Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Haihong(陈海红), CHEN Weizhou(陈伟洲), SHI Jingyi(石经仪), CHEN Zepan(陈泽攀), ZHANG Yi(张毅)
- Isolation and callus formation of Gracilariopsis bailiniae (Gracilariales, Rhodophyta) protoplasts
- Chinese Journal of Oceanology and Limnology, 36(6): 2268-2277
- http://dx.doi.org/10.1007/s00343-019-7217-y
Article History
- Received Jul. 14, 2017
- accepted in principle Sep. 8, 2017
- accepted for publication Dec. 24, 2017
Gracilariopsis bailiniae shows fast growth and heat-resistance (Zhong et al., 2014). It is a food for abalones and a raw material for agar extraction that is farmed in Fujian and Guangdong Provinces, China (Hurtado-Ponce, 1992; Pan and Li, 2010). Agarophytes (Gelidium, Gracilaria and Gracilariopsis) have considerable industrial importance since they are the principal source of raw material for the agar industry worldwide (Zemke-White and Ohno, 1999; Smit, 2004). In recent years, there has been increasing global demand for agar, which is used widely for microbial culture and in the food, health care products, medical and chemical industries, because of its gel, thickening and stabilization properties (Liu et al., 2013). The supply of agarophytes from wild stocks can no longer meet the demand for agar. Thus, large-scale cultivation of agarophytes is required (Gupta et al., 2013). The continuous supply of seed material is a key step for successful aquaculture (Saminathan et al., 2015). However, the limitations of traditional seed production methods for most agarophytes have restricted exploitation of these seaweeds for industrial use (Mantri, 2009). Thus, development of seed stock methods for agarophytes is crucial to achieve largescale production of seedlings.
Protoplasts are living plant cells devoid of cell walls which can be applied in somatic hybridization, proteomics, metabolomics, cybridization and protoclonal variation studies (Fujita and Saito, 1990; Davey et al., 2005; Reddy et al., 2008). Theoretically, a protoplast can lead to the regeneration of one or more plants from a single cell due to the totipotence of plant cells (Huddy et al., 2013). Moreover, many protoplasts can be isolated from a small fragment of algal thallus, so protoplasts can be excellent tools for seed stock production and plant breeding (Gupta et al., 2011; Huddy et al., 2013; Wang et al., 2014). Chen (1998) and Chen and Shih (2000) developed methods for producing stocks of seedlings using protoplasts from green algae (Ulva fasciata and Monostroma latissimum). Protoplasts from Monostroma and Porphyra have also been successfully tested for seeding and regeneration in laboratory conditions (Kito et al., 1998; Dipakkore et al., 2005; Reddy et al., 2006), and protoplasts from some species of Gracilaria and Gracilariopsis have successfully been regenerated to whole thalli (Cheney, 1990; Yan and Wang, 1993; Reddy et al., 2008; Yeong et al., 2008; Wang et al., 2014; Huddy et al., 2015). These studies indicated that using protoplasts as seed stocks for cultivation is feasible. However, methods for protoplast isolation and regeneration of agarophyte species have not been sufficiently established (Wang, 1994; Baweja et al., 2009). There are very few reports about protoplast isolation from Gracilaria and Gracilariopsis species (Reddy et al., 2010; Gupta et al., 2011; Huddy et al., 2013; Wang et al., 2014).
In the present study, we used a marine bacterium, Marinomonas sp. YS-70, that can produce agarase. The crude agarase solution from this bacterium mixed with commercial enzymes (cellulase and macerozyme R-10) as a cell wall hydrolase produced good yields of protoplasts from Gracilariopsis bailiniae. This paper reports the optimization of the protoplast isolation conditions and the callus formation of these protoplasts.
2 MATERIAL AND METHOD 2.1 Experimental algaeThalli of G. bailiniae were obtained from the breeding pond of Hainan Ocean and Fisheries Sciences Research Base, Qionghai, Hainan, China. In the laboratory, the seaweed samples were cleaned of mud and epiphytes using a soft brush and filtered seawater. Then, the thalli were maintained in ventilated tanks at 27℃ and 40-60 μmol photons/ (m2·s) (12 h light:12 h dark) (Zhong et al., 2014).
2.2 Preparation of agaraseMarinomonas sp. YS-70, which was isolated from red algae, was used for the preparation of agarase. The bacteria were inoculated into 100 mL of 2216E medium in a 500-mL conical flask. The 2216E medium was composed of 5 g tryptone (OXOID), 1 g yeast extract (OXOID), 0.01 g FePO4 (Sangon) and 0.2% agar (OXOID), dissolved in 1 000 mL aged seawater (final pH adjusted to 7.3). After incubating at 26℃ for 48 h in a reciprocal shaker (120 r/min), the bacterial solution was centrifuged at 10 444 r/min for 30 min at 4℃. The supernatant was collected and stored at -80℃ until use in protoplast isolation.
