Institute of Oceanology, Chinese Academy of Sciences
Article Information
- JIANG Weiwei, DU Meirong, FANG Jianguang, GAO Yaping, MAO Yuze, CHEN Qionglin, LIN Fan, JIANG Zengjie
- Response of Yesso scallop Patinopecten yessoensis to acute temperature challenge: physiological and biochemical parameters
- Journal of Oceanology and Limnology, 37(1): 321-329
- http://dx.doi.org/10.1007/s00343-019-7245-7
Article History
- Received Aug. 24, 2017
- accepted in principle Nov. 8, 2017
- accepted for publication Jan. 22, 2018
2 Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266200, China
The Yesso scallop, Patinopecten yessoensis, is a cold-tolerant species distributed in the coastal areas such as the North Pacific Ocean, the south Okhotsk Sea and the Japan Sea (Kosaka, 2016). P. yessoensis has become an economically important mollusk in the northern parts of China, and production has developed quickly since its introduction in 1982. However, the scallop aquaculture industry has been thwarted by mass summer mortality in the northern Yellow Sea (Xiao et al., 2005; Guo and Luo, 2016). Thermocline in the north Yellow Sea is often influenced by the Northern Yellow Sea Cold Water Mass (NYSCWM), creating water temperature variations of up to 7℃ within the same layer, and the cultivation Yesso scallops framework is often laid in this layer (Lan, 1990). Experimental data indicated that water temperature changes, including acute changes or frequent fluctuations, can be a primary environmental stressor affecting scallops in growth, metabolism and immune function (Pilditch and Grant, 1999; Chen et al., 2007). It is possible that water temperature changes related to scallops physiology and immunity is an important contributing factor to summer mortalities in Yellow Sea.
Water temperature can be an important environmental stressor affecting growth, reproduction and immunization activities in poikilothermic organisms (Le Moullac and Haffner, 2000). The physiological activities of ectothermic organisms, including respiration and excretion (Somero, 2002) and ingestion (Fearman and Moltschaniwskyj, 2010) are usually strongly limited by the ambient temperature (Van Der Have, 2002). Environmental temperature changes may also induce modification in the antioxidant status of ectothermic organisms (Vosloo et al., 2013b). In the Antarctic intertidal limpet Nacella concinna, significant enhancement in superoxide dismutase (SOD) and catalase (CAT) activities was observed in gills and digestive tissues when water temperature increased from 0℃ to 4℃ and 9℃ (Abele et al., 1998). SOD and CAT are key enzymatic players in antioxidant defenses that detoxify O2- and H2O2, respectively (Hermes-Lima et al., 1998). Other immune enzymes and terminal products such as acid phosphatase (ACP), which is a typical lysosomal enzyme involved in degrading foreign particles in bivalves (Chen et al., 2007); and malondialdehyde (MDA), which is formed during the process of lipid peroxidation (Kehrer, 1993), also seem to be sensitive to water temperature fluctuations.
SODs (EC 1.15.1.1) are classified into three distinct groups depending on their redox-active metals: iron SOD (Fe-SOD), manganese SOD (Mn-SOD) and copper/zinc SOD (Cu/Zn-SOD). Cu/Zn-SOD is very significant because of its physiological function and therapeutic potential (Ni et al., 2007). Thermal stress has been considered a serious stimulator that regulates the expression of the Cu/Zn-SOD gene. In disk abalone, Haliotis discus, the mRNA levels of Cu/ZnSOD genes were highly modulated in gill, muscle and hepatopancreas from juvenile abalones by thermal treatment (Kim et al., 2007). However, little is known about the Cu/Zn-SOD gene responses of Yesso scallop to water temperature fluctuations.
In this study, the physiological activities (oxygen consumption and ammonia-N excretion rates), enzyme parameters (SOD, CAT, ACP and MDA), and Cu/Zn-SOD gene expression were analyzed while Yesso scallops were exposed to acute temperature changes. Understanding the physiological and biochemical responses of Yesso scallops to temperature stressors in summer will provide further information about their thermal tolerance mechanism and may contribute to the management of scallop mortality.
