. 2010. Spatial patterns and light-driven variation of microbial population gene expression in surface waters of the oligotrophic open ocean. Environmental Microbiology, 12(27): 1 940-1 956.
|
Humayoun S B, Bano N, Hollibaugh J T. 2003. Depth distribution of microbial diversity in Mono Lake, a meromictic soda lake in California. Applied and Environmental Microbiology, 69(2): 1 030-1 042.
|
Hut R A, Beersma D G M. 2011. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1574): 2 141-2 154.
DOI:10.1098/rstb.2010.0409 |
Imhoff J F. 2016. New dimensions in microbial ecology – functional genes in studies to unravel the biodiversity and role of functional microbial groups in the environment. Microorganisms, 4(2): 19.
DOI:10.3390/microorganisms4020019 |
Johnson D B, Hallberg K B. 2009. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Advances in Microbial Physiology, 54: 201-255.
|
Koizumi Y, Kojima H, Fukui M. 2004a. Dominant microbial composition and its vertical distribution in saline meromictic Lake Kaiike (Japan) as revealed by quantitative oligonucleotide probe membrane hybridization. Applied and Environmental Microbiology, 70(8): 4 930-4 940.
DOI:10.1128/AEM.70.8.4930-4940.2004 |
Koizumi Y, Kojima H, Oguri K, Kitazato H, Fukui M. 2004b. Vertical and temporal shifts in microbial communities in the water column and sediment of saline meromictic Lake Kaiike (Japan), as determined by a 16S rDNA-based analysis, and related to physicochemical gradients. Environmental Microbiology, 6(6): 622-637.
DOI:10.1111/emi.2004.6.issue-6 |
Lanzén A, Simachew A, Gessesse A, Chmolowska D, Jonassen I, Øvreås L. 2013. Surprising prokaryotic and eukaryotic diversity, community structure and biogeography of Ethiopian Soda Lakes. PLoS One, 8(8): e72577.
DOI:10.1371/journal.pone.0072577 |
Lauro F M, De Maere M Z, Yau S, Brown M V, Ng C, Wilkins D, Raftery M J, Gibson J A, Andrews-Pfannkoch C, Lewis M, Hoffman J M, Thomas T, Cavicchioli R. 2011. An integrative study of a meromictic lake ecosystem in Antarctica. ISME Journal, 5(5): 879-895.
DOI:10.1038/ismej.2010.185 |
Leboulanger C, Agogué H, Bernard C, Bouvy M, Carré C, Cellamare M, Duval C, Fouilland E, Got P, Intertaglia L, Lavergne C, Le Floc'h E, Roques C, Sarazin G. 2017. Microbial diversity and cyanobacterial production in Dziani Dzaha crater Lake, a unique tropical thalassohaline environment. PLoS One, 12(1): e0168879.
DOI:10.1371/journal.pone.0168879 |
Lehours A C, Bardot C, Thenot A, Debroas D, Fonty G. 2005. Anaerobic microbial communities in Lake Pavin, a unique meromictic Lake in France. Applied and Environmental Microbiology, 71(11): 7 389-7 400.
DOI:10.1128/AEM.71.11.7389-7400.2005 |
Lehours A C, Evans P, Bardot C, Joblin K, Gérard F. 2007. Phylogenetic diversity of archaea and bacteria in the anoxic zone of a meromictic lake (Lake Pavin, France). Applied and Environmental Microbiology, 73(6): 2 016-2 019.
DOI:10.1128/AEM.01490-06 |
Lindström E S, Langenheder S. 2012. Local and regional factors influencing bacterial community assembly. Environmental Microbiology Reports, 4(1): 1-9.
DOI:10.1111/j.1758-2229.2011.00257.x |
Lozupone C A, Knight R. 2007. Global patterns in bacterial diversity. Proceedings of the National Academy of Sciences of the United States of America, 104(27): 11 436-11 440.
DOI:10.1073/pnas.0611525104 |
Matyugina E B, Borzenko S V, Matafonov P V, Belkova N L. 2014. A laboratory experiment for meromixis in an integrated sample of soda Lake Doroninskoye (Transbaikalia). Current Research in Microbiology and Biotechnology, 2(3): 398-401.
|
Matyugina E, Belkova N. 2015. Distribution and diversity of microbial communities in meromictic soda Lake Doroninskoe (Transbaikalia, Russia) during winter. Chinese Journal of Oceanology and Limnology, 33(6): 1 378-1 390.
DOI:10.1007/s00343-015-4355-8 |
Meuser J E, Baxter B K, Spear J R, Peters J W, Posewitz M C, Boyd E S. 2013. Contrasting patterns of community assembly in the stratified water column of Great Salt Lake, Utah. Microbial Ecology, 66(2): 268-280.
DOI:10.1007/s00248-013-0180-9 |
Oksanen J, Blanchet F G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin P R, O'Hara R B, Simpson G L, Solymos P, Stevens M H H, Szoecs E, Wagner H. 2018. Vegan: Community Ecology Package.
|
Ottesen E A, Young C R, Gifford S M, Eppley J M, Marin III R, Schuster S C, Scholin C A, DeLong E F. 2014. Ocean microbes. Multispecies diel transcriptional oscillations in open ocean heterotrophic bacterial assemblages. Science, 345(6193): 207-212.
DOI:10.1126/science.1252476 |
Overmann J, Beatty J T, Hall K J, Pfennig N, Northcote T G. 1991. Characterization of a dense, purple sulfur bacterial layer in a meromictic salt lake. Limnology and Oceanography, 36(5): 846-859.