2.3 Agarase assayAgarase assay was carried out by estimating the reducing sugar released using the 3, 5-dinitrosalisylic acid (DNS) method (Tang, 2012). The activity of agarase was determined at pH 3.5, 4.5, 5.5, 6.5, 7.5, 8.5, 9.5 and 10.5. The reaction mixture was prepared from 0.1 mL crude agarase solution and 0.9 mL buffer (0.05 mol/L) containing 20 g/L agar; the buffers used were citric acid (pH 3.5-5.5), phosphate (pH 6.5-7.5), and glycine-NaOH (pH 8.5-10.5). The reaction mixture was incubated at 40℃ for 30 min. Then, 1 mL of DNS was mixed with 1 mL of the reaction mixture, heated for 5 min in a boiling water bath, and then cooled. The release of reducing sugar was determined by measuring the absorbance at 540 nm against a standard curve for galactose. One unit of agarase activity was defined as the amount of enzyme (mL) that produced reducing sugar equivalent to 1 μg D-galactose per min in these conditions.
2.4 Isolation of protoplasts from G. bailiniaeFor the optimization of protoplast isolation, thalli were cleaned with a soft brush and rinsed three times with filtered seawater. After cleaning, G. bailiniae thalli were cut into 2-3 mm long pieces using a sterile scalpel blade in a culture dish and rinsed three times in filtered seawater. Then, approximately 0.5 g of seaweed pieces were incubated in 5 mL of enzyme solution in a 50-mL conical flask placed in the dark on a rotary shaker (90 r/min) for 4 h at pH 6.5. The initial enzyme solution (before various parameters were optimized) contained 1% (w/v) cellulase and 0.8 mol/L sorbitol in 40% base solution (deionized water containing 25 mmol/L 2-(N-morpholino) ethanesulfonic acid-Tris [MES-Tris] and 25 mmol/L CaCl2·2H2O) and 60% crude agarase solution. After incubation, the enzyme mixture was filtered through 45 μm nylon mesh to remove undigested algal pieces, and the remaining filtrate was centrifuged at 1 247 r/min for 8 min at 25℃. After that, 80% of the supernatant was discarded, and the protoplasts were resuspended in 5 mL MES medium containing 0.6 mol/L D-sorbitol and this procedure was repeated twice. Then, protoplast numbers were determined in a blood counting chamber. Each set of conditions was tested with three replicates.
For protoplast regeneration experiments, the thalli of G. bailiniae were prepared more stringently. First, thallus tips (within 3 cm from the apex) were selected, cleaned with a soft brush and rinsed three times with filtered seawater. After that, the thallus tips were treated with an ultrasonic cleaner (KQ-250DB) at 100% power for 3 min before soaking in sterile seawater containing 1.5% KI for 10 min. Finally, the thallus tips were immersed in sterile seawater containing 0.1 g/L ampicillin sulfate, 0.1 g/L kanamycin sulfate, 0.02 g/L neomycin sulfate and 2 mg/L GeO2 for 48 h at 25℃. Then, the G. bailiniae thalli were used to isolate protoplasts, as described above.
2.5 Optimization of protoplast isolation parametersFor protoplast isolation from G. bailiniae, first, the base solution was optimized from among seawater (base-solution 1), deionized water (base-solution 2), and deionized water containing 25 mmol/L MES-Tris and 25 mmol/L CaCl2·2H2O (base-solution 3). Then, the concentration of cellulase (0%, 1%, 2%, 3% or 4% w/v), macerozyme R-10 (0%, 0.5%, 1% or 1.5% w/v) and sorbitol (0.2, 0.4, 0.6, 0.8 or 1.0 mol/L), and the incubation time (2, 3, 4, 5 or 6 h), temperature (25, 28 or 31℃) and enzyme solution pH (5.9, 6.1, 6.3, 6.5 or 6.7), were also optimized, in order. The optimal conditions identified in the proceeding tests were used in subsequent tests.
2.6 Protoplast stainingTo confirm the viability of protoplasts, we used 0.5% w/v Evans blue (Biotopped, Beijing, China) to stain protoplasts, which were observed under a light microscope (Zhang et al., 2014). The viable protoplast yields were determined as follows:
Viable protoplast yield=total number of protoplasts×survival rate.
To confirm true protoplasts lacking a cell wall, 0.01% (w/v) Fluorescent Brightener 28 (Sigma) was used to stain protoplasts, and they were observed under a fluorescence microscope (LEICA DMI3000 B) with UV light (Wang et al., 2014).
2.7 Culture of protoplastsProtoplasts of G. bailiniae were dispensed into 2 mL MES medium containing 0.6 mol/L sorbitol in 35 mm×10 mm Petri dishes with a protoplast density of 5×104-1×105 cells/mL. Then, protoplasts were cultured at 26℃ with a 12 h:12 h light:dark cycle (16 μmol/(m2·s)). After culturing for 2 days, the culture medium was replaced with ½ MES medium containing 0.6 mol/L sorbitol. Then, the ½ culture medium was replaced with MES medium when the protoplasts had been cultured for a total of 4 days. After that, 50% of the culture medium was replaced every 3 days with MES medium.
