Institute of Oceanology, Chinese Academy of Sciences
Article Information
- THIRUNAVUKKARASU Muralisankar, PERIYAKALI Saravana Bhavan, SUBRAMANIAN Radhakrishnan, PERUMAL Santhanam
- Influence of two different dietary zinc sources in freshwater prawn Macrobrachium rosenbergii post larvae
- Journal of Oceanology and Limnology, 37(1): 290-299
- http://dx.doi.org/10.1007/s00343-018-7253-z
Article History
- Received Sep. 20, 2017
- accepted in principle Nov. 19, 2017
- accepted for publication Feb. 22, 2018
2 Crustacean Biology Laboratory, Department of Zoology, Bharathiar University, Coimbatore-641 046, Tamil Nadu, India;
3 Department of Arid Land Agriculture, College of Food & Agriculture, United Arab Emirates University, Al Ain-15551, UAE;
4 Marine Planktonology and Aquaculture Laboratory, Department of Marine Science, Bharathidasan University, Tiruchirappalli-620 024, Tamil Nadu, India
The giant river prawn, Macrobrachium rosenbergii is an economically important crustacean species due to its ability to tolerant wide variety of environments, higher market value, delicious taste and presence of significant nutrients like protein, essential amino acids (EAA), saturated fatty acids, minerals and vitamins which plays an essential role in human health.
The nutritional status of feeds plays an important role for regulating the productivity of farmed animals. Zinc (Zn) is an essential trace mineral nutrient required for a wide array of metabolic functions and it is essential for stabilizing cellular membranes, tissue component, and organ fluids of an organism (Nockels, 1994; Kidd et al., 1996; Yamaguchi, 1998). It regulates growth, cell division, synthesis of proteins, energy, metabolism of nutrients (vitamin A, carbohydrates, and lipid), fertility and immune system of all organisms. Zn acts as a cofactor in many enzyme systems and component of a large number of metalloenzymes (alkaline phosphatase, creatine kinase, carbonic anhydrase, carboxypeptidase, alcohol dehydrogenase, glutamic dehydrogenase, calcium-ATPase, thymidine kinase and D-superoxide dismutase etc.) (Hays and Swenson, 1985; Salgueiro et al., 2000; Arinola, 2008). Zn related biological and physiological functions are greatly influenced by its transport and storage. The hepatic metallothionein proteins are regulates the storage and metabolism of Zn (Tan and Mai, 2001; Gammanpila et al., 2007; Herland et al., 2011). Zn is essential for several enzymes, proteins and transcription factors which regulate the vital cellular functions like DNA replication, DNA repair, response to oxidative stress, apoptosis and cell cycle progression (Kumaresan et al., 2017). Zn also essential for optimal innate immune functions, the nutritional deficiency of Zn leads to increase the susceptibility to pathogenic infections and it can suppress the physiological functions, signaling of molecules, and DNA replication and repair of an organism (Witkiewicz-Kucharczyk and Bal, 2006; Yan et al., 2008; Djoko et al., 2015). The optimum level of dietary Zn regulates the survival, growth performance, muscle composition, nutrient utilization, reproductive performance and nonspecific immune response of fishes and crustaceans have been reported by previous studies (Herland et al., 2011; Hasnat et al., 2012; Muralisankar et al., 2015a).
Recent years, aquaculture industries have showed potential interest on using nanotechnology to revolutionize their activities, such as uptake of hormones, vaccines, drugs, nutrients etc. (Rather et al., 2011). The influence of nanoparticles (NPs), such as Zn-NPs, Se-NPs, FeO-NPs, Cu-NPs, MgO-NPs, MnO-NPs etc., in aquatic organisms have been reported (Zhou et al., 2009; Muralisankar et al., 2014, 2016; Asaikkutti et al., 2016; Srinivasan et al., 2016, 2017). Hence, present study was performed to evaluate the effects of two different forms of dietary Zn sources, such as bulk zinc [B-Zn (size 10 μm)] and zinc nanoparticles [Zn-NPs (size 50 nm)] on the survival, growth, amino acids composition, fatty acids composition, and total and differential haemocytes count (THC and DHC) of M. rosenbergii post larvae.
2 MATERIAL AND METHOD 2.1 Collection of experimental prawnsPost larvae (PL) of M. rosenbergii (age PL five; 0.07±0.01 g) were obtained from M/s. Aqua Hatchery, Mugaiyur Village in Kanchipuram District of Tamil Nadu, India. Prawn PL were safely transported to the laboratory conditions using polythene bags half filled with oxygenated mother water and acclimatized to ambient laboratory conditions for three weeks in a cement tank (capacity 1 000 L) with ground water with optimal level of physico-chemical characteristics (temperature, 27±1.14℃; dissolved oxygen, 7.29±0.21 mg/L; pH, 7.53±0.20; total dissolved solids, 0.59±0.05 g/L; biological oxygen demand, 19.60±1.25 mg/L; chemical oxygen demand, 66.0±2.40 mg/L; ammonia, 0.015±0.002 mg/L; suspended Zn, 25.00±3.61 μg/L). During acclimatization period, adequate aeration was provided to the prawn larvae and they were fed with boiled egg albumin, Artemia nauplii (Artemia salina) and control feed (prepared with basal feed ingredients) thrice (at 06:00 h, 12:00 h, and 18:00 h respectively) per day, and 80% of rearing tap water was renewed daily.