2 MATERIAL AND METHOD 2.1 Animals and rearing conditionsIn July 2015, healthy adult P. yessoensis, with average shell height of 5.91±0.69 cm and body weight of 24.63±5.73 g, were collected from Xunshan Fishery Group (Weihai, Shandong, China). Scallops were cleaned of epibionts, and maintained in flowthrough rearing systems containing 750-L grit-filtered seawater (salinity, 30.00; pH, 7.97±0.20) for 7 days before the challenge experiments at 23±0.5℃. Animals were fed daily with Phaeodactylum tricornutum during the period of acclimation.
2.2 Experimental designFor acute temperature decrease treatment (Tdec treatment), scallops were cold-shocked for 72 h by transferring them from 23℃ to 15℃. Thereafter, the same animals were moved abruptly from 15℃ to 23℃ in the following 72 h (acute temperature increase treatment, Tinc treatment). Design of the acute temperature change treatments was based on the abnormal water temperature fluctuations in the Yellow Sea caused by the NYSCWM in June 2014, which caused water temperature variations of about 8℃ in 72 h (unpublished data). Simultaneously, scallop culture has suffered from severe mortalities in Zhangzidao Fishery Group, a leading Chinese company in scallop aquaculture. Three replicate tanks were set up for each treatment. After each temperature fluctuation, five scallops were arbitrarily selected at 0, 6, 24, 48 and 72 h for the oxygen consumption and ammonia-N excretion rates analyses, and another five scallops were sampled randomly to evaluate ingestion rate. Nine scallops were sampled at 0, 3, 6, 9, 12, 24, 48 and 72 h to examine the activities of SOD, CAT and ACP; the content of MDA in the hepatopancreas; and the Cu/Zn-SOD gene expression in the gills. Scallops acclimated to the constant temperature of 23℃ were sampled as controls.
2.3 Measurement of physiological parametersOne scallop from each replicate was placed in a sealed plastic respirometric chamber containing 2-L seawater, and kept in a water bath at 23℃ or 15℃ for 2 h. At the end of the experiment, the concentration of dissolved oxygen (DO) in each respiration chamber must be kept in a relatively high level no less than 60% of the initial DO concentration (based on a preliminary experiment) to avoid a negatively effect on normal physiological activities. DO was measured with a smarTROLL Multiparameter Handheld (InSitu Inc., Andersonville, USA). Ammonia-N was determined colorimetrically according to the Chinese National Standard (GB/T 12763.4-2007). The soft tissue of each scallop was excised and dried for 48 h to constant weight at 60℃. Oxygen consumption and ammonia-N excretion rates were detected by the difference between the experimental and control (without animals) chambers.
Oxygen consumption rate (O2 mg/(g·h)) and ammonia-N excretion rate (μmol/(g·h)) were calculated by multiplying the difference in dissolved oxygen (mg/L) and ammonia-N (μmol/L) by the volume of seawater in each respirometric chamber (L), and by dividing the result by the dry weight of soft tissue (g) and time lapse (h). The atomic ratios of oxygen to nitrogen (O/N) were calculated by dividing the amount of oxygen consumed by the ammonium nitrogen excreted in each scallop (Mayzaud and Conover, 1988).
2.4 Enzyme activity assaysThe hepatopancreas samples were dissected from scallops, shock-frozen in liquid nitrogen and then stored at -80℃ for subsequent enzyme activity analysis. A portion of 500 mg of the hepatopancreas was homogenized in 10 volumes of 0.01 mol/L phosphate buffered saline (pH 7.4) on ice with a chilled glass homogenizer. The samples were centrifuged at 10 000×g at 4℃ for 30 min. After centrifugation, the supernatants were obtained and used immediately for determination of the activities of different enzymes.
SOD, CAT and ACP activities, and MDA content, were measured using assay kits (Nanjing Jiancheng Chemical Industries, Nanjing, Jiangsu, China). Details of the assays have been described previously (Góth, 1991; Cong et al., 2008; Wang et al., 2008).
2.5 Tissue expression analyses 2.5.1 Tissue sampling, RNA extraction and cDNA synthesisGills were removed from three individuals and pooled together as a replicate sample. Three replicates were used per treatment. Tissues samples were stored in liquid nitrogen until RNA extraction.
