Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MCCLOUD Clayton Leigh, ISMAIL Hasnun Nita, SEURONT Laurent
- Cue hierarchy in the foraging behaviour of the brackish cladoceran Daphniopsis australis
- Chinese Journal of Oceanology and Limnology, 36(6): 2050-2060
- http://dx.doi.org/10.1007/s00343-018-7335-y
Article History
- Received Jan. 19, 2018
- accepted in principle Mar. 13, 2018
- accepted for publication Jul. 6, 2018
2 School of Biology, Universiti Teknologi MARA, Selangor, Malaysia;
3 CNRS, Univ. Lille, Univ. Littoral Côte d'Opale, UMR 8187, LOG, Laboratoire d'Océanologie et de Géosciences, F 62930 Wimereux, France
Cladocerans are an integral component of the planktonic and benthic crustacean fauna in freshwater ecosystems such as lakes and ponds (Mergeay et al., 2006). These organisms are the primary herbivores of these ecosystems, eating algae and bacteria and contributing to the recycling of nutrients in the water column (Dodson and Frey, 2001). They also provide a large proportion of the diets of planktivorous fish and invertebrates (Brancelj et al., 2012; Bledzki and Rybak, 2016). Cladocerans are subject to both bottomup and top-down forces, hence play an essential role in carbon and energy transfer through the food web (Brancelj et al., 2012; Bledzki and Rybak, 2016). The interactions of cladocerans with their biotic and abiotic environment at micro-scales (i.e. typically < 1 m) have often been overlooked, but can influence the structure of entire ecosystems. As stressed in the context of the behavioural ecology of freshwater and marine copepods, swimming behaviour has increasingly been considered as a stepping-stone to explain phenomena ranging from individual encounter rates to population dynamics (Kiørboe, 2008; Seuront, 2015a).
The swimming behaviour of cladocerans, in particular Daphnia sp. (i.e. the so-called water flea), has received a great deal of attention (e.g. Brewer, 1998; Seuront et al., 2004a, b; Garcia et al., 2007; Da S. Ferrão Filho et al., 2008; Nihongi et al., 2011, 2016; Ziarek et al., 2011; La et al., 2014; Uttieri et al., 2014; Hinow et al., 2015). The regular beating of the second set of antennae enables these crustaceans to swim with characteristic hops (Dees et al., 2008). Daphnia swimming behaviour is dependent on body size (Dodson and Ramcharan, 1991) and may also be affected by various factors such as light, water temperature, salinity, and the presence of both food and predators (Baylor and Smith, 1953; O'Keefe et al., 1998; Hamza and Ruggiu, 2000; Ziarek et al., 2011). Noticeably, the alteration of Daphnia swimming behaviour under various conditions of chronic water contamination is now acknowledged as one of the most sensitive biomarkers in toxicity assessment; see Bownik (2017) for a review.
In contrast, the behaviour of the genus Daphniopsis (Cladocera: Daphniidae; Benzie, 2005) is still unknown despite its global distribution in the inland saline waters of Asia (Sars, 1903), Australia (Sergeev and Williams, 1985), North America (Schwartz and Hebert, 1987) and South America (Hann, 1986). More specifically, D. australis (Sergeev and Williams, 1985) is endemic to South-eastern Australia (Hebert and Wilson, 2000), where it is commonly found in ephemeral saline lakes and swamps for salinity ranging from 4 to 30 (Sergeev and Williams, 1985). The occurrence of D. australis is highly seasonal, with high and low abundances respectively observed in spring and winter, while it is undetectable during summer and autumn (Campbell, 1994). Previous studies on D. australis have essentially focused on their systematics (Colbourne et al., 2006), morphology (Aladin, 1991), diversity (Hebert and Wilson, 2000), seasonal dynamics (Campbell, 1994), biogeography (Timms, 2007), osmoregulation (Aladin and Potts, 1995), thermal and halo tolerance (Aladin, 1991; Ismail et al., 2010a), reproductive biology (Ismail et al., 2010b), and life history as a function of biotic parameters (food quality; Ismail et al., 2011a) and abiotic parameters such as temperature and salinity (Ismail et al., 2011b, c).
As all cladocerans, D. australis females reproduce sexually (epiphial female), but when conditions are favourable, asexual reproduction occurs via parthenogenesis. Males and epiphial females are produced by parthenogenetic females when the environment deteriorates due to e.g. overcrowding, starvation or anoxy (Dodson and Frey, 2001). Epiphial females produce resting eggs that are capable of surviving harsh conditions such as summer droughts and winter frost (Schwartz and Hebert, 1987). D. australis is one species that has been successful in adapting to the extreme temperature and salinity of the Australian climate. However, despite their potential role in the structure and function of South Australian inland water ecosystems, there have been few ecological studies on this genus and none on this particular species. In this context, the objectives of this study were to quantify the swimming behaviour of D.australis males and females (both parthenogenetic and epiphial) in the absence and presence of cues from food and conspecifics to assess if this species has comparable sensory abilities to detect food items, and conspecifics using chemical and/or mechanical cues than the well studied Daphnia sp. and freshwater and marine copepods, and the subsequent ability to modify their swimming behaviour accordingly (e.g. Brewer, 1998; Nihongi et al., 2011; La et al., 2014; Uttieri et al., 2014; Nihongi et al., 2016).
2 MATERIAL AND METHOD 2.1 Daphniopsis australis cultureIndividuals of D. australis were cultured in 20-L containers, and fed daily Isochrysis affinis galbana (Tahiti isolate, obtained from the Australian National Algae Culture Collection, CSIRO, Hobart, Tasmania) at a density of 106 cells/mL, which is in the optimal range reported for most Daphniidae species (Delbare and Dhert, 1996). The continuous culture was maintained under constant conditions of temperature (22℃) and salinity (22) and kept on a 12-h light/12-h dark cycle with an irradiance of 300 W/m2 (Light Intensity Recorder MDS-MkV, Alec Electronics Inc., Kobe, Japan). In order to avoid overcrowding and population crash, the population density was controlled at less than 1 000 ind./L in the stock culture (Ismail et al., 2010a, b).