2.2 Experimental feed formulationExperimental feeds were prepared using locally available feed ingredients (Table 1). Feed ingredients were purchased from local shops. For preparation of these experimental feeds, fishmeal and soybean meal, wheat flour and tapioca flour, and cod liver oil were used as protein, carbohydrate and lipid sources respectively. Also, tapioca flour and egg albumin were used as binding agents, and vitamins (B complex with vitamin C) and Zn free mineral mix were also added. The optimum concentrations (60 mg/kg) of dietary B-Zn and Zn-NPs were selected for M. rosenbergii PL according to previous reports of Muralisankar et al.(2014, 2015a). In the present study, 99.99% pure B-Zn (size 10 μm) and Zn-NPs (size 50 nm) were purchased from Sigma-Aldrich Chemicals Pvt. Limited, Bangalore, India. These two different sources of Zn were supplemented in the basal feeds at the concentration of 60 mg/kg separately. Feed without supplementation of Zn source was served as control. According to the method of Muralisankar et al. (2014), about 3.0±0.84 mm dia feeds were prepared and stored in plastic containers until used for feeding experiments.
The proximate compositions of formulated feeds were analyzed according to standard methods (Table 1). Briefly, the amounts of crude protein, lipid, fiber, moisture and ash contents were analyzed according to standard procedures of AOAC (1995). The content of nitrogen-free extracts (NFE) and gross energy were calculated according to Natarajan (2006). Briefly, NFE (%)=100–(% moisture+% crude protein+% crude lipid+% total ash+% crude fiber). Gross energy was calculated using the energy values contributed by the protein, NFE and lipid fractions of feed with multiplying of generalised physiological energy values of proteins (19 kJ), NFE (15 kJ) and lipids (36 kJ) respectively, the detailed calculation as follows:
energy contributed by crude protein (kJ/g)=protein content of feed (%)×19=x,
energy contributed by NFE (kJ/g)=NFE content of feed (%)×15=z,
energy contributed by crude lipid (kJ/g)=lipid content of feed (%)×36=y,
gross energy (GE kJ/g)=(x+y+z)/100.
2.4 Experimental procedureIn the study, three groups of M. rosenbergii PL ranged from 1.44±0.37 cm length and 0.19±0.02 g weight were assigned for this experiment in triplicate (three independent groups per feed) for a period of 90 days. One group was served as a control and fed the control feed (without supplementation of any Zn source). The remaining two groups were fed feeds supplemented with 60 mg/kg B-Zn and Zn-NPs, respectively. The prawn groups were separately maintained in a 40-L aquarium [size 27" (width)×11" (length)×10" (height) and the water level was maintained 8" height] with stocking density of one larva /L. During the experimental period, the 3/4th of aquarium water was renewed every day by siphoning method with minimum disturbance to the prawns and sufficient aeration was also provided. Prawns were fed these experimental feeds at 10% of body weight (body weight was measured every 30 days interval for adjusting the feeding) twice per day (at 06:00 and 18:00). During the experimental period, prawn larvae were maintained on a 12 h light:12 h dark photoperiod. The unfed feed, feces and moults were removed by siphoning filtration during the feeding trial while renewing aquarium water.
2.5 Analysis of Zn content in feeds and prawnsThe concentration of Zn in experimental feeds and prawns were determined using the atomic absorption spectrophotometer (Perkin-Elmer; Model 2380) in air acetylene flame by adopting the triple acid digestion method. To attain this, feed/sacrificed prawns were digested in 9:3:1 ratio of concentrated nitric acid, sulphuric acid, and nitric acid using Kjeldhal digestion chamber at 400℃ for 2 h. The digested samples were allowed to cool at room temperature and diluted with deionized water (AOAC, 1995).
2.6 Survival, growth and food index analysisThe survival percent, growth performance [length gain (LG), weight gain (WG) and specific growth rate (SGR)] and food index parameters, such as feed intake (FI) and feed conversion ratio (FCR) were calculated by the following equations:
survival (%)=No. of live prawns/ No. of prawns introduced×100,
LG (cm)=final length (cm)–initial length (cm),
WG (g)=final weight (g)–initial weight (g),
SGR (% /day)= log final weight (g)– log initial weight (g)/total number of days×100,
FI (g/day)=feed eaten (g)/total number of days,
FCR=feed intake (g)/weight gain (g).
2.7 Amino acids analysisThe amino acids profile of experimental prawns were analyzed using high-performance thin-layer chromatography (HPTLC) method described by Hess and Sherma (2004). Sample preparation, standard amino acids and working procedures were adopted according to previous studies (Radhakrishnan et al., 2015). Accurately, 1 μL (41.6 μg of sample or standard amino acids) of solution was loaded and processed in the instrument. The peak area of the sample was matched with standard amino acids and quantified. The results of amino acid concentrations were expressed as percent in dry weight of prawn sample.
2.8 Fatty acids analysisThe fatty acids profile of the experimental prawns were analyzed using the gas chromatography (GC) method (Nichols et al., 1993). Fatty acids from sacrificed prawns were obtained from lipids by saponification using NaOH dissolved in the methanolwater mixture (hydrolysis with alkali). They were converted into fatty acid-methyl ester using HCl and methanol mixture which can be easily detectable by GC. Nitrogen was used as the carrier gas and the chromatogram was used for the calculation. Standard fatty acids were also analyzed simultaneously. Each fatty acid was indentified based on the retention time and peak area. The amount of fatty acids expressed as percent per 2 μL of methylated fatty acid sample of prawn.