Total RNA was isolated from gills using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocols. The quality and purity of RNA were checked by agarose gel electrophoresis and absorbance spectrophotometry to confirm suitability for cDNA synthesis. Total RNA (1 μg) was reverse transcribed by using Anchoredoligo(dT)18 primers and the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's protocol. cDNA mix was diluted 3-fold and stored at -80℃ until subsequent real-time PCR analyses were made.
2.5.2 Quantification of Cu/Zn-SOD gene expression levelsPrimers for Cu/Zn-SOD gene and Gapdh used in this study were described by Jiang et al. (2011) and Bao et al. (2011), respectively. Cu/Zn-SOD-F: ATG TAA TGG CTG GAA ACG ATG G; Cu/Zn-SOD-R: ATC ACC AAG GTT CTG CCG ATG; Gapdh-F: TGG TAT GGC TTT CCG TGT GC; Gapdh-R: TCC TCT GTG TAA CCA AGG AAC C. Gapdh was used as an endogenous control for its expression stability.
cDNA (1 μL of a 1:3 dilution) was subjected to quantitative real-time PCR on the LightCycler 480 Ⅱ system (Roche Diagnostics, Basel, Switzerland) by using SYBR Green I Master (Roche Applied Science, Mannheim, Germany), containing 5 μL Master Mix, 0.5 μL each primer and 3 μL water, PCR-grade. A real-time PCR program was performed as follows: initial denaturation at 95℃ for 5 min, 45 cycles at 95℃ for 10 s, 55℃ for 15 s and 72℃ for 10 s. Each quantitative real-time PCR amplification was performed in triplicate. Transcript levels of the Cu/ Zn-SOD gene were calculated relative to Gapdh transcript levels by using the 2-ΔΔCt method, and the value indicated an n-fold change relative to the control group.
2.6 Statistical analysisAll data were presented as mean±standard deviation, and subjected to analysis of variance (ANOVA) followed by Duncan's multiple range test. The enzyme activities and Cu/Zn-SOD gene expression levels were analyzed by two-way ANOVA. A value of P < 0.05 was considered significant.
3 RESULT 3.1 Physiological activities 3.1.1 Oxygen consumptionOxygen consumption rates of P. yessoensis decreased significantly with an acute decrease in ambient water temperature, and increased with a sudden rise in water temperature (Fig. 1a). Oxygen consumption rates of P. yessoensis in the Tdec treatment were significantly lower than those in control groups at 0, 6, 48 and 72 h (P < 0.05). By contrast, the highest oxygen consumption rate was observed at 6 h in the Tinc treatment, which was significantly higher than that of all other groups (P < 0.05). No differences were found between the Tinc treatment and the control groups at 0, 24, 48 and 72 h (P>0.05).
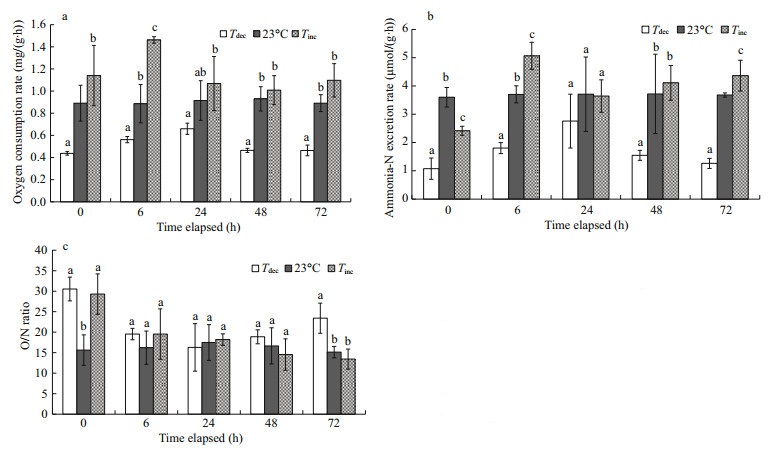
|
| Fig.1 Changes in oxygen consumption rates (a), ammonia-N excretion rates (b) and O/N ratios (c) of Patinopecten yessoensis suddenly exposed to water temperature changes Values were expressed as means±SD. Bars without shared letters for the same time indicate significant differences from each other (P<0.05). Tdec: acute temperature decrease; Tinc: acute temperature increase. |
Ammonia-N excretion rates of P. yessoensis were dramatically influenced by water temperature fluctuations (Fig. 1b). Scallops exposed suddenly to 15℃ had a significantly lower ammonia-N excretion rate than those in the control groups (P < 0.05), but this increased gradually thereafter and reached the maximum at 24 h. The ammonia-N excretion rate initially decreased significantly as temperature changed rapidly from 15℃ to 23℃ (P < 0.01), but increased significantly at 6 h and 72 h (P < 0.05).