2.8 Statistical analysisAnalysis of variance was used for the comparison of results in different conditions. P < 0.05 was considered significant.
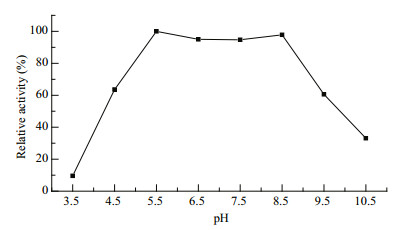
3 RESULT 3.1 Agarase activity assayMarinomonas sp. YS-70 agarase activity was high at pH 5.5 to 8.5 (Fig. 1) and the highest enzyme activity was observed at pH 5.5. Lower and higher pH values resulted in lower enzyme activity.
|
| Fig.1 pH-activity curve of Marinomonas sp. YS-70 agarase |
As Fig. 2 shows, the protoplast yield was significantly (P < 0.05) increased by using base-solution 3 and the highest protoplast yield ((12.5±1.3)×105 cells/g fresh weight [f. wt]) was obtained with this treatment. There was no significant difference in the protoplast yield between treatment with base-solution 1 and base-solution 2. The lowest protoplast yield ((1.08±0.59)×105 cells/g f. wt) was obtained on treatment with base-solution 1.

|
| Fig.2 Effect of different base solutions on protoplast yield of G. bailiniae thalli 1. seawater. 2. deionized water. 3. deionized water containing 25 mmol/L MES-Tris and 25 mmol/L CaCl2·2H2O. Different lower-case letters indicate a significant difference (P < 0.05) between sample means. |
As Fig. 3a and b show, the protoplast yields from G. bailiniae thalli were significantly affected by the concentration of added enzymes (P < 0.05). The protoplast yield was highest when the enzyme mixture contained 60% crude Marinomonas sp. YS-70 agarase solution, 2% w/v cellulase and 1% w/v macerozyme R-10. Alarge quantity of protoplasts could be obtained when the mixture of enzymes consisted of agarase andcellulaseonly (Fig. 3a), i.e., when theconcentration of macerozyme R-10 was 0%. However, few protoplasts were released from G. bailiniae thalli when agarase was mixed with macerozyme R-10 without cellulase (Fig. 3b).

|
| Fig.3 Effect of enzyme constituents and concentrations on protoplast yield from G. bailiniae thalli a. effects of different concentrations of macerozyme R-10 tested in combination with 1% w/v cellulase and 60% crude agarase; b. effects of different concentrations of cellulase with 1% macerozyme R-10 and 60% crude agarase. Different lower-case letters indicate a significant difference (P < 0.05) between sample means. |
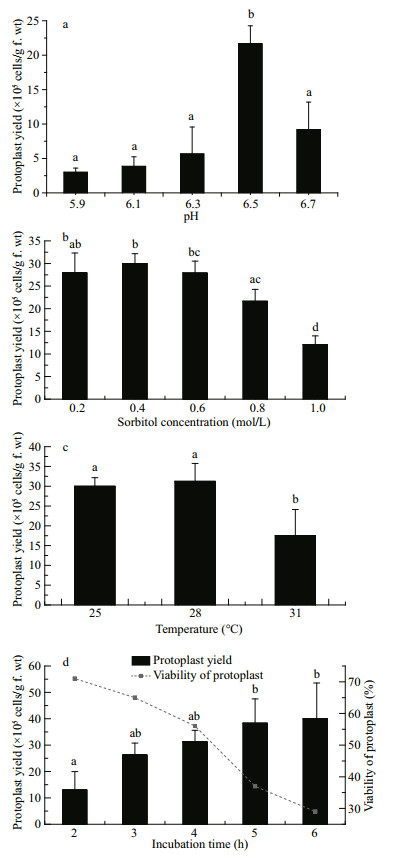
pH 6.5 was found to be the optimal pH for protoplast yield of G. bailiniae, with a significantly increased yield compared with the other pH values assessed (P < 0.05) (Fig. 4a).
|
| Fig.4 Effects of (a) different incubation pH, (b) sorbitol concentration, (c) incubation temperature, and (d) time on protoplast yield from G. bailiniae thalli Different lower-case letters indicate a significant difference (P < 0.05) between sample means. |
The protoplast yields were not significantly different with sorbitol treatments of 0.2, 0.4 and 0.6 mol/L (Fig. 4b); the highest protoplast yield was obtained when the sorbitol concentration was 0.4 mol/L. The yield of protoplast decreased significantly (P < 0.05) when the sorbitol concentration was > 0.6 mol/L. Based on these results, a sorbitol concentration of 0.4 mol/L was considered optimal for protoplast yield.
Protoplast yields from G. bailiniae were not significantly different between the incubation temperatures of 25 and 28℃, and the optimal yield was obtained at 28℃. The protoplast yield decreased significantly (P < 0.05) at 31℃ (Fig. 4c).