2.2 Experimental design and behavioural observationsOne control and six different water treatments were used to provide D. australis with a hierarchy of cues (Table 1). Control artificial seawater was made with milli-Q water and Instant Ocean Sea Salt (Instant Ocean®, Blacksburg, VA, USA) to 22. Treatment solutions based on I. affinis galbana culture (hereafter Isochrysis culture) and D. australis culture (hereafter Daphniopsis culture) were made by diluting Isochrysis and Daphniopsis cultures with artificial seawater at a ratio of 1:1. Isochrysis culture water contained both I. affinis galbana cells and their exudates. Similarly, Daphniopsis culture water contained both D. australis and their specific chemicals, and I. affinis galbana cells and their exudates. We also created pheromone-conditioned water incubating males, parthenogenetic females and epiphial females in control seawater during 24 h at a concentration of 20 individuals per litre (Seuront and Stanley, 2014). Cues were subsequently hierarchized through behavioural experiments conducted using filtered and unfiltered Isochrysis and Daphniopsis culture waters, and both pheromone-conditioned seawater in the presence and absence of conditioning individuals (Table 1). All filtrations were conducted through Isopore membranefi lters (Millipore; 0.8 μm porosity) using a vacuum air pump. All water types used in the experiments were maintained at the temperature and salinity of the cultures (i.e. 22℃ and 22). All water treatments made from Isochrysis and Daphniopsis cultures were prepared on the same day to avoid any bias related to putative cumulative effects of both I. affinis galbana and D. australis exudation and excretion.
|
Individual D. australis were collected from the culture using a wide-tip pipette to prevent physical damage to the organisms and identified under a dissecting microscope to determine sex and female stage (Fig. 1). Males were smaller (1.00-1.30 mm), whilst both parthenogenetic and epiphial females sizes were similar and ranged between 1.60-2.16 mm and 1.68-2.25 mm, respectively. The three adult types were then separated into 250 mL beakers filled with control seawater, and placed in a culture cabinet (22℃) until used in the video experiments. All video recordings of freely swimming D. australis occurred within one hour after the organisms were taken from the culture. For each adult type (n=3) and water treatment (n=7, including control), 3 individuals were transferred into a 3 375-mL (i.e. 15 cm×15 cm×15 cm) glass behavioural container, placed inside the culture cabinet (22℃) and allowed to acclimatise for 15 min (Seuront, 2006, 2013). This was replicated 13 times for each sex, under control conditions in the absence of any cues, and under each of the six treatment types (i.e. 21 treatments replicated 13 times). Sex and treatments were randomized (Seuront, 2006). Threedimensional trajectories of D. australis were recorded at a rate of 25 frame/s using two synchronized infrared digital cameras (Sony Handycam; DCR-PC120E) placed orthogonally and each facing one side of the experimental container. Six arrays of 72 infrared lightemitting diodes provided the only light source from the bottom of the experimental container (Seuront, 2013). Note that because infrared light-emitting diodes generate heat from the bottom of the experimental container, we gently ventilated the culture cabinet during the behavioural experiments to prevent the formation of a temperature gradient. This was further controlled through measurements of the temperature of the cabinet, and the temperature of the bottom and surface waters of the experimental container at the beginning and the end of the experiments. No significant changes in temperature were ever recorded.

|
| Fig.1 D. australis male (a), epiphial female (b) and parthenogenetic female (c) The dashed white bars indicate how the size of individuals was measured, and the black scale bar indicates 0.5 mm. |
Each behavioural experiment lasted 30 min, after which the resulting 273 video recordings were transferred to a computer, and (x, y, z) coordinates of D. australis were subsequently automatically extracted using LabTrack software (DiMedia, Kvistgård, Denmark). All behavioural analyses were based on trajectories in which D. australis individuals were swimming at least two body lengths away from a conspecific, and any chamber walls or the surface of the water (Seuront, 2006, 2013). The experiments were consistently run under the same conditions of temperature (22℃) and salinity (22) in the dark and at night (between 22:00 and 02:00) to avoid any potential behavioural artifact related to the diel cycle of D. australis (Seuront, 2011). To work on statistically consistent swimming paths, paths of similar durations (i.e. 55-60 s) were selected according to the abovementioned criteria, and the same number (n=25) of swimming paths was considered for the behavioural analysis of each experimental conditions, i.e. a total of 525 paths.
Daphniopsis australis motion behaviour was subsequently quantified using three metrics widely used in zooplankton behavioural studies: swimming speed, net-to-gross displacement ratio (NGDR) and swimming activity index; see Seuront et al. (2004c) for a review. The maximum speed was also determined. The distance d (mm) between two points in a threedimensional space was computed from the (x, y, z) coordinates as d=((xt+1-xt)2+(yt+1-yt)2(zt+1-zt)2)1/2, where (xt, yt, zt) and (xt+1, yt+1, zt+1) are the positions of a D. australis individual at time t and t+1, respectively. The swimming speed v (mm/s) was subsequently calculated as v=df, where f is the sampling rate of the camera, i.e. f=25 frame/s. The net-to-gross displacement ratio (NGDR) was calculated as NGDR=ND/GD, where ND (mm) is the net displacement of a D. australis or the shortest distance between starting and ending points and GD (mm) is the gross displacement of a D. australis or the actual distance travelled between starting and ending points. The NGDR describes the linearity of D. australis swimming paths, with a high ratio relating to a straight path and a low NGDR relating to a curvy trajectory (Buskey, 1984). The activity of D. australis was estimated using an activity index Ai defined as Ai=100×ttrack/tswim, where ttrack and tswim are the duration of a swimming path and the time D. australis spent swimming, respectively.
2.4 Statistical analysisNon-parametric statistics were used throughout this work as criteria for normality and homogeneity of variance were not met. Comparisons between sexes and treatments were conducted using the Kruskal-Wallis test (KW test hereafter), and when appropriate were followed by a multiple comparison procedure based on the Tuckey test (Zar, 2010) to identify significant differences between sexes and cue treatments. All statistical tests were run using homemade Fortran routines coded from the procedures described in Zar (2010).