3.1.3 O/N ratioThe O/N ratios of P. yessoensis were estimated to be 13.44−30.52 under different water temperature treatments (P < 0.05; Fig. 1c). Scallops presented significantly higher O/N ratios in response to an acute temperature change from 23℃ to 15 than did those in the control groups at 0 and 72 h (P < 0.05). However, no differences were found between the Tinc treatment and the control groups at 6, 24, 48 and 72 h (P>0.05).
3.2 Enzyme activities 3.2.1 Antioxidant enzymatic activity enhanced by acute water temperature changeSignificant increases in activities of antioxidant enzymes were observed in P. yessoensis exposed to abrupt water temperature changes (P < 0.05) (Fig. 2). The activity of SOD increased significantly for scallops transferred from 15℃ to 23℃ for exposures of 3, 6, 9, 24 and 48 h, and reached the maximum of 13.63 U/mg prot (P < 0.05; Fig. 2a). CAT activity ranged from 9.52 to 13.22 U/mg prot (Fig. 2b). A significant increase in CAT activity occurred in the Tdec treatment after 12 h exposure, whereas CAT activity decreased significantly at 12 h in the Tinc treatment (P < 0.01). No differences in CAT activity levels were observed between the Tdec treatment and the control group after 3, 6, 9 or 48 h exposures (P>0.05).
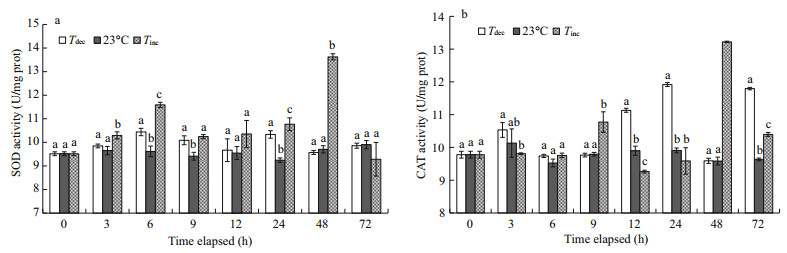
|
| Fig.2 Changes in superoxide dismutase (SOD) (a) and catalase (CAT) (b) activities of P. yessoensis suddenly exposed to water temperature changes Values were expressed as means±SD. Bars without shared letters for the same time indicate significant differences from each other (P<0.05). |
Figure 3 shows that the levels of ACP activity in the hepatopancreas of all experimental scallops were significantly increased compared with the control (P < 0.05). Significant increases in hepatopancreatic ACP activity were found in scallops after 6 h exposure in the Tdec treatment, and reached the maximum value of 67.98 U/g protat 24 h (P < 0.01). In the Tinc treatment, significantly higher values of ACP activity were also observed at 3, 9, 12, 24 and 48 h in scallops as compared with those of the control (P < 0.01), and then returned to near the initial level.
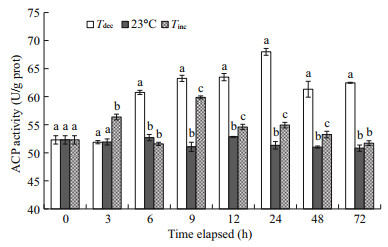
|
| Fig.3 Changes in acid phosphatase (ACP) activities of P. yessoensis suddenly exposed to water temperature changes Values were expressed as means±SD. Bars without shared letters for the same time indicate significant differences from each other (P<0.05). |
The change in MDA concentration in the hepatopancreas was more sensitive to acute water temperature decrease (Fig. 4). Compared with the control, MDA content in the Tdec treatment increased significantly after evisceration, and the maximum value (12.11 nmol/mg prot) occurred after 6 h exposure (P < 0.01). Furthermore, there was a significant increase in MDA content in the Tinc treatment following 3, 9, 12 and 24 h exposures to 23℃ (P < 0.01), and no significant difference was found at 6, 48 and 72 h, respectively (P>0.05).