Protoplasts were released from G. bailiniae as early as 2 h after the start of incubation of the thalli in enzyme solution. Protoplast yields increased with incubation time (Fig. 4d). However, the survival rate of the protoplasts decreased as the incubation time increased. As Fig. 4d shows, the protoplast yield from G. bailiniae obtained after 4.0 h was (31.3±4.5)×105 cells/g f.wt and the survival rate was 56%, the highest viable yield among the tested incubation times. Therefore, an incubation period of 4 h was considered optimal for viable protoplast yield.
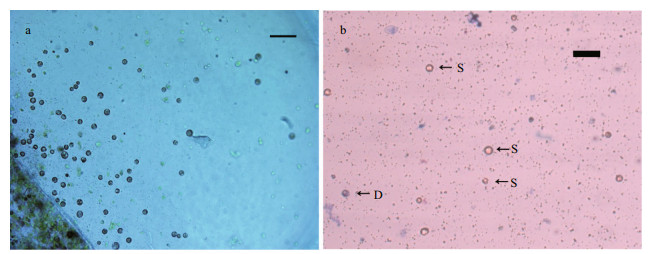
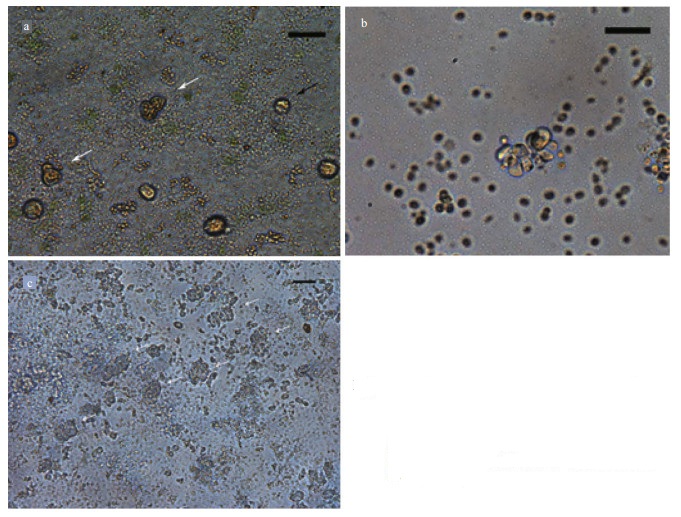
3.5 Protoplast cell wall regeneration and cell divisionFreshly isolated protoplasts from G. bailiniae were spherical with diameter from 7 to 35 μm (Fig. 5a). Living protoplasts appeared yellow and dead protoplasts were dark blue when stained with Evans blue (Fig. 5b). True protoplasts were red and undigested cell walls were green when they were stained with Fluorescent Brightener 28 and observed under UV light (Fig. 6a). Regeneration of the cell wall began from one pole of the protoplast (Fig. 6b). After being cultured for 48 h, the surface of most protoplasts was covered with regenerated cell wall and then the cell wall thickened. After 60 h of culture, the cell wall regeneration was almost complete (Fig. 6c). We found that the first division of most protoplasts occurred on the third day after protoplasts were isolated. There were two division types for the first division of protoplasts from G. bailiniae, asymmetric division and symmetric division (Fig. 7a). In the former, protoplasts produced a small bud from one side of the cell, and in the latter, protoplasts divided into two cells from the cell middle. After the first division, the divided cells could develop into callus masses over several days of culture (Fig. 7b, c).
|
| Fig.5 Protoplasts of G. bailiniae a. protoplasts isolated from G. bailiniae thalli (scale bar=50 μm); b. fresh protoplasts stained with Evans blue (D: dead protoplasts; S: viable protoplasts; scale bar=20 μm). |

|
| Fig.6 Cell wall regeneration of protoplasts from G. bailiniae a. freshly isolated protoplasts (shown in red); b. protoplasts after 24 h of culture; c. protoplasts after 60 h of culture; d. protoplasts after 72 h of culture. Scale bar=20 μm. |
|
| Fig.7 Protoplast regeneration of G. bailiniae a. dividing cells from protoplast after 3 days of culture (white arrow: asymmetrically divided cell; black arrow: symmetrically divided cell); b. cell mass from protoplast after 8 days of culture; c. cell masses from protoplasts after 17 days of culture. Scale bar=20 μm. |
Common sources of algal cell wall degrading enzymes are digestive gland juices of herbivorous marine invertebrates, culture filtrates of marine bacteria, and commercial preparations (Yan and Wang, 1993; Araki et al., 1998; Mussio and Rusig, 2006; Reddy et al., 2008; Yeong et al., 2008; Gupta et al., 2013; Wang et al., 2014; Zhang et al., 2014). However, the enzyme activity of digestive gland juices from marine invertebrates varies with sampling time, sampling species and production batch of enzymes, and this material has highly deleterious effects on protoplast viability (Wang, 1994; Zhao et al., 2005). Commercial cell wall degrading enzyme preparations have sometimes been assessed for protoplast isolation from Gracilaria species. Yeong et al. (2008) and Huddy et al. (2013) obtained a large quantity of protoplasts from Gracilaria changii and Gracilaria gracilis using commercial cellulase, macerozyme and agarase. In our tests, protoplasts could not be isolated effectively from Gracilariopsis bailiniae using commercial agarase (TAKARA), cellulase and macerozyme R-10 (data not shown), but we obtained many protoplasts from G. bailiniae when the commercial agarase was replaced by crude agarase solution from Marinomonas sp. YS-70. This might be because the commercial agarase is purer and could not completely degrade the complex amorphous matrix including sulfated polygalactans (agarcolloids or agars) in the cell wall of G. bailiniae, resulting in non-release of protoplasts.