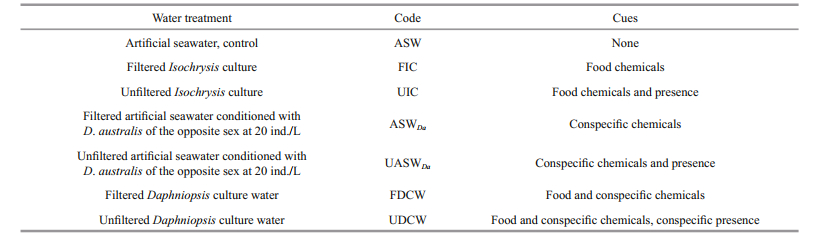
3 RESULT 3.1 Swimming behaviour of Daphniopsis australis in the absence of cuesExemplars of D. australis two-dimensional swimming trajectories are shown in Fig. 2. Each sex displayed irregular and erratic motions including strong jumps. Males displayed the most extensive style of swimming, exploring all areas of the container, and swimming in a series of relatively straight trajectories (Fig. 2a). In contrast, females typically exhibited a hop-and-sink motion characterised by the alternation between short bursts of swimming and sinking phases. Both types of females spent long periods near the bottom of the container, but the epiphial females (Fig. 2b) appeared to be more active than parthenogenetic ones who rarely made an excursion in the water column (Fig. 2c).
|
| Fig.2 Two-dimensional projections of the typical swimming behaviour exhibited by a male (a), epiphial female (b) and parthenogenetic female (c) of the cladoceran D. australis in a 150 mm×150 mm×150 mm experimental container The three trajectories shown here all have a duration of 60 s. |
More specifically, D. australis swimming speed significantly differs between sexes (KW test, P < 0.05). A subsequent Tuckey test showed that epiphial females swam significantly faster (7.35±0.26 mm/s; x±SD) than both males (6.38±0.26 mm/s) and parthenogenetic females (5.73±0.44 mm/s). Note, however, that the maximum swimming speed reached by males was significantly higher (P < 0.05; 92± 5.8 mm/s) than those observed for both parthenogenetic females (57±5.5 mm/s) and epiphial females (63±3.9 mm/s), which could not be statistically distinguished (P > 0.05). Finally, both the net-to-gross displacement ratio and the activity index Ai significantly differed between all sexes (P < 0.05). Specifically, males swam following significantly more linear trajectories (NGDR=0.13±0.07) than epiphial females (NGDR=0.07±0.02) and parthenogenetic females (NGDR=0.03±0.01). Epiphial females were the most active (Ai =87.2%±3.3%), followed by parthenogenetic females (71.7%±5.2%) and males (60.2%±4.0%).
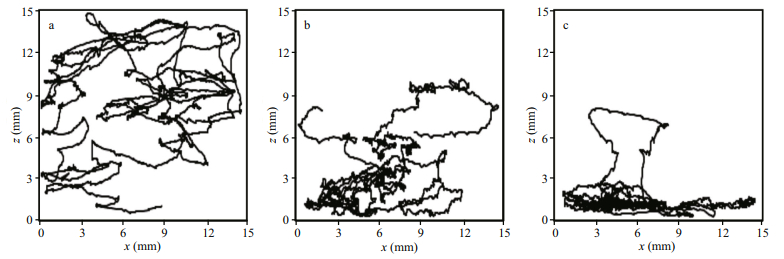
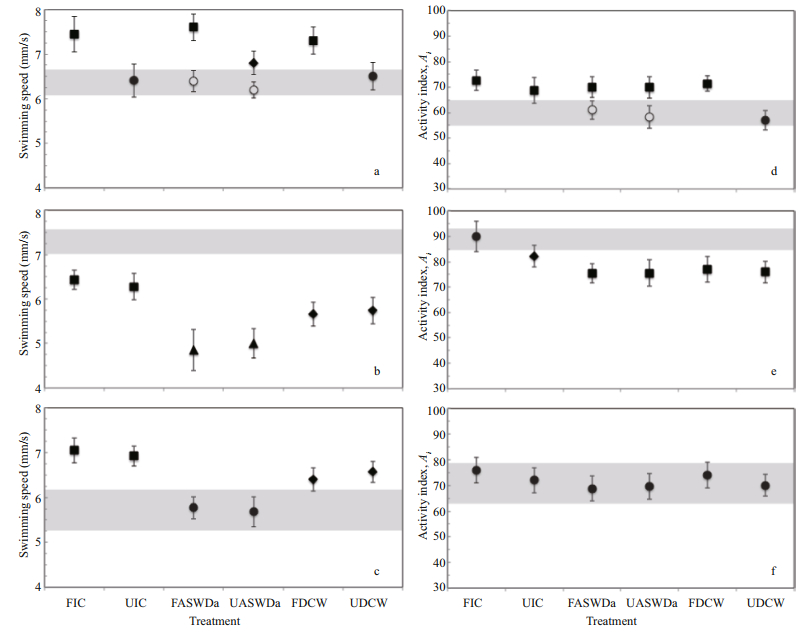
3.2 Swimming behaviour of Daphniopsis australis in the presence of cues 3.2.1 MalesMale swimming speeds significantly differ between treatments (KW test, P < 0.05). Specifically, experimental males swam significantly faster than control males only in filtered Isochrysis and Daphniopsis culture water, and in pheromone-conditioned water in the absence and presence of epiphial females (Fig. 3a). The other treatments had no effect on male swimming speed, which did not significantly differ (P > 0.05) from the speed of males swimming in control seawater. Note that males swimming in filtered pheromone-conditioned water swam significantly faster than males swimming in unfiltered pheromone-conditioned water than is in the presence of epiphial females. The presence of parthenogenetic females had no significant effect on male swimming speed. No significant difference between treatments was found (KW test, P > 0.05) in net-to-gross displacement ratio NGDR (Fig. 4a), suggesting that the tortuosity of the trajectories of freely swimming D. australis males was independent on the quality of the water. In contrast, the activity index Ai (Fig. 3d) significantly differed between treatments (KW test, P < 0.05); males swimming in filtered and unfiltered Isochrysis culture water, in filtered and unfiltered pheromone-conditioned water conditioned with epiphial females, and in filtered Daphniopsis culture water were significantly more active than the others, which did not significantly differ from control males.