2.9 Enumeration of total and differential haemocytesThe total and differential heamocytes counts were performed at the end of 90 days experiment. Briefly, 0.1 mL of haemolymph was collected from the ventral sinus in the first abdominal segment of prawns using a 26-gauge hypodermic needle on a 1-mL syringe. The syringe was pre-filled with 0.2 mL of anticoagulant (10 mmol/L Tris-HCl, 250 mmol/L sucrose, 100 mmol/L sodium citrate, pH 7.6). The anticoagulated haemolymph was made up to 1 mL with more anticoagulant, followed by fixed with 10% formalin (1:1) for 30 min for determination of THC and DHC.
THC was determined by diluting of haemolymph with ice-cold phosphate buffer saline (PBS, 20 mmol/L, pH 7.2) at 1:2 ratio (v/v). The diluted haemolymph was stained with 0.2 mL of Rose Bengal (1.2%), followed by incubated at room temperature for 15 to 20 min. The hemocytometer (Neubauer improved, Germany) was used to determine the THC under the light microscope (Labomed, CXR2) and calculated as THC (×106 cells/mL)=counted cells×depth of chamber×dilution factor/ number of 1 mm square. DHC was determined by staining of fixed haemolymph with Rose Bengal (10%) for 10 min at room temperature and smeared on a clean slide. The three different haemocytes were characterised (Tsing et al., 1989) and 350–400 cells from each smear were counted under a Trinocular Inverted Microscope (INVERSO 3000).
2.10 Statistical analysisThe results were presented as means±SD of three replicates. Data from each treatment were subject to one-way analysis of variance (ANOVA), followed by Duncan's multiple range test (DMRT) using SPSS (version 20) to compare the differences among treatments where significant differences (P < 0.05) were observed. The data of survival, amino acid and fatty acid were arcsine transformed prior to one-way analysis.
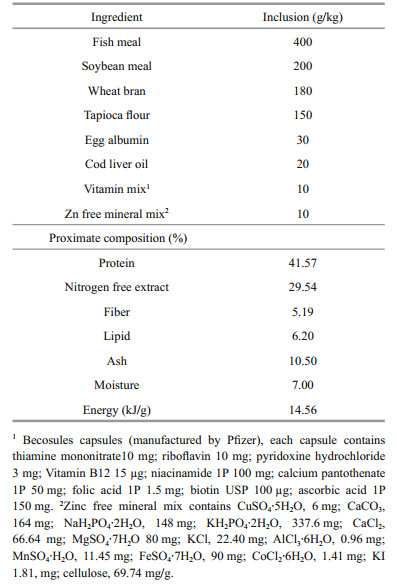
3 RESULT 3.1 Zn content in experimental feeds and prawnsIn the present study, the analysed Zn content was significantly (P < 0.05) elevated in both B-Zn and ZnNPs supplemented feeds when compared to control feed, whereas, insignificant difference (P>0.05) was noted between B-Zn and Zn-NPs supplemented feeds (Table 2). In context, in the case of experimental prawns, the analysed Zn content was significantly increased (P < 0.05) in prawns fed Zn-NPs supplemented feeds, followed by B-Zn when compared with the control.
|
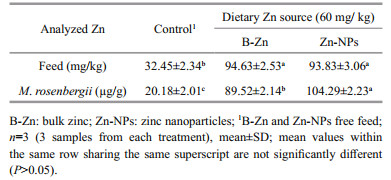
Survival rate was significantly (P < 0.05) higher in B-Zn and Zn-NPs supplemented feeds fed prawns group when compared to control feed fed prawns group, however, the insignificant (P>0.05) difference was observed between B-Zn and Zn-NPs supplemented feeds fed prawns (Table 3). Present study, LG, WG, SGR and FI were significantly (P < 0.05) higher in ZnNPs supplemented feed fed prawns group, followed by B-Zn fed prawns when compared to control feed fed prawns, whereas, the FCR was significantly (P < 0.05) decreased in Zn-NPs supplemented feed fed prawns group when compared to the control.
|
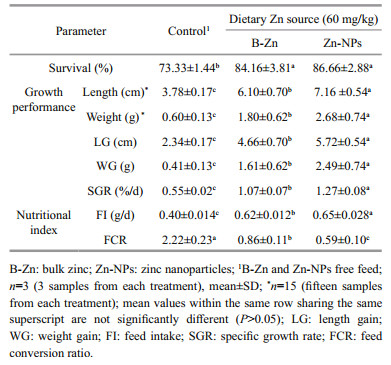
Current study, nine indispensable amino acids and five dispensable amino acids were noted in prawns fed control, B-Zn and Zn-NPs supplemented feeds (Table 4). Among the EAA, arginine, methionine, isoleucine and cystine were found to be significantly higher (P < 0.05) in prawns fed on Zn-NPs supplemented feeds when compared to B-Zn and control feed fed prawns. In context, tryptophan, leucine and phenylalanine were significantly increased in both B-Zn and Zn-NPs supplemented feed fed prawns compared to control. Whereas, the insignificant elevations were observed in lysine and histidine contents of prawns fed with control, B-Zn and Zn-NPs supplemented feeds. Also, the insignificant (P>0.05) difference was observed in all nonessential amino acids (NAA) between control and both Zn sources supplemented feeds fed prawn groups. The ratio of EAA /NEAA was found to be higher in prawns fed to Zn-NPs supplemented feed, followed by B-Zn and control.