Studies have shown that red algae have a wide variety of matrix polysaccharides in their cell walls, which vary in amount depending on the species (Bellanger et al., 1990; Graham and Wilcox, 2000; Reddy et al., 2008). In our study, cellulase and agarase were necessary for protoplast isolation. We could not obtain a good protoplast yield from G. bailiniae with a single cell wall degrading enzyme or enzyme mixtures lacking agarase or cellulase, which suggests the cell wall of G. bailiniae is complex. Macerozyme R-10 presumably enhanced the protoplast yield because macerozyme has been shown to digest pectin and polygalactans in many studies of protoplast isolation from agarophyte species (Evans and Bravo, 1983; Yeong et al., 2008; Gupta et al., 2011; Huddy et al., 2013). However, the commercial enzyme is partially purified and contains (a) toxic substance(s) (Inoue et al., 2011), so the optimization of cell wall degrading enzyme concentration was necessary. A combination of 2% w/v cellulase, 1% w/v macerozyme and 60% crude agarase prepared from Marinomonas sp. YS-70 resulted in the highest protoplast yield among the combinations and concentrations of enzymes we tested.
pH is an important parameter for protoplast isolation. In general, the pH used in published reports of algal protoplast isolation ranges between pH 5.8 and 7.0 (Cheney et al., 1986; Björk et al., 1990; Yan and Wang, 1993; Mussio and Rusig, 2006; Reddy et al., 2006; Yeong et al., 2008; Lafontaine et al., 2011). However, Gupta et al. (2011) reported an optimal pH of 7.5 for the isolation of protoplasts from Gracilaria verrucosa and Gracilaria dura. In our study, the optimal pH was 5.0 for commercial cellulase activity and 5.0 to 6.0 for macerozyme R-10, while the crude agarase from Marinomonas sp. YS-70 had its highest activity at pH 5.5-8.5 (Fig. 1). Thus, weakly acidic or neutral solution (pH 5-7) could maintain relatively high activity of all three enzymes. The optimal pH of 6.5 for protoplast yield observed in this study is consistent with this conclusion.
Protoplast yield is influenced by many physicochemical factors, especially the constituents of the enzyme solution. In some previous studies of protoplast isolation from seaweed, CaCl2·2H2O, MES buffer, or both, were added into the enzyme solution (Dipakkore et al., 2005; Mussio and Rusig, 2006; Yeong et al., 2008; Huddy et al., 2013), but other studies did not use these reagents (Björk et al., 1990; Wang et al., 2014). In the present study, the protoplast yield was significantly enhanced by base-solution 3 (deionized water containing 25 mmol/L MES-Tris buffer and 25 mmol/L CaCl2·2H2O). It is possible that the MES-Tris buffer maintained a stable pH for enzyme function and CaCl2·2H2O as a plasma membrane stabilizer protected the plasmalemma of the protoplasts (Wang, 1994). We found that a deionized water-based solution gave a higher protoplast yield than seawater based-solution. Similar results were also reported in studies of protoplast isolation from Ulva, Enteromorpha and Monostroma (Dipakkore et al., 2005). This phenomenon was possibly because ions in the seawater result in reduced activity of cell wall lytic enzymes (Dipakkore et al., 2005).
In this study, 0.4 mol/L sorbitol was found to be the optimal concentration for protoplast yield. Similarly, sorbitol was used as the osmotic stabilizer by Wang et al. (2014) in protoplast isolation from Gracilariopsis lemaneiformis, but the concentration of sorbitol in their study was 0.8 mol/L. Osmotic conditions help protoplasts to maintain their integrity after the cell walls are removed (Compton et al., 2000). Sugars and sugar alcohols, such as mannitol, sorbitol and glucose, are commonly used as osmotic stabilizers in protoplast isolation (Araki et al., 1998; Lafontaine et al., 2011; Wang et al., 2014; Zhang et al., 2014). Use of the inorganic osmoticum NaCl was also reported in protoplast isolation from Laminaria species (Butler et al., 1989).
In our experiments, the optimal incubation temperature for protoplast yield from G. bailiniae was 28℃, close to the optimal growth temperature of G. bailiniae in nature (Zhong et al., 2014). An optimal incubation temperature for protoplast isolation close to the optimal growth temperature of the algae was also observed for the tropical species Gracilaria changii and the temperate species Gracilaria Verrucosa (Araki et al., 1998; Yeong et al., 2008; Gupta et al., 2011).