|
| Fig.3 Quantification of the swimming behaviour of male (a, d), epiphial female (b, e) and parthenogenetic female (c, f) of the cladoceran, Daphniopsis australis through their swimming speed (a-c) and activity index Ai (d-f) under various conditions of water conditioning FIC: filtered Isochrysis culture; UIC: unfiltered Isochrysis culture; FASWDa: filtered artificial seawater conditioned during 24 h with conspecifics of the opposite sex at a concentration of 20 individuals per litre, i.e. partenogenetic females (open symbols) and epiphial females (black symbols) for males (a, d), and males for partenogenetic females (b, e) and epiphial females (c, f); UASWDa: unfiltered artificial seawater conditioned during 24 h with conspecifics of the opposite sex at a concentration of 20 individuals per litre, i.e. partenogenetic females (open symbols) and epiphial females (black symbols) for males (a), and males for partenogenetic females (b) and epiphial females (c); FDCW: filtered Daphniopsis culture: UDCW: unfiltered Daphniopsis culture. The grey surfaces defined the 95% confidence intervals of swimming speed and NGDR obtained in artificial seawater in the absence of cues, and the error bars represent the 95% confidence intervals. Symbols with different shapes indicate statistical differences significant at the 5% confidence level. |
|
| Fig.4 Net-to-gross displacement ratio (NGDR) of the swimming behaviour of males (a), epiphial females (b) and parthenogenetic females (c) of the cladoceran Daphniopsis australis under various conditions of water conditioning FIC: filtered Isochrisis culture; UIC: unfiltered Isochrisis culture; FASWDa: filtered artificial seawater conditioned during 24 h with conspecifics of the opposite sex at a concentration of 20 individuals per litre, i.e. partenogenetic females (open symbols) and epiphial females (black symbols) for males (a), and males for partenogenetic females (b) and epiphial females (c); UASWDa: unfiltered artificial seawater conditioned during 24 h with conspecifics of the opposite sex at a concentration of 20 individuals per litre, i.e. partenogenetic females (open symbols) and epiphial females (black symbols) for males (a), and males for partenogenetic females (b) and epiphial females (c); FDCW: filtered Daphniopsis culture: UDCW: unfiltered Daphniopsis culture. The grey surfaces defined the 95% confidence intervals of the NGDR obtained in artificial seawater in the absence of cues, and the error bars represent the 95% confidence intervals. |
The swimming speed of epiphial females (Fig. 3b) significantly differed between treatments (KW test, P < 0.01), and was consistently significantly slower than in the control experiment (P < 0.01). Specifically, they were significantly faster in filtered and unfiltered Isochrysis culture water (6.36±0.26 mm/s) than in both filtered and unfiltered Daphniopsis culture water (5.70±0.29 mm/s), and they were the slowest in pheromone-conditioned water either in the presence or the absence of males (4.93±0.40 mm/s). In contrast to the observations conducted on males, NGDR significantly differs between treatments (KW test, P > 0.05). NGDR only significantly differed, however, in unfiltered Isochrysis water where the tortuosity was significantly lower (i.e. higher NGDR) than in all the other treatments, including the control experiments (Fig. 4b). The activity index Ai (Fig. 3e) significantly differed between treatments (KW test, P < 0.05). The activity of epiphial females in filtered Isochrysis culture water did not significantly differ from the activity of control females, they were significantly less active in unfiltered Isochrysis culture water, where they were significantly more active than filtered and unfiltered male pheromone-conditioned water and filtered and unfiltered Daphniopsis culture water, which did not significantly differ from each other.
3.2.3 Parthenogenetic femalesThe swimming speed of parthenogenetic females (Fig. 3c) significantly differed between treatments (KW test, P < 0.05). Specifically, these females were the slowest in control experiments (5.73±0.40 mm/s) and in pheromone-conditioned water in the presence (5.77±0.25 mm/s) and absence (5.68±0.33 mm/s) of males. Parthenogenetic females were the fastest in filtered Isochrysis culture water (7.05±0.28 mm/s) and in unfiltered Isochrysis culture water (6.92± 0.22 mm/s), where they swam significantly faster than in filtered Daphniopsis culture water (6.40± 0.26 mm/s) and in unfiltered Daphniopsis culture water (6.57±0.24 mm/s). As reported for epiphial females, NGDR never significantly differ from each other nor from the NGDR obtained in the absence of cues, except for females swimming in unfiltered Isochrysis culture water which exhibited significantly less tortuous trajectories (i.e. higher NGDR; Fig. 4c). Finally, the activity of parthenogenetic females did not significantly differ between control and experimental treatments (KW test, P > 0.05).
4 DISCUSSION 4.1 Sex-specific innate swimming behaviour in D. australisIn the absence of cues, the adult stages of D. australis display a variety of swimming strategies characterised by swimming speeds, trajectory tortuosity and activity levels that are clearly sex-specific (Figs. 2 and 3). Specifically, the swimming behaviours of males and females fundamentally differed as the former (Fig. 2a) is more space-filling and the related swimming trajectories more rectilinear, while the latter (Fig. 2b, 2c) are less space-filling (especially parthenogenetic females; Fig. 2c) and characterised by the alternation between short bursts of swimming and sinking phases. Male behaviour is consistent with search models showing that swimming in linear paths and reducing tight turns increases the probability of encountering randomly distributed particles, either food or other individuals (Dusenbery, 1992). Males are then likely to be more tuned towards efficient searching strategies towards food and females, though such a strategy would also increase predation risk. Parthenogenetic females who spend most of their time in close proximity to the bottom (Fig. 2c) exhibit a behaviour that is consistent with their biology as it implicitly limits the probability of unneeded encounters with males, while both maximising grazing rates on benthic algae and minimizing the predation risk of laid eggs. The behaviour of sexually reproducing epiphial females then lies somewhere between those of males and parthenogenetic females, and may somehow represent a compromise between optimising encounter rates with males and the need to remain close to the bottom to lay their resting eggs and minimising the related predation risk. These observations further suggest that (ⅰ) mate-searching may essentially be a male business, and (ⅱ) males may be filter-feeder, while females (especially parthenogenetic ones) might essentially rely on resources lying on the bottom or in the benthic microlayer. Assessing these hypothesis are, however, far beyond the scope of the present work, and warrant the need for further work on this still seldom known and understood cladoceran species.