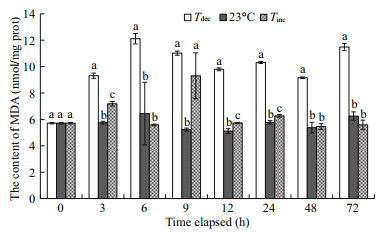
|
| Fig.4 Changes in malondialdehyde (MDA) content of P. yessoensis suddenly exposed to water temperature changes Values were expressed as means±SD. Bars without shared letters for the same time indicate significant differences from each other (P<0.05). |
The temporal expressions of the Cu/Zn-SOD gene in gills from the control and all treated groups were relatively quantified by real-time PCR with Gapdh as internal control (Fig. 5). Results showed that the Cu/ Zn-SOD gene expression levels in scallops changed significantly in response to acute water temperature changes (P < 0.05). Cu/Zn-SOD gene expression in gills increased significantly compared with those in the control group and reached the maximum at 6 h (23.12-fold of the control group) in the Tdec treatment (P < 0.01). Scallops exposed to 72 h heat shock from 15℃ to 23℃ also exhibited significant increases in the expression levels of Cu/Zn-SOD gene after 3 h recovery (P < 0.01).
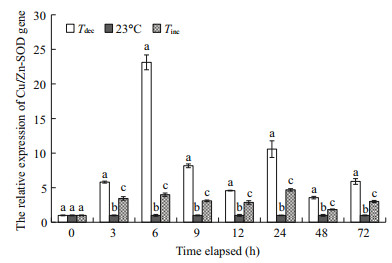
|
| Fig.5 The Cu/Zn-SOD gene expressions of P. yessoensis suddenly exposed to water temperature changes Values were expressed as means±SD. Bars without shared letters for the same time indicate significant differences from each other (P<0.05). |
Water temperature changes affect the physiological and biochemical activities of marine mollusks, as described for the mussel Dreissena polymorpha, Pacific oyster Crassostrea gigas and South African abalone H. midae (Vosloo and Vosloo, 2010). Results of the present study support previous conclusions and show the significant effects of temperature change on Yesso scallops. The significantly lower oxygen consumption and ammonia-N excretion rates in the Tdec treatment might be related to the effect of water temperature on the activities of enzymes involved in normal metabolism (Bougrier et al., 1995). Indeed, the increased normal enzyme metabolic rate would be synchronous with the increasing water temperature. On the other hand, oxygen consumption rates of P. yessoensis in the Tinc treatment were significantly increased at 6 h (P < 0.05; Fig. 1). Saucedo et al. (2004) showed that some mollusks are capable of performing metabolic compensation in oxygen consumption as a response to acute water temperature fluctuation over moderate temperature changes, especially at warmer temperatures. The increases in oxygen consumption rates in the Tinc treatment may be explained by partial metabolic compensation (Tian et al., 2004). Interestingly, a significant decrease in the ammonia-N excretion rate was detected in the Tinc treatment at 0 h (P < 0.01; Fig. 1b), suggesting that an acute temperature increase could induce the ammonia efflux regulation of scallops. According to Sadok et al. (1999), reducing the ammonia efflux rate under environmental stress is a strategy for energy conservation. In this study, to some extent the conserved ammonia or energy seemed to be important in restoring the normal levels of some amino acids in scallops after exposure to different thermal stressors.
Identification of energy storage and use is usually determined by tissue biochemical composition analysis, but can also be provided by the O/N ratio of the organisms (Corner and Cowey, 1968). Mayzaud (1973) and Ikeda (1974) reported a minimum value of O/N ratio is 7 for strictly proteinic catabolism, and an O/N ratio is about 24 for metabolizing protein and lipid catabolism. An O/N ratio between 24 and infinity corresponds to a carbohydrate- and lipid- dominated catabolism, as indicated by Mayzaud and Conover (1988). In the present study, an O/N ratio between 13.44 and 23.41 from 6 h to 24 h is indicative of a protein and lipid metabolism. Interestingly, scallops presented significantly higher O/N ratios in response to an acute temperature decrease from 23℃ to 15℃ at 0 h and 72 h (P < 0.05; Fig. 1c), suggesting that the level of carbon-based substrate metabolism increases after an acute temperature decrease.