|
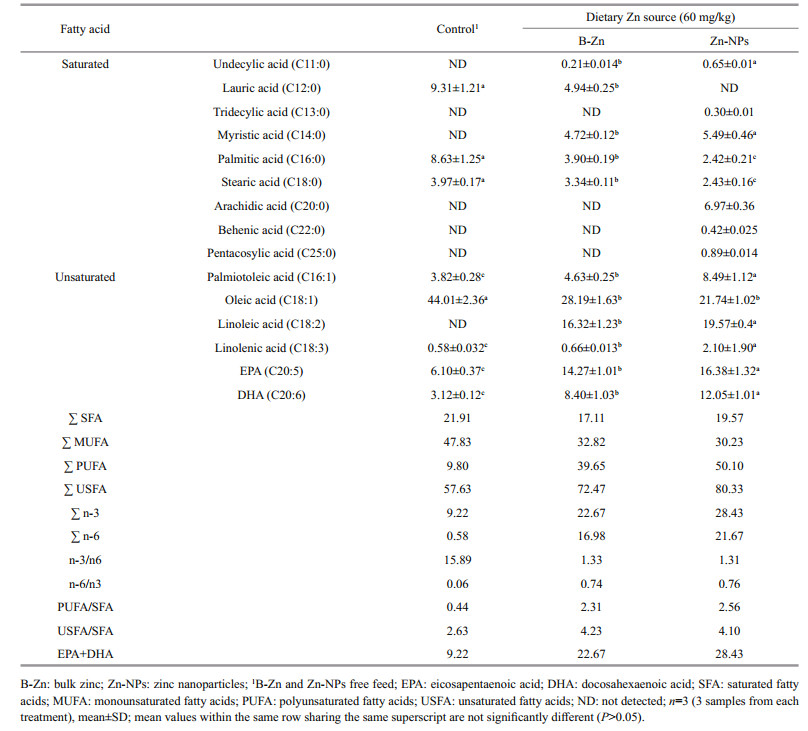
The saturated fatty acids (SFA), such as lauric acid, palmitic acid and stearic acids were significantly elevated (P < 0.05) in prawns fed with control feed when compared to B-Zn and Zn-NPs supplemented feeds fed prawn groups (Table 5). Whereas, undeceylic acid and myristic acid were found to be significantly (P < 0.05) higher in prawns fed on Zn-NPs supplemented feed. While, tridecylic acid, arachidic acid, behenic acid and pentacosylic acid were detected in prawns fed Zn-NPs alone. The monounsaturated fatty acids (MUFA), palmiotoleic acid and oleic acid were found to be significantly (P < 0.05) higher in Zn (B-Zn and Zn-NPs) supplemented feeds fed prawns and control feed fed prawns group respectively. In context, the polyunsaturated fatty acids (PUFA) like linoleic acid, linolenic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) were significantly (P < 0.05) elevated in prawns fed with Zn-NPs supplemented feed, followed by B-Zn when compared with the control. Further, the sum of unsaturated fatty acids (UFA), PUFA, n-3, n-6 fatty acids and EPA+DHA were found to be higher in ZnNPs supplemented feed fed prawns. Whereas, the sum of SFA and MUFA were higher in control feed fed prawns. In context, the ratio of n-6/n-3 and PUFA/ SFA were elevated in control feed fed prawns. While, the USFA/SFA ratio was found to be higher in B-Zn supplemented feed fed prawns.
|
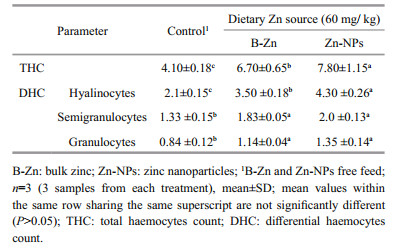
Present study, THC was significantly higher in ZnNPs supplemented feed fed prawns, followed by B-Zn and control feed fed prawns (Table 6). The DHC, such as hyalinocytes, semigranulocytes, and granulocytes were significantly (P < 0.05) elevated in B-Zn and Zn-NPs supplemented feed fed prawns compared to control feed fed prawns, however, the insignificant(P>0.05) difference were noted in semigranulocytes, and granulocytes between B-Zn and Zn-NPs supplemented feed fed prawns.
|
Zn is crucial for normal growth and the reproduction of all organisms. It plays a key role on physiology, growth and fulfills an immune function (Frassinetti et al., 2006). Zn in cells bound to proteins, peptides, and amino acids and it is required for different biological functions including enzymatic reactions, metabolism, neurotransmission, cell signaling, DNA synthesis and gene expression (Beyersmann, 2002; Murakami and Hirano, 2008). The dietary requirements of Zn have been studied for variety of aquatic species including crustaceans through feeding experiments with semipurified feeds (NRC, 2011). In the present study, the significant elevation of Zn in B-Zn and Zn-NPs supplemented feeds indicates that the bioavailability of Zn was increased due to supplementation. Further, the significant elevation of Zn content in prawns fed on B-Zn and Zn-NPs supplemented feeds indicates that the supplementation of both Zn sources can increase the Zn concentration in experimental prawns. While, between these two different Zn sources, ZnNPs produced significantly higher level of Zn contents in experimental prawns, it might be due their more penetration to cells at nano-scale level, followed by more storage in tissues. Dietary supplementations of minerals like Zn, Zn-NPs, Cu and Cu-NPs showed significant elevations in the muscle Zn and Cu contents in M. rosenbergii post larvae have been reported earlier (Muralisankar et al., 2014, 2015a, b, 2016).