4.2 Sex-specific cue hierarchy in D. australis swimming behaviourThe responses observed in D. australis males and females in the presence of cues from food, conspecifics of the opposite sex, and a mixture of food and conspecific indicate a clear sex-specific cue hierarchy in their swimming behaviour. The implications of these results in the behavioural ecology of D. australis are discussed hereafter.
4.2.1 Lack of cue hierarchy in D. australis male behaviourDaphniopsis australis males did not significantly modify the tortuosity of their swimming paths in relation to any of the cues (Fig. 4). This result indicates that the available cues did not trigger any change in their search strategies, and contrasts with the widely reported extensive and intensive search strategies expected in food-depleted and food-rich habitats in a variety of organisms ranging from microcrustaceans to megafauna (Seuront and Vincent, 2008; Humphries et al., 2012; Sims et al., 2012; Seuront and Stanley, 2014). This absence of changes in search strategies under various conditions of food and conspecific cues in D. australis may suggest a lack of sensory perception in these organisms. However, D. australis males significantly increase their activity level in all treatments, except in both filtered and unfiltered male pheromone-conditioned water and in unfiltered Daphniopsis culture water (Fig. 3d). This result confirms that D. australis males are sensitive to chemical cues from both food and conspecifics. More specifically, D. australis males significantly increased their swimming speed in filtered Isochrysis culture water, filtered Daphniopsis culture water and filtered water conditioned with epiphial females. In contrast, their speed did not significantly differ from the speed observed in the absence of cues when they were observed in unfiltered Isochrysis culture water, unfiltered Daphniopsis culture water and unfiltered water conditioned with epiphial females. These results have several fundamental implications in terms of our understanding of the sensory ecology of D. australis males:
1) they have the ability to detect the infochemicals released by phytoplankton cells and conspecifics of the opposite sex. This is consistent with previous observations conducted on the sensory abilities of a variety of microcrustaceans such as cladocerans (Brewer, 1998; La et al., 2014; Nihongi et al., 2016) and copepods (e.g. Woodson et al., 2007; Seuront and Vincent, 2008; Yen et al., 2011; Seuront, 2013; Seuront and Stanley, 2014);
2) they increase their swimming speed in response to chemical exudates of phytoplankton and epiphial females, compared to when exposed to the presence of food and/or epiphial females. This observation is consistent with the widely observed increase in swimming speed in response to physical and chemical cues (Cowles, 2004; Woodson et al., 2007), though exceptions exist (e.g. Seuront, 2013; Seuront and Stanley, 2014). This clear behavioural shift suggests that whilst food and females are actually available males decreases their speed as an active search for food is no longer required, and females may be located through their hydrodynamic trails (Wickramarathna et al., 2014);
3) the lack of significant differences in swimming speed in the presence of cues from phytoplankton exudates (filtered Isochrysis culture water), epiphial females exudates and pheromones (filtered female-conditioned water) and a mixture of phytoplankton and conspecifics (filtered Daphniopsis culture water) further suggests the absence of cue hierarchy between food and conspecific infochemicals in the sensory ecology of D. australis males;
4) finally, the lack of observed behavioural changes when D. australis males were swimming in filtered and unfiltered water conditioned with parthenogenetic females further suggest that parthenogenetic females do not generate any infochemicals susceptible to trigger a mate-searching strategy in males or that males have the ability to relate infochemicals to the sexual status of the females that produced them. The slower swimming speed of parthenogenetic females in the absence of cues (and in the presence of filtered male cues; Fig. 3c) also suggest that their hydrodynamic trails may be less conspicuous and/or distinct from those of epiphial females. D. australis males may hence also have the ability to recognise the sexual status of females based on their hydrodynamic trails.
4.2.2 Cue hierarchy in D. australis female behaviour is driven by sexual statusBoth epiphial and partenogenetic females shared a lack of significant difference in the observed NGDR between control and experimental treatments, except in unfiltered Isochrysis culture water where NGDR significantly increased (Fig. 4). This increased tortuosity of their swimming paths indicates a switch towards an intensive search strategy, that is expected in food-rich habitats; see e.g. Seuront and Vincent (2008), Sims et al. (2012) and Humphries et al. (2012). In contrast, epiphial females consistently decreased their activity level in all treatments, except in filtered Isochrysis culture water (Fig. 3e), while parthenogenetic females never modified their activity level (Fig. 3f).
The response pattern of epiphial and parthenogenetic females swimming speed to experimental treatments further showed clear, though significantly distinct, cue hierarchies that are specific to their sexual status. As observed for males, parthenogenetic females swam significantly faster in Isochrysis and Daphniopsis culture water than in control experiments (Fig. 3c). This indicates an increase in searching activity in the presence of infochemichals originating respectively from food and from a mixture of food and conspecific cues that are in agreement with previous behavioural studies (e.g. Woodson et al., 2007) and the optimal foraging hypothesis (Pyke, 1984). In agreement with their asexual status, parthenogenetic females did not exhibit any change in their swimming speed in male conditioned water (Fig. 3c). These results suggest that parthenogenetic females (ⅰ) detect the infochemicals released by phytoplankton cells, (ⅱ) do not detect infochemicals from conspecifics, which alternatively may deter their ability to detect phytoplankton infochemicals, and (ⅲ) ignore conspecifics, but consistently actively foraged for food, even when it was readily available as shown by the lack of significant difference in swimming speed between filtered and unfiltered Isochrysis culture water and filtered and unfiltered Daphniopsis culture water.