The protoplast yield increased, but the protoplast survival percentage decreased, when the incubation time increased during isolation. A similar result was found in the protoplast isolation from Kappaphycus alvarezii (Zhang et al., 2014). Optimal incubation times for protoplast isolation from many algae are between 2.5 and 3 h, for example 2.5 h for Gracilaria verrucosa (Araki et al., 1998), and 3 h for Gracilaria changii (Yeong et al., 2008), Gracilaria gracilis (Huddy et al., 2013), Monostroma nitidum and Porphyra yezoensis (Kito et al., 1998). Incubation times > 6 h cause the cells to be overdigested, resulting in a significant reduction of the protoplast yield (Yeong et al., 2008).
Cell wall resynthesis of protoplasts was observed to start within 12 h (data not shown) after protoplast isolation from G. bailiniae. Cell wall regeneration began at one pole of the protoplast. A similar pattern of cell wall deposition, beginning at a single pole of the protoplast, was also noted for Gracilaria gracilis (Huddy et al., 2013) and K. alvarezii (Zablackis et al., 1993). Two division types for the first division— asymmetric division and symmetric division—were first reported in the protoplast regeneration of Gracilariopsis genera. After the first division, the divided cells can develop into callus-like masses. In the present study, many callus masses were obtained in the cultivation process, which might have potential for application in production of metabolites of G. bailiniae. Meanwhile, some seaweeds, such as Kappaphycus alvarezii (Reddy et al., 2003), have regenerated whole plants from their calli, so the callus masses obtained in our study might have the potential to produce seed material for G. bailiniae.
In previous reports, protoplasts of Gracilaria changii and Gracilaria gracilis regenerated after 2 months of culture (Yeong et al., 2008; Huddy et al., 2015), andprotoplastsof Gracilariopsis lemaneiformis regenerated after 90 days of culture (Wang et al., 2014). However, we have been unable to regenerate protoplasts of G. bailiniae into whole plants. The capacity for protoplast regeneration might be related to the growth conditions of the algal thalli. Using algae which are in good condition could improve the yield and the survival rate of protoplasts, and be good for protoplast regeneration (Björk et al., 1990; Wang et al., 2014). The thalli of G. bailiniae used in our study had been cultured for 1 month before protoplast isolation, so they might have been in poor condition and unsuitable for subsequent regeneration. In addition, the culture medium used in our study might not be suitable for protoplast regeneration of G. bailiniae. Huddy et al. (2015) regenerated Gracilaria gracilis protoplasts into whole plants in Provasoli's enriched seawater (PES) medium. However, Yeong et al. (2008) obtained whole Gracilaria changii plants from protoplasts only in MES medium but not PES medium. Moreover, the densities of protoplasts in our culture system were 5×104-1×105 cells/mL, which might not be suitable for protoplast regeneration. Thus, further studies, especially to determine the optimum conditions for the growth of protoplasts into whole G. bailiniae plants, need to be conducted.
5 CONCLUSIONIn this study, we established a protocol for protoplast isolation from G. bailiniae and investigated primary development of the protoplasts. This study thus provides a theoretical basis for seedling production of G. bailiniae, and a protoplast isolation method for protoplast fusion and hybridization work. Furthermore, a large number of callus masses could be obtained in our study, which might have potential application in production of G. bailiniae metabolites.
6 ACKNOWLEDGEMENTThe authors thank Professor LIU Tao (Ocean University of China), Professor MEI Zhiping (Shantou University), Dr. WANG Hui (Shantou University) and Ms. WANG Zhongxia (Ocean University of China) for providing much advice and kind assistance.
Araki T, Lu Z, Morishita T. 1998. Optimization of parameters for isolation of protoplasts from Gracilaria verrucosa(Rhodophyta). Journal of Marine Biotechnology, 6(3): 193-197.
|
Baweja P, Sahoo D, García-Jiménez P, Robaina R R. 2009. Review:seaweed tissue culture as applied to biotechnology:problems, achievements and prospects. Phycological Research, 57(1): 45-58.
DOI:10.1111/pre.2009.57.issue-1 |
Bellanger F, Verdus M C, Henocq V, Christiaen D. 1990. Determination of the composition of the fibrillar part of Gracilaria verrucosa (Gracilariales, Rhodophyta) cell wall in order to prepare protoplasts. Hydrobiologia, 204-205(1): 527-531.
DOI:10.1007/BF00040281 |
Björk M, Ekman P, Wallin A, Pedersén M. 1990. Effects of growth rate and other factors on protoplast yield from four species of Gracilaria (Rhodophyta). Botanica Marina, 33(5): 433-439.
|
Butler D M, Østgaard K, Boyen C, Evans L V, Jensen A, Kloareg B. 1989. Isolation conditions for high yields of protoplasts from Laminaria saccharina and L. digitata (Phaeophyceae). Journal of Experimental Botany, 40(11): 1 237-1 246.
DOI:10.1093/jxb/40.11.1237 |
Chen Y C, Shih H C. 2000. Development of protoplasts of Ulva fasciata (Chlorophyta) for algal seed stock. Journal of Phycology, 36(3): 608-615.