The quantitative and qualitative productions of aquaculture can be determined by assessing the survival and growth performance of cultivable species. In the current study, the significant elevations in survival, growth (LG, WG and SGR) and FI in both B-Zn and Zn-NPs supplemented feeds fed prawns suggests that the supplementation of these two different Zn sources have ability to promote survival, growth, feed intake of M. rosenbergii. Further, the significant decreases of FCR in Zn-NPs and B-Zn supplemented feeds indicates the better quality of experimental feeds than control which shows that given feeds were efficiently utilized by prawns towards growth. Similar findings have been reported in giant river prawn (M. rosenbergii), giant tiger prawn (Penaeus monodon) and Chinese mitten crab (Eriocheir sinensis) fed with Zn included feed (Davis et al., 1993; Shiau and Jiang, 2006; Li et al., 2010; Muralisankar et al., 2015a). In this present study, among the two different Zn sources, Zn-NPs produced significantly better performance in growth (LG, WG and SGR) and FI, it suggests that the nano-scale level of Zn has more influence than that of bulk form due to their higher surface area. Previously, Muralisankar et al. (2014) reported that provide of Zn-NPs had shown significant positive impacts on survival, growth and FI of M. rosenbergii. Also, dietary administration of magnesium oxide nanoparticles (MgO-NPs), iron oxide nanoparticles (FeO-NPs) and manganese oxide nanoparticles (MnO-NPs) nanoparticles produced better survival, growth, SGR and PER in M. rosenbergii have also been reported (Asaikkutti et al., 2016; Srinivasan et al., 2016, 2017).
Amino acids are the simplest units of proteins and they form the building blocks of protein structure. Each amino acid has its own biological function and metabolism in living organisms (Wu, 2009). In the present study, the elevations in EAA, such as lysine, cystine, arginine, methionine, isoleucine, tryptophan, leucine and phenylalanine in prawns fed on B-Zn and Zn-NPs supplemented feeds indicates that two different forms of this trace element have potent to regulate the production of essential amino acids by M. rosenbergii, it led to produced better muscle meat quality. Also, the elevated level of EAA/NEAA ratio in B-Zn and Zn-NPs supplemented feeds fed prawn indicates that the amino acids were well balanced by prawns. The influence of B-Zn and Zn-NPs on synthesis of total amino acids has been reported earlier in M. rosenbergii (Muralisankar et al., 2014, 2015a). The effects of Zn forms on proline uptake by American lobster, Homarus americanus has also been reported earlier (Monteilh-Zoller et al., 1999). Present study, among these two mineral forms, Zn-NPs supplemented feed produced significantly more elevation on these essential amino acids, it indicates that nano forms of Zn (Zn-NPs) has more influence on these amino acids production. The similar results have been in M. rosenbergii PL fed with FeO-NPs and MgO-NPs (Srinivasan et al., 2016, 2017). The insignificant alterations of nonessential amino acids (NEAA) like proline, glysine, glutamine, alanine, and glutamic acid between control and experimental (BZn and Zn-NPs) feeds fed prawns suggests that these metal forms did not produce any adverse effects on these amino acids production in M. rosenbergii. The insignificant difference of nonessential amino acids between control and nanoparticles (FeO-NPs and MgO-NPs) supplemented feed prawns have also been reported earlier (Srinivasan et al., 2016, 2017).
Fatty acids are important constituents of lipid in crustaceans and it is well known that the fatty acid composition of dietary lipids is metabolic significance (Del Rosario Gonzalez-Baro and Pollero, 1998). The role of essential trace elements on lipid metabolism in the living organisms have been reported by some nutritional investigations (Petering et al., 1977; Engle et al., 2000a, b, c). In the current study, the significant elevations of SFA (myristic acid and undecylic acid) and USFA (palmiotoleic acid, linoleic acid, linolenic acid, EPA and DHA) in prawns fed on B-Zn and ZnNPs supplemented feeds indicates that the supplementation of these two different forms of Zn had influence on synthesis of fatty acids in M. rosenbergii. Synthesis of these essential fatty acids (EFA) might have regulation on survival and growth in experimental prawns. The role of EFA on survival and growth of crustacean (Litopenaeus vannamei, P. monodon and E. sinensis) post larvae have been reported previously (Rees et al., 1994; Lim et al., 1997; Wen et al., 2006). In context, the SFA, such as arachidic acid, pentacosylic acid, tridecylic acid, and behenic acid were noted in prawns fed Zn-NPs supplemented feed alone, it indicates that fact Zn-NPs have potent to synthesis of these particular fatty acids in M. rosenbergii. While, undetectable level of lauric acid and significant decrease in oleic acid in prawns fed Zn-NPs supplemented feed suggests that Zn-NPs might be suppressed synthesis of this particular fatty acid in M. rosenbergii. Among these two different Zn sources, Zn-NPs produced significantly better performance on overall fatty acids synthesis, this might be due to its higher surface area which led to more activities on fatty acids synthesis. The poor performance of fatty acids synthesis in prawns fed control feed indicates the deficiency of Zn in feeds. Dietary Zn mediated fatty acid synthesis has been reported in E. sinensis (Li et al., 2010). Influence of trace elements like B-Zn, Zn-NPs, Cu and Cu-NPs on muscle total lipid contents have also been reported in M. rosenbergii (Muralisankar et al., 2014, 2015a, b, 2016). Further, the influences of dietary FeO-NPs and MgO-NPs on fatty acid synthesis in M. rosenbergii have been reported earlier by Srinivasan et al.(2016, 2017). In the present study, the elevation in sum (Ʃ) of USFA, PUFA, n-3 and n-6 fatty acids, EPA+DHA in prawns fed with Zn-NPs supplemented feeds indicates that Zn-NPs had remarkable influence on these fatty acids synthesis. Also, the increased PUFA/SFA and n-6/n-3 ratios in Zn-NPs and B-Zn supplemented feed fed prawn groups shows their better fatty acids balance.