In sharp contrast to observations conducted on parthenogenetic females and males, epiphial females consistently decreased their swimming speed compared to control experiments run in the absence of cues (Fig. 3b). The intensity of the observed behavioural changes was the smallest in Isochrysis culture water, the greatest in male conditioned water, and intermediate in Daphniopsis culture water (Fig. 3b). These observations suggest that epiphial females (ⅰ) have the ability to detect the infochemicals released by both phytoplankton cells and conspecifics of the opposite sex, (ⅱ) hierarchically adjust their behavioural response depending on the nature of the cue, and (ⅲ) prioritise sexual reproduction to feeding as reducing speed is likely to increase the conspicuousness of both their chemical and hydrodynamic trails. This hypothesis is consistent with trail-following experiments showing that D. daphniopsis males were more efficient to trail-follow and subsequently capture slow swimming epiphial females (Seuront, unpublished data).
5 CONCLUSIONIn the present study, we have shown that Daphniopsis australis males, epiphial females, and parthenogenetic females share the ability to detect the infochemicals released by phytoplankton cells and conspecifics of the opposite sex. However, males do not exhibit any hierarchy in their behavioural response to infochemicals from food or conspecifics. In contrast, both epiphial and parthenogenetic females exhibited a clear hierarchy in their behavioural responses to food and conspecific cues. The behaviour of epiphial and parthenogenetic females was then respectively driven by sex-related cues and food-related cues, in agreement with their reproductive status. Note that further behavioural analyses based on the use of robust metrics that are very sensitive to subtle behavioural changes such as fractals and multifractals (Seuront, 2015b) may provide further insights into our understanding of the role of behavioural changes in shaping critical processes such as sexual encounters. In particular, they may allow moving beyond the relatively limiting consideration of mean swimming speed in quantifying encounter rates (Seuront and Stanley, 2014).
More generally, because behavioural changes in microcrustaceans can alter entire biological food webs through e.g. modifications in their trophodynamics, energetics and mating rates (Banse, 1995; Kiørboe, 2008), the results of the present work imply that the understanding of the biology and ecology of cladoceran species, especially in the context of the relatively seldom studied genus Daphniopsis, will benefit from detailed investigations of their behavioural properties in response to various biotic cues as food and conspecific infochemicals. This issue is even more critical in an era of global change as Australian inland water bodies which are expected to become drier and more saline. In this context, furthering our understanding of the interplay between temperature and salinity in the resilience of aquatic species to global change may be the key to the successful development and implementation of management and conservation plans.
6 DATA AVAILABILITY STATEMENTThe data used in this work are available upon request to the corresponding author, L. Seuront (laurent.seuront@cnrs.fr).
7 ACKNOWLEDGMENTThis research was supported by an Honours Scholarship from Flinders University to C. McCloud, and an Australian Research Council's Discovery Projects funding scheme (project DP0664681). Professor Seuront is the recipient of an Australian Professorial Fellowship (project DP0988554). This work is a contribution to the CPER research project CLIMIBIO. The authors thank the French Ministère de l'Enseignement Supérieur et de la Recherche, the Hauts de France Region and the European Funds for Regional Economical Development for their financial support to this project.
Aladin N V. 1991. Salinity tolerance and morphology of the osmoregulation organs in Cladocera with special reference to Cladocera from the Aral Sea. Hydrobiologia, 225(1): 291-299.
DOI:10.1007/BF00028407 |
Aladin N V, Potts W T W. 1995. Osmoregulatory capacity of the Cladocera. J. Comp. Physiol. B., 164(8): 671-683.
DOI:10.1007/BF00389810 |
Banse K. 1995. Zooplankton:pivotal role in the control of ocean production:Ⅰ. Biomass and production. ICES J. Mar. Sci., 52(3-4): 265-277.
DOI:10.1016/1054-3139(95)80043-3 |
Baylor E R, Smith F E. 1953. The orientation of Cladocera to polarized light. Am. Nat., 87(833): 97-101.
DOI:10.1086/281761 |
Benzie J A H. 2005. The Genus Daphnia (Including Daphniopsis): Anomopoda: Daphniidae (Guides to the Identification of the Microinvertebrates of the Continental Waters of the World). Kenobi Productions, Ghent.
|
Bledzki L A, Rybak J I. 2016. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda(Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis. Springer, New York. 918p.
|
Bownik A. 2017. Daphnia swimming behaviour as a biomarker in toxicity assessment:a review. Sci. Total Environ., 601-602: 194-205.
DOI:10.1016/j.scitotenv.2017.05.199 |
Brancelj A, De Meester L, Spaak P. 2012. Cladocera: the Biology of Model Organisms: Proceedings of the Fourth International Symposium on Cladocera, Held in Postojna, Slovenia, 8-15 August 1996. Springer, New York. 303p.
|
Brewer M C. 1998. Mating behaviours of Daphnia pulicaria, a cyclic parthenogen:comparisons with copepods. Philos. Trans. Roy. Soc. B: Biol. Sci., 353(1369): 805-815.
DOI:10.1098/rstb.1998.0244 |
Buskey E J. 1984. Swimming patterns as an indicator of the roles of copepod sensory systems in the recognition of food. Mar. Biol., 79(2): 165-175.
DOI:10.1007/BF00951825 |
Campbell C E. 1994. Seasonal zooplankton fauna of salt evaporation basins in South Australia. Austr. J. Mar.Freshw. Res., 45(2): 199-208.
DOI:10.1071/MF9940199 |
Colbourne J K, Wilson C C, Hebert P D N. 2006. The systematics of Australian Daphnia and Daphniopsis(Crustacea:Cladocera):a shared phylogenetic history transformed by habitat-specific rates of evolution. Biol. J.Linn. Soc., 89(3): 469-488.