DOI:10.1046/j.1529-8817.2000.99128.x |
Chen Y C. 1998. Development of protoplasts from holdfasts and vegetative thalli of Monostroma latissimum(Chlorophyta, Monostromatacae) for algal seed stock. Journal of Phycology, 34(6): 1 075-1 081.
DOI:10.1046/j.1529-8817.1998.341075.x |
Cheney D P, Mar E, Saga N, van der Meer J. 1986. Protoplast isolation and cell division in the agar-producing seaweed Gracilaria (Rhodophyta). Journal of Phycology, 22(2): 238-243.
|
Cheney D P. 1990. Genetic improvement of seaweeds through protoplast fusion. In: Yarish C, Penniman C A, Van Patten P eds. Economically Important Marine Plants of the Atlantic: their Biology and Cultivation. Connecticut Sea Grant College Program, Groton, CT, USA. p.15-25.
|
Compton M E, Saunders J A, Veilleux R E. 2000. Use of protoplast for plant improvement. In: Trigiano R N, Gray D J eds. Plant Tissue Culture Concepts and Laboratory Exercise. CRC Press, Boca Raton, USA. p.249-261.
|
Davey M R, Anthony P, Power J B, Lowe K C. 2005. Plant protoplasts:status and biotechnological perspectives. Biotechnology Advances, 23(2): 131-171.
DOI:10.1016/j.biotechadv.2004.09.008 |
Dipakkore S, Reddy C R K, Jha B. 2005. Production and seeding of protoplasts of Porphyra okhaensis (Bangiales, Rhodophyta) in laboratory culture. Journal of Applied Phycology, 17(4): 331-337.
DOI:10.1007/s10811-005-7291-8 |
Evans D A, Bravo J E. 1983. Protoplast isolation and culture.In: Evans D A, Sharp W R, Ammirato P V, Yamada Y eds.Handbook of Plant Cell Culture, Vol 1. Techniques for Propagation and Breeding. Macmillan, New York, USA.p.124-176. http://agris.fao.org/openagris/search.do?recordID=US8619832
|
Fujita Y, Saito M. 1990. Protoplast isolation and fusion in Porphyra (Bangiales, Rhodophyta). Hydrobiologia, 204-205(1): 161-166.
DOI:10.1007/BF00040228 |
Graham L E, Wilcox L W. 2000. Red algae. In: Graham L E, Wilcox L W eds. Algae. Prentice-Hall, Upper Saddle River, NJ. p.343-396.
|
Gupta V, Kumar M, Kumari P, Reddy C R K, Jha B. 2011. Optimization of protoplast yields from the red algae Gracilaria dura (C. Agardh) J. Agardh and G. verrucosa(Huds.) Papenfuss. Journal of Applied Phycology, 23(2): 209-218.
DOI:10.1007/s10811-010-9579-6 |
Gupta V, Trivedi N, Kumar M, Reddy C R K, Jha B. 2013. Purification and characterization of exo-β-agarase from an endophytic marine bacterium and its catalytic potential in bioconversion of red algal cell wall polysaccharides into galactans. Biomass and Bioenergy, 49: 290-298.
DOI:10.1016/j.biombioe.2012.12.027 |
Huddy S M, Meyers A E, Coyne V E. 2013. Protoplast isolation optimization and regeneration of cell wall in Gracilaria gracilis (Gracilariales, Rhodophyta). Journal of Applied Phycology, 25(2): 433-443.
DOI:10.1007/s10811-012-9877-2 |
Huddy S M, Meyers A E, Coyne V E. 2015. Regeneration of whole plants from protoplasts of Gracilaria gracilis(Gracilariales, Rhodophyta). Journal of Applied Phycology, 27(1): 427-435.
DOI:10.1007/s10811-014-0278-6 |
Hurtado-Ponce A Q. 1992. Rheological properties of agar from Gracilariopsis heteroclada (Zhang et Xia) Zhang et Xia(Gracilariales, Rhodophyta) treated with powdered commercial lime and aqueous alkaline solution. Botanica Marina, 35(5): 365-370.
|
Inoue A, Mashino C, Kodama T, Ojima T. 2011. Protoplast preparation from Laminaria japonica with recombinant alginate lyase and cellulase. Marine Biotechnology, 13(2): 256-263.
|
Kito H, Kunimoto M, Kamanishi Y, Mizukami Y. 1998. Protoplast fusion between Monostroma nitidum and Porphyra yezoensis and subsequent growth of hybrid plants. Journal of Applied Phycology, 10(1): 15-21.
DOI:10.1023/A:1008063415548 |
Lafontaine N, Mussio I, Rusig A M. 2011. Production and regeneration of protoplasts from Grateloupia turuturu Yamada (Rhodophyta). Journal of Applied Phycology, 23(1): 17-24.
DOI:10.1007/s10811-010-9527-5 |
Liu M Q, Yang X Q, Qi B, et al. 2013. Present situation and prospect of polysaccharide and phycobiliprotein from Gracilaria. Science and Technology of Food Industry, 34(13): 338-341.