The hematological assays like total and differential blood cell counts are the primary parameters to determine the health status of aquatic animals. Minerals play an essential role on immune system of aquatic organisms (Ward et al., 1993; Lee and Shiau, 2002; Shiau and Su, 2003; Cheng et al., 2005; Liu et al., 2010). Haemocytes are the primary mediators of immunity in invertebrates which carrying out the significant immune function like phagocytic and pathogens trapping that protect the animals against infection without specific antibodies. In this study, the significant elevation of THC and DHC in prawns fed with B-Zn and Zn-NPs supplemented feeds indicates that both Zn source had influenced the production of haemocytes in experimental prawns. Similarly, the significant elevations in haemocytes production of M. rosenbergii and E. sinensis when fed to different level of dietary Cu, Cu-NPs, and MgO-NPs have been reported (Sun et al., 2013; Muralisankar et al., 2016; Srinivasan et al., 2017).
6 CONCLUSIONResults of present study indicates that the dietary supplementation of 60 mg/kg B-Zn and Zn-NPs could enhance the survival, growth, composition of essential amino acids, unsaturated fatty acids, and haemocytes population of the freshwater prawn, M. rosenbergii. However, among these two forms of Zn sources (BZn and Zn-NPs), the nano forms of zinc (Zn-NPs) produced significantly better performance in prawns due to its higher surface area which led to increased more biological functions. Hence, 60 mg/kg Zn-NPs can be used as dietary Zn source instead of B-Zn for better culture of freshwater prawns.
7 DATA AVAILABILITY STATEMENTAll data are provided in full in the results and table sections of this paper.
AOAC. 1995. Official Methods of Analysis of AOAC International. 16thedn. AOAC International, Gaithersburg, USA.
|
Arinola O G. 2008. Essential trace elements and metal binding proteins in Nigerian consumers of alcoholic beverages. Pak. J. Nutr., 7(6): 763-765.
DOI:10.3923/pjn.2008.763.765 |
Asaikkutti A, Bhavan P S, Vimala K, Karthik M, Cheruparambath P. 2016. Dietary supplementation of green synthesized manganese-oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii. J. Trace Elem. Med. Biol., 35: 7-17.
DOI:10.1016/j.jtemb.2016.01.005 |
Beyersmann D. 2002. Homeostasis and cellular functions of zinc. Materwiss. Werksttech., 33(12): 764-769.
DOI:10.1002/(ISSN)1521-4052 |
Cheng W, Liu C H, Kuo C M, Chen J C. 2005. Dietary administration of sodium alginate enhances the immune ability of white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol., 18(1): 1-12.
DOI:10.1016/j.fsi.2004.03.002 |
Davis D A, Lawrence A L, Gatlin Ⅲ D M. 1993. Evaluation of the dietary zinc requirement of Penaeus vannamei and effects of phytic acid on zinc and phosphorus bioavailability. J. World Aquacult. Soc., 24(1): 40-47.
DOI:10.1111/jwas.1993.24.issue-1 |
Del Rosario Gonzalez-Baro M, Pollero R J. 1998. Fatty acid metabolism of Macrobrachium borellii:dietary origin of arachidonic and eicosapentaenoic acids. Comp. Biochem.Physiol. A Mol. Integr. Physiol., 119(3): 747-752.
DOI:10.1016/S1095-6433(98)01028-9 |
Djoko K Y, Ong C I Y, Walker M J, McEwan A G. 2015. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem., 290(31): 18 954-18 961.
DOI:10.1074/jbc.R115.647099 |
Engle T E, Spears J W, Armstrong T A, Wright C L, Odle J. 2000a. Effects of dietary copper source and concentration on carcass characteristics and lipid and cholesterol metabolism in growing and finishing steers. J. Anim. Sci., 78(4): 1 053-1 059.
DOI:10.2527/2000.7841053x |
Engle T E, Spears J W, Fellner V, Odle J. 2000c. Effects of soybean oil and dietary copper on ruminal and tissue lipid metabolism in finishing steers. J. Anim. Sci., 78(10): 2 713-2 721.
DOI:10.2527/2000.78102713x |
Engle T E, Spears J W, Xi L, Edens F W. 2000b. Dietary copper effects on lipid metabolism and circulating catecholamine concentrations in finishing steers. J. Anim. Sci., 78(10): 2 737-2 744.