DOI:10.1111/j.1095-8312.2006.00687.x |
Cowles T J. 2004. Planktonic layers: physical and biological interactions on the small scale. In: Seuront L, Strutton P G eds. Handbook of Scaling Methods in Aquatic Ecology: Measurements, Analysis, Simulation. CRC Press, Boca Raton, FL. p.31-49. https://www.researchgate.net/publication/262402995_Planktonic_layers_physical_and_biological_interactions_on_the_small_scale
|
Da S., Ferrão Filho A, Da Costa S M, Ribeiro M G L, Azevedo S M F O. 2008. Effects of a saxitoxin-producer strain of Cylindrospermopsis raciborskii (cyanobacteria) on the swimming movements of cladocerans. Environ. Toxicol., 23(2): 161-168.
DOI:10.1002/(ISSN)1522-7278 |
Dees N D, Bahar S, Garcia R, Moss F. 2008. Patch exploitation in two dimensions:from Daphnia to simulated foragers. J. Theor. Biol., 252(1): 69-76.
DOI:10.1016/j.jtbi.2008.01.026 |
Delbare D, Dhert P. 1996. Cladocerans, nematodes and trochophora. In: Laverns P, Sorgeloos P eds. Manual on the Production and Use of Live Food for Aquaculture. Food and Agriculture Organization of United Nation, Rome.
|
Dodson S I, Frey D G. 2001. Cladocera and other branchiopoda.In: Thorp J H, Covich A P eds. Ecology and Classification of North American Freshwater Invertebrates. 2nd edn.Academic Press, San Diego. p.723-786. https://www.sciencedirect.com/science/article/pii/B9780123748553000200
|
Dodson S, Ramcharan C. 1991. Size-specific swimming behavior of Daphnia pulex. J. Plankton Res., 13(6): 1367-1379.
DOI:10.1093/plankt/13.6.1367 |
Dusenbery D B. 1992. Sensory Ecology: How Organisms Acquire and Respond to Information. WH Freeman, New York.
|
Garcia R, Moss F, Nihongi A, Strickler J R, Göller S, Erdmann U, Schimansky-Geier L, Sokolov I M. 2007. Optimal foraging by zooplankton within patches:the case of Daphnia. Mathem. Biosci., 207(2): 165-188.
|
Hamza W, Ruggiu D. 2000. Swimming behaviour of Daphnia galeata × hyalina as a response to algal substances and to opaque colours. Int. Rev. Hydrobiol., 85(2-3): 157-166.
DOI:10.1002/(ISSN)1522-2632 |
Hann B J. 1986. Revision of the genus Daphniopsis Sars, 1903(Cladocera:Daphniidae) and a description of Daphniopsis chilensis, new species, from South America. J. Crustacean Biol., 6(2): 246-263.
DOI:10.1163/193724086X00073 |
Hebert P D, Wilson C C. 2000. Diversity of the genus Daphniopsis in the saline waters of Australia. Can. J.Zool., 78(5): 794-808.
DOI:10.1139/z99-253 |
Hinow P, Nihongi A, Strickler J R. 2015. Statistical mechanics of zooplankton. PLoS One, 10(8): e0135258.
DOI:10.1371/journal.pone.0135258 |
Humphries N E, Weimerskirch H, Queiroz N, Southall E J, Sims D W. 2012. Foraging success of biological Lé vy flights recorded in situ. Proc. Natl. Acad. Sci. U.S.A., 109(19): 7169-7174.
DOI:10.1073/pnas.1121201109 |
Ismail H N, Qin J G, Seuront L, Adams M. 2010b. Impacts of male and food density on female performance in the brackish cladoceran Daphniopsis australis. Hydrobiologia, 652(1): 277-288.
DOI:10.1007/s10750-010-0359-8 |
Ismail H N, Qin J G, Seuront L. 2010a. Thermal and halo tolerance of a brackish cladoceran Daphniopsis australis(Sergeev & Williams). In: Martorino L, Puopolo K eds.New Oceanography Research Developments: Marine Chemistry, Ocean Floor Analyses and Marine Phytoplankton. Nova Science Publisher, New York.p.213-230. https://www.researchgate.net/publication/281330860_Thermal_and_halo_tolerance_of_a_brackish_cladoceran_Daphniopsis_australis_Sergeev_Williams
|
Ismail H N, Qin J G, Seuront L. 2011a. Dietary responses of the brackish cladoceran Daphniopsis australis fed on different algal species. J. Exp. Mar. Biol. Ecol., 409(1-2): 275-282.
DOI:10.1016/j.jembe.2011.09.008 |
Ismail H N, Qin J G, Seuront L. 2011b. Regulation of life history in the brackish cladoceran, Daphniopsis australis(Sergeev and Williams, 1985) by temperature and salinity. J. Plankton Res., 33(5): 763-777.
DOI:10.1093/plankt/fbq145 |
Ismail H N, Qin J G, Seuront L. 2011c. The survival and reproductive performance of Daphniopsis australis(Cladocera:Daphniidae) in response to temperature changes. Jurnal Intelek, 6(1): 70-76.
|
Kiørboe T. 2008. A Mechanistic Approach to Plankton Ecology. Princeton University Press, Princeton.
|
La G H, Choi J Y, Chang K H, Jang M H, Joo G J, Kim H W. 2014. Mating behavior of Daphnia:impacts of predation risk, food quantity, and reproductive phase of females. PLoS One, 9(8): e104545.
DOI:10.1371/journal.pone.0104545 |
Mergeay J, Declerck S, Verschuren D, De Meester L. 2006. Daphnia community analysis in shallow Kenyan lakes and ponds using dormant eggs in surface sediments. Freshw. Biol., 51(3): 399-411.
DOI:10.1111/fwb.2006.51.issue-3 |
Nihongi A, Ziarek J J, Nagai T, Uttieri M, Zambianchi E, Strickler J R. 2011. Daphnia pulicaria hijacked by Vibrio cholera: altered swimming behaviour and predation risk implications. In: Kattel G ed. Zooplankton and Phytoplankton: Types, Characteristics and Ecology. Nova Science Publishers, New York. p.181-192. https://www.researchgate.net/publication/286360707_Daphnia_pulicaria_hijacked_by_Vibrio_cholerae_Altered_swimming_behaviour_and_predation_risk_implications
|
Nihongi A, Ziarek J J, Uttieri M, Sandulli M, Zambianchi E, Strickler J R. 2016. Behavioural interseasonal adaptations in Daphnia pulicaria (Crustacea:Cladocera) as induced by predation infochemicals. Aquat. Ecol., 50(4): 667-684.