(in Chinese with English abstract) |
Mantri V A. 2009. Studies on Biology of Gracilaria dura (C.Agardh) J. Agardh. Bhavnagar University, Bhavnagar, India.
|
Mussio I, Rusig A M. 2006. Isolation of protoplasts from Fucus serratus and F. vesiculosus (Fucales, Phaeophyceae):factors affecting protoplast yield. Journal of Applied Phycology, 18(6): 733-740.
DOI:10.1007/s10811-006-9079-x |
Pan J Q, Li S D. 2010. Development and utilization of Gracilaria resources. Chinese Journal of Tropical Agriculture, 30(10): 47-50, 89.
(in Chinese with English abstract) |
Reddy C R K, Dipakkore S, Kumar G R, Jha B, Cheney D P, Fujita Y. 2006. An improved enzyme preparation for rapid mass production of protoplasts as seed stock for aquaculture of macrophytic marine green algae. Aquaculture, 260(1-4): 290-297.
DOI:10.1016/j.aquaculture.2006.06.034 |
Reddy C R K, Gupta M K, Mantri V A, Jha B. 2008. Seaweed protoplasts:status, biotechnological perspectives and needs. Journal of Applied Phycology, 20(5): 619-632.
DOI:10.1007/s10811-007-9237-9 |
Reddy C R K, Gupta V, Jha B. 2010. Developments in biotechnology of red algae. In: Chapman D J, Seckbach J eds. Red Algae in Genomic Age. Springer, New York.p.307-341. http://link.springer.com/10.1007/978-90-481-3795-4_17
|
Reddy C R K, Kumar G R K, Siddhanta A K, Tewari A, Eswaran K. 2003. In vitro somatic embryogenesis and regeneration of somatic embryos from pigmented callus of Kappaphycus alvarezii (Doty) Doty (Rhodophyta, Gigartinales). Journal of Phycology, 39(3): 610-616.
DOI:10.1046/j.1529-8817.2003.02092.x |
Saminathan K R, Ashok K S, Veeragurunathan V, Mantri V A. 2015. Seedling production in the industrially important agarophyte Gracilaria dura (Gracilariales, Rhodophyta). Journal of Applied Phycology, 27(4): 1 541-1 548.
DOI:10.1007/s10811-014-0450-z |
Smit A J. 2004. Medicinal and pharmaceutical uses of seaweed natural products:a review. Journal of Applied Phycology, 16(4): 245-262.
DOI:10.1023/B:JAPH.0000047783.36600.ef |
Tang Z X. 2012. Preparation, Purification and Characterization of Agrase from Paenibacillus sp. Zhejiang University of Technology, Hangzhou, Zhejiang, China. (in Chinese with English abstract)
|
Wang S J. 1994. Seaweed Biotechnology. Shanghai Science and Technology Press, Shanghai, China. p.49-60.
(in Chinese)
|
Wang Z X, Sui Z H, Hu Y Y, Zhang S, Pan Y L, Ju H R. 2014. A comparison of different Gracilariopsis lemaneiformis(Rhodophyta) parts in biochemical characteristics, protoplast formation and regeneration. Journal of Ocean University of China, 13(4): 671-676.
DOI:10.1007/s11802-014-2217-1 |
Yan X H, Wang S J. 1993. Regeneration of whole plants from Gracilaria asiatica Chang et Xia protoplasts(Gracilariaceae, Rhodophyta). Hydrobiologia, 260(1): 429-436.
|
Yeong H Y, Khalid N, Phang S M. 2008. Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta). Journal of Applied Phycology, 20(5): 641-651.
DOI:10.1007/s10811-007-9249-5 |
Zablackis E, Vreeland V, Kloareg B. 1993. Isolation of protoplasts from Kappaphycus alvarezii var. tambalang (Rhodophyta) and secretion of i-carrageenan fragments by cultured cells. Journal of Experimental Botany, 44(9): 1 515-1 522.
DOI:10.1093/jxb/44.9.1515 |
Zemke-White W L, Ohno M. 1999. World seaweed utilisation:an end-of-century summary. Journal of Applied Phycology, 11(4): 369-376.
DOI:10.1023/A:1008197610793 |
Zhang S, Liu C, Jin Y M, Chi S, Tang X M, Chen F X, Fang X, Liu T. 2014. Studies on the isolation and culture of protoplasts from Kappaphycus alvarezii. Acta Oceanologica Sinica, 33(10): 114-123.
DOI:10.1007/s13131-014-0546-y |
Zhao Y, Zhang N, Li B W, Huang Q, Chen Y X. 2005. Comparison on activities of cytodern hydrolase of seaweed from three species of gastropoda. Journal of Xiamen University (Natural Science), 44(2): 276-278.
(in Chinese with English abstract) |
Zhong Z H, Huang Z J, Chen W Z. 2014. Effects of various environmental factors on growth and biochemical components of Gracilaria bailinae. Progress in fishery sciences, 35(3): 98-104.
(in Chinese with English abstract) |
 2018, Vol. 36
2018, Vol. 36