DOI:10.2527/2000.78102737x |
Frassinetti S, Bronzetti G L, Caltavuturo L, Cini M, Croce C D. 2006. The role of zinc in life:a review. J. Environ.Pathol. Toxicol. Oncol., 25(3): 597-610.
DOI:10.1615/JEnvironPatholToxicolOncol.v25.i3 |
Gammanpila M, Age A Y, Bart A N. 2007. Evaluation of the effects of dietary vitamin C, E and zinc supplementation on reproductive performance of Nile tilapia (Oreochromis niloticus). Sri Lanka J. Aquat. Sci., 12: 39-60.
|
Hasnat A, Rani B, Kohli M P S, Chandraprakash G. 2012. Zinc supplementation and its effect on thermal stress resistance in Carassius auratus fry. Isr.J. Aquacult. Bamidgeh, 64: 779.
|
Hays V W, Swenson M J. 1985. Minerals and bones. In: Dukes' Physiology of Domestic Animals. 10th edn. Cornell University, Ithaca, Greece. p.449-466.
|
Herland H, Cooper M, Esaiassen M, Olsen R L. 2011. Effects of dietary mineral supplementation on quality of fresh and salt-cured fillets from farmed Atlantic cod, Gadus morhua. J. World Aquacult. Soc., 42(2): 261-267.
DOI:10.1111/jwas.2011.42.issue-2 |
Hess B, Sherma J. 2004. Quantification of arginine in dietary supplement tablets and capsules by silica gel highperformance thin-layer chromatography with visible mode densitometry. Acta Chromatogra., 14: 60-69.
|
Kidd M T, Ferket P R, Qureshi M A. 1996. Zinc metabolism with special reference to its role in immunity. Worlds Poult. Sci. J., 52(3): 309-324.
DOI:10.1079/WPS19960022 |
Kumaresan V, Palanisamy R, Pasupuleti M, Arockiaraj J. 2017. Impacts of environmental and biological stressors on immune system of Macrobrachium rosenbergii. Rev Aquacult., 9(3): 283-307.
DOI:10.1111/raq.2017.9.issue-3 |
Lee M H, Shiau S Y. 2002. Dietary copper requirement of juvenile grass shrimp, Penaeus monodon, and effects on non-specific immune responses. Fish Shellfish Immunol., 13(4): 259-270.
DOI:10.1006/fsim.2001.0401 |
Li W W, Gong Y N, Jin X K, He L, Jiang H, Ren F, Wang Q. 2010. The effect of dietary zinc supplementation on the growth, hepatopancreas fatty acid composition and gene expression in the Chinese mitten crab, Eriocheir sinensis(H. Milne-Edwards) (Decapoda:Grapsidae). Aquacult.Res, 41(11).
|
Lim C, Ako H, Brown C L, Hahn K. 1997. Growth response and fatty acid composition of juvenile Penaeus vannamei fed different sources of dietary lipid. Aquaculture, 151(1-4): 143-153.
DOI:10.1016/S0044-8486(96)01500-1 |
Liu T L, Wen H, Jiang M, Yuan D N, Gao P, Zhao Y J, Wu F, Liu W. 2010. Effect of dietary Chromium picolinate on growth performance and blood parameters in grass carp fingerling, Ctenopharyngodon idellus. Fish Physiol.Biochem., 36(3): 565-572.
DOI:10.1007/s10695-009-9327-5 |
Monteilh-Zoller M K, Zonno V, Storelli C, Ahearn G A. 1999. Effects of zinc on L-[3H]proline uptake by lobster(Homarus americanus) hepatopancreatic brush-border membrane vesicles. J. Exp. Biol., 202(21): 3 003-3 010.
|
Murakami M, Hirano T. 2008. Intracellular zinc homeostasis and zinc signaling. Cancer Sci., 99(8): 1 515-1 522.
DOI:10.1111/cas.2008.99.issue-8 |
Muralisankar T, Bhavan P S, Radhakrishnan S, Seenivasan C, Manickam N, Srinivasan V. 2014. Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn, Macrobrachium rosenbergii. Biol. Trace Elem.Res., 160(1): 56-66.
DOI:10.1007/s12011-014-0026-4 |
Muralisankar T, Bhavan P S, Radhakrishnan S, Seenivasan C, Srinivasan V, Santhanam P. 2015a. Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions, and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture, 448: 98-104.
DOI:10.1016/j.aquaculture.2015.05.045 |
Muralisankar T, Bhavan P S, Radhakrishnan S, Seenivasan C, Srinivasan V. 2016. The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachium rosenbergii post larvae. J. Trace Elem. Med. Biol., 34: 39-49.
DOI:10.1016/j.jtemb.2015.12.003 |
Muralisankar T, Bhavan P S, Srinivasan V, Radhakrishnan S, Seenivasan C, Manickam N. 2015b. Effects of dietary copper on the growth physiology and biochemistry of the freshwater prawn Macrobrachium rosenbergii post larvae. Int. J. Pure Appl. Biosci., 3(2): 509-518.
|
Natarajan M. 2006. Analysis of shrimp/fish Feeds for proximate composition. In: Ali S A ed. Training Manual on Shrimp and Fish Nutrition and Feed Management. CIBA Special Publication. p.104-109.
|
Nichols D S, Nichols P D, McMeekin T A. 1993. Polyunsaturated fatty acids in Antarctic bacteria. Antarct.Sci., 5(2): 149-160.
|
Nockels C F. 1994. Micornutrients and the immune response.In: Montana Nutrition Conference Proceedings. Bozeman, Montana, USA. p.3.1.
|
NRC. 2011. Nutrient Requirements of Fish and Shrimp. National Academies Press, Washington, D.C, USA.
|
Petering H G, Murthy L, O'Flaherty E. 1977. Influence of dietary copper and zinc on rat lipid metabolism. J. Agric.Food Chem., 25(5): 1 105-1109.