DOI:10.1007/s10452-016-9585-0 |
O'Keefe T C, Brewer M C, Dodson S I. 1998. Swimming behavior of Daphnia:its role in determining predation risk. J. Plankton Res., 20(5): 973-984.
DOI:10.1093/plankt/20.5.973 |
Pyke J H. 1984. Optimal foraging theory:a critical review. Ann. Rev. Ecol. Syst., 15: 523-575.
DOI:10.1146/annurev.es.15.110184.002515 |
Sars G O. 1903. On the crustacean fauna of Central Asia. 2.Cladocera. Ann. Mus. zool. Acad. Sci. St. Petersbourg, 8: 157-194.
|
Schwartz S S, Hebert P D N. 1987. Breeding system of Daphniopsis ephemeralis:adaptations to a transient environment. Hydrobiologia, 145(1): 195-200.
DOI:10.1007/BF02530280 |
Sergeev V, Williams W D. 1985. Daphniopsis australis nov.sp. (Crustacea:Cladocera), a further daphniid in Australian salt lakes. Hydrobiologia, 120(2): 119-128.
DOI:10.1007/BF00032132 |
Seuront L, Brewer M C, Strickler J R. 2004c. Quantifying zooplankton swimming behavior: the question of scale.In: Seuront L, Strutton P G eds. Handbook of Scaling Methods in Aquatic Ecology: Measurement, Analysis, Simulation. CRC Press, Boca Raton. p.333-359. https://www.researchgate.net/publication/265109149_Quantifying_Zooplankton_Swimming_Behavior_The_Question_of_Scale
|
Seuront L, Schmitt F G, Brewer M C, Strickler J R, Souissi S. 2004b. From random walk to multifractal random walk in zooplankton swimming behavior. Zool. Stud., 43(2): 498-510.
|
Seuront L, Stanley H E. 2014. Anomalous diffusion and multifractality enhance mating encounters in the ocean. Proc. Natl. Acad. Sci. U.S.A., 111(6): 2206-2211.
DOI:10.1073/pnas.1322363111 |
Seuront L, Vincent D. 2008. Increased seawater viscosity, Phaeocystis globosa spring bloom and Temora longicornis feeding and swimming behaviours. Mar. Ecol. Progr.Ser., 363: 131-145.
DOI:10.3354/meps07373 |
Seuront L, Yamazaki H, Souissi S. 2004a. Hydrodynamic disturbance and zooplankton swimming behavior. Zool.Stud., 43(2): 376-387.
|
Seuront L. 2006. Effect of salinity on the swimming behaviour of the estuarine calanoid copepod Eurytemora affinis. J.Plankton Res., 28(9): 805-813.
DOI:10.1093/plankt/fbl012 |
Seuront L. 2011. Behavioral fractality in marine copepods:endogenous rhythms versus exogenous stressors. Phys. A:Stat. Mech. Appl., 390(2): 250-256.
DOI:10.1016/j.physa.2010.09.025 |
Seuront L. 2013. Chemical and hydromechanical components of mate-seeking behaviour in the calanoid copepod Eurytemora affinis. J. Plankton Res., 35(4): 724-743.
DOI:10.1093/plankt/fbt039 |
Seuront L. 2015a. On uses, misuses and potential abuses of fractal analysis in zooplankton behavioral studies:a review, a critique and a few recommendations. Phys. A:Stat. Mech. Appl., 432: 410-434.
DOI:10.1016/j.physa.2015.03.007 |
Seuront L. 2015b. Copepods:Diversity, Habitat and Behavior. Nova Science Publishers, New York. 291p.
|
Sims D W, Humphries N E, Bradford R W, Bruce B D. 2012. Lé vy flight and Brownian search patterns of a free-ranging predator reflect different prey field characteristics. J.Anim. Ecol., 81(2): 432-442.
DOI:10.1111/j.1365-2656.2011.01914.x |
Timms B V. 2007. The biology of the saline lakes of central and eastern inland of Australia:a review with special reference to their biogeographical affinities. Hydrobiologia, 576(1): 27-37.
DOI:10.1007/s10750-006-0290-1 |
Uttieri M, Sandulli R, Spezie G, Zambianchi E. 2014. From small to large scale: a review of the swimming behaviour of Daphnia. In: El-Doma M ed. Daphnia: Biology and Mathematics Perspectives. Nova Science Publishers, New York. p.309-312. https://www.researchgate.net/publication/265373513_From_small_to_large_scale_A_review_of_the_swimming_behaviour_of_Daphnia
|
Wickramarathna L N, Noss C, Lorke A. 2014. Hydrodynamic trails produced by Daphnia:size and energetics. PLoS One, 9(3): e92383.
DOI:10.1371/journal.pone.0092383 |
Woodson C B, Webster D R, Weissburg M J, Yen J. 2007. Cue hierarchy and foraging in calanoid copepods:ecological implications of oceanographic structure. Mar. Ecol.Progr. Ser., 330: 163-177.
DOI:10.3354/meps330163 |
Yen J, Sehn J K, Catton K, Kramer A, Sarnelle O. 2011. Pheromone trail following in three dimensions by the freshwater copepod Hesperodiaptomus shoshone. J.Plankton Res., 33(6): 907-916.
DOI:10.1093/plankt/fbq164 |
Zar J H. 2010. Biostatistical Analysis. 5th edn. Prentice-Hall, Upper Saddle River, NJ.
|
Ziarek J J, Nihongi A, Nagai T, Uttieri M, Strickler J R. 2011. Seasonal adaptations of Daphnia pulicaria swimming behaviour:the effect of water temperature. Hydrobiologia, 661(1): 317-327.
DOI:10.1007/s10750-010-0540-0 |
 2018, Vol. 36
2018, Vol. 36