The ability of mollusks to increase antioxidant enzymatic activity is an indication of their capacity to tolerate environmental stressors (Regoli and Principato, 1995; Wang et al., 2012; Vosloo et al., 2013a). In our study, the activities of antioxidant enzymes (i.e., SOD and CAT) in the hepatopancreas were significantly influenced by water temperature fluctuations. Similar results have also been reported in the mussel Mytilus edulis (Power and Sheehan, 1996; Lesser et al., 2010), indicating that SOD and CAT activities (to some degree) play a considerable role in protecting scallops against oxidative damage caused by thermal stress. The antioxidant enzyme SOD converts superoxide anion O2- to H2O2, after which CAT catalyzes H2O2 to H2O and O2 (Matés and Sánchez-Jiménez, 1999).
A rising oxygen consumption rate increases the release of mitochondrial reactive oxygen species (ROS), as reported by Boveris et al. (1976). In the Tinc treatment, the significant trend of increased hepatopancreatic SOD activity with increased oxygen consumption levels may indicate that more ROS were present (Lurman et al., 2007). Significant increases in SOD and CAT activities were also found in the Tdec treatment (P < 0.01; Fig. 2), which has been demonstrated for other marine invertebrates such as the Zhikong scallop Chlamys farreri (Chen et al., 2007) and the sea cucumber A. japonicus (Wang et al., 2008). The high antioxidant enzymatic activities would serve to prepare the organisms for potential oxidative stress upon re-oxygenation (Hermes-Lima and Zenteno-Savı́n, 2002; Vosloo et al., 2013a). Significant depressed CAT activity was also found in the Tinc treatment (Fig. 2b, P < 0.01). These results probably suggest that CAT activity in scallops was greater in the Tinc treatment after 12 h exposure (sensitive to an increase in water temperature), and that lengthy exposure to high temperatures would cause cell damage (Wang et al., 2012).
Phagocytosis is considered one of the most important mechanisms of internal defense in bivalve mollusks (Wootton et al., 2003). As a typical enzyme in lysosomes of phagocytes, ACP activity in thermally stressed scallops was significantly higher than that in the control group (P < 0.05; Fig. 3), and could help to destroy and clear foreign particles more effectively. This result is consistent with a reported rise in haemocyte lysate in the Zhikong scallop C. farreri (Chen et al., 2007). Moreover, the activity of ACP in the Tdec treatment was significantly higher than that in the Tinc treatment. When animals are exposed to a low temperature, unsaturated lipids will increase in the membrane components, resulting in more permeability to the exogenous or endogenous substrates (Camus et al., 2000; Zhang et al., 2006). The enhanced ACP activity in scallops challenged at 15℃ might be due to the destabilized lysosomal membrane (Camus et al., 2000).
Lipid peroxidation (measured as MDA) is considered a biomarker of oxyradical stress and the level of oxidative damage in an organism (Wei and Yang, 2015). In the present study, the content of MDA in thermally stressed scallops increased significantly after evisceration (P < 0.05; Fig. 4), suggesting that acute water temperature fluctuations had induced a disordered antioxidant system and caused oxidative damage. From the above discussion it is clear that extreme water temperature changes can activate the antioxidant defense system and the acute phase response system, including increasing SOD and CAT activities in Yesso scallop, P. yessoensis, to maintain homeostasis. However, when scallops were acutely exposed to 23℃ again, significantly depressed CAT activity, together with significantly increased MDA content, was observed at 12 h (P < 0.01; Figs. 2, 4), indicating that lengthy exposure to thermal stress may lead to lipid peroxidation and cell damage (Wang et al., 2012).
To compare sensitivity to thermal stress, the gills were selected to study P. yessoensis Cu/Zn-SOD gene expression after exposure to different temperature stressors. Real-time PCR analysis demonstrated that the Cu/Zn-SOD transcripts in gills had increased significantly compared with those in the control group (P < 0.01; Fig. 5). The up-regulation in the Cu/Zn-SOD gene is likely linked to its role in protecting against oxidative damage caused by thermal stress. The expression pattern of Cu/Zn-SOD