DOI:10.1021/jf60213a051 |
Radhakrishnan S, Bhavan P S, Seenivasan C, Muralisankar T, Shanthi R. 2015. Effects of native medicinal herbs(Alternanthera sessilis, Eclipta alba and Cissus quadrangularis) on growth performance, digestive enzymes and biochemical constituents of the monsoon river prawn Macrobrachium malcolmsonii. Aquacult.Nutr., 21(4): 496-506.
DOI:10.1111/anu.2015.21.issue-4 |
Rather M A, Sharma R, Aklakur M, Ahmad S, Kumar N, Khan M, Ramya V L. 2011. Nanotechnology:a novel tool for aquaculture and fisheries development a prospective mini-review. Fish Aquacult. J., 16: 1-5.
|
Rees J F, Curé S, Piyatiratitivorakul P, Sorgeloos P, Menasveta P. 1994. Highly unsaturated fatty acid requirements of Penaeus monodon postlarvae:an experimental approach based on Artemia enrichment. Aquaculture, 122(2-3): 193-207.
DOI:10.1016/0044-8486(94)90510-X |
Salgueiro M, Zubillaga M, Lysionek A, Sarabia M I, Caro R, De Paoli T, Hager A, Weill R, Boccio J. 2000. Zinc as an essential micronutrient:a review. Nutr. Res., 20(5): 737-755.
DOI:10.1016/S0271-5317(00)00163-9 |
Shiau S Y, Jiang L C. 2006. Dietary zinc requirements of grass shrimp, Penaeus monodon, and effects on immune responses. Aquaculture, 254(1-4): 476-482.
DOI:10.1016/j.aquaculture.2005.10.033 |
Shiau S Y, Su L W. 2003. Ferric citrate is half as effective as ferrous sulfate in meeting the iron requirement of juvenile tilapia, Oreochromis niloticus×O. aureus. J. Nutr., 133(2): 483-488.
DOI:10.1093/jn/133.2.483 |
Srinivasan V, Bhavan P S, Rajkumar G, Satgurunathan T, Muralisankar T. 2016. Effects of dietary iron oxide nanoparticles on the growth performance, biochemical constituents and physiological stress responses of the giant freshwater prawn Macrobrachium rosenbergii postlarvae. Int. J. Fish Aquat. Stud., 4(2): 170-182.
|
Srinivasan V, Bhavan P S, Rajkumar G, Satgurunathan T, Muralisankar T. 2017. Dietary supplementation of magnesium oxide (MgO) nanoparticles for better survival and growth of the freshwater prawn Macrobrachium rosenbergii post-larvae. Biol. Trace Elem. Res., 177(1): 196-208.
DOI:10.1007/s12011-016-0855-4 |
Sun S M, Qin J G, Yu N, Ge X P, Jiang H B, Chen L Q. 2013. Effect of dietary copper on the growth performance, nonspecific immunity and resistance to Aeromonas hydrophila of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol., 34(5): 1195-1201.
DOI:10.1016/j.fsi.2013.01.021 |
Tan B P, Mai K S. 2001. Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture, 192(1): 67-84.
DOI:10.1016/S0044-8486(00)00435-X |
Tsing A, Arcier J M, Brehélin M. 1989. Hemocytes of penaeid and palaemonid shrimps:morphology, cytochemistry, and hemograms. J. Invert. Pathol., 53(1): 64-77.
DOI:10.1016/0022-2011(89)90075-X |
Ward J D, Spears J W, Kegley E B. 1993. Effect of copper level and source (copper lysine vs copper sulfate) on copper status, performance, and immune response in growing steers fed diets with or without supplemental molybdenum and sulfur. J. Anim. Sci., 71(10): 2748-2755.
DOI:10.2527/1993.71102748x |
Wen X B, Chen L Q, Ku Y M, Zhou K Y. 2006. Effect of feeding and lack of food on the growth, gross biochemical and fatty acid composition of juvenile crab, Eriocheir sinensis. Aquaculture, 252(2-4): 598-607.
DOI:10.1016/j.aquaculture.2005.07.027 |
Witkiewicz-Kucharczyk A, Bal W. 2006. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol. Lett., 162(1): 29-42.
|
Wu G Y. 2009. Amino acids:metabolism, functions, and nutrition. Amino Acids, 37(1): 1-17.
|
Yamaguchi M. 1998. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med., 11(2-3): 119-135.
DOI:10.1002/(ISSN)1520-670X |
Yan M, Song Y, Wong C P, Hardin K, Ho E. 2008. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J. Nutr., 138(4): 667-673.
DOI:10.1093/jn/138.4.667 |
Zhou X X, Wang Y B, Gu Q, Li W F. 2009. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture, 291(1-2): 78-81.
DOI:10.1016/j.aquaculture.2009.03.007 |
 2019, Vol. 37
2019, Vol. 37