DOI:10.4319/lo.1991.36.5.0846 |
Poretsky R S, Hewson I, Sun S L, Allen A E, Zehr J P, Moran M A. 2009. Comparative day/night metatranscriptomic analysis of microbial communities in the North Pacific subtropical gyre. Environmental Microbiology, 11(6): 1 358-1 375.
DOI:10.1111/emi.2009.11.issue-6 |
R Development Core Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
|
Robertson C E, Harris J K, Wagner B D, Granger D, Browne K, Tatem B, Feazel L M, Park K, Pace N R, Frank D N. 2013. Explicet: Graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics, 29(23): 3 100-3 101.
DOI:10.1093/bioinformatics/btt526 |
Shilova I N, Robidart J C, DeLong E F, Zehr J P. 2016. Genetic diversity affects the daily transcriptional oscillations of marine microbial populations. PLoS One, 11(1): e0146706.
DOI:10.1371/journal.pone.0146706 |
Silveira C B, Gregoracci G B, Coutinho F H, Silva G G Z, Haggerty J M, de Oliveira L S, Cabral A S, Rezende C E, Thompson C C, Francini-Filho R B, Edwards R A, Dinsdale E A, Thompson F L. 2017. Bacterial community associated with the reef coral Mussismilia braziliensis's momentum boundary layer over a diel cycle. Frontiers in Microbiology, 8: 784.
DOI:10.3389/fmicb.2017.00784 |
Sorokin D Y, Foti M, Pinkart H C, Muyzer G. 2007. Sulfuroxidizing bacteria in Soap Lake (Washington State), a meromictic, haloalkaline lake with an unprecedented high sulfide content. Applied and Environmental Microbiology, 73(2): 451-455.
DOI:10.1128/AEM.02087-06 |
Sorokin D Y, Gorlenko V M, Tourova T P, Tsapin A I, Nealson K H, Kuenen G J. 2002. Thioalkalimicrobium cyclicum sp. nov. and Thioalkalivibrio jannaschii sp. nov., novel species of haloalkaliphilic, obligately chemolithoautotrophic sulfur-oxidizing bacteria from hypersaline alkaline Mono Lake (California). International Journal of Systematic and Evolutionary Microbiology, 52(3): 913-920.
|
Tagkopoulos I, Liu Y C, Tavazoie S. 2008. Predictive behavior within microbial genetic networks. Science, 320(5881): 1 313-1 317.
DOI:10.1126/science.1154456 |
Thorpe C L, Morris K, Boothman C, Lloyd J R. 2012. Alkaline Fe(Ⅲ) reduction by a novel alkali-tolerant Serratia sp. isolated from surface sediments close to Sellafield nuclear facility, UK. FEMS Microbiology Letters, 327(2): 87-92.
DOI:10.1111/fml.2012.327.issue-2 |
Tonolla M, Peduzzi R, Hahn D. 2005. Long-term population dynamics of phototrophic sulfur bacteria in the chemocline of Lake Cadagno, Switzerland. Applied and Environmental Microbiology, 71(7): 3 544-3 550.
DOI:10.1128/AEM.71.7.3544-3550.2005 |
Vila-Costa M, Sharma S, Moran M A, Casamayor E O. 2013. Diel gene expression profiles of a phosphorus limited mountain lake using metatranscriptomics. Environmental Microbiology, 15(4): 1 190-1 203.
DOI:10.1111/emi.2013.15.issue-4 |
Volkov I I, Zhabina N N. 1990. Method of determination of reduced sulfur-compounds in the sea-water. Okeanologiya, 30(5): 778-782.
|
Wang J J, Yang D M, Zhang Y, Shen J, van der Gast C, Hahn M W, Wu Q L. 2011. Do patterns of bacterial diversity along salinity gradients differ from those observed for macroorganisms?. PLoS One, 6(11): e27597.
DOI:10.1371/journal.pone.0027597 |
Wang Q, Garrity G M, Tiedje J M, Cole J R. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73(16): 5 261-5 267.
DOI:10.1128/AEM.00062-07 |
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, de Vos P, Verstraete W, Boon N. 2009. Initial community evenness favours functionality under selective stress. Nature, 458(7238): 623-626.
DOI:10.1038/nature07840 |
Zamana L V, Borzenko S V. 2007. Hydrogen sulfide and other reduced forms of sulfur in oxic waters of Lake Doroninskoe, Eastern Transbaikalia. Doklady Earth Sciences, 417(1): 1 268-1 271.
DOI:10.1134/S1028334X07080314 |
Zerkle A L, Kamyshny A Jr, Kump L R, Farquhar J, Oduro H, Arthur M A. 2010. Sulfur cycling in a stratified euxinic lake with moderately high sulfate: constraints from quadruple S isotopes. Geochimica et Cosmochimica Acta, 74(17): 4 953-4 970.
DOI:10.1016/j.gca.2010.06.015 |
Zhang F, Vicente J, Hill R T. 2014. Temporal changes in the diazotrophic bacterial communities associated with Caribbean sponges Ircinia stroblina and Mycale laxissima. Frontiers in Microbiology, 5: 561.
|
Zhong Z P, Liu Y, Miao L L, Wang F, Chu L M, Wang J L, Liu Z P. 2016. Prokaryotic community structure driven by salinity and ionic concentrations in Plateau Lakes of the Tibetan plateau. Applied and Environmental Microbiology, 82(6): 1 846-1 858.
DOI:10.1128/AEM.03332-15 |
 2018, Vol. 36
2018, Vol. 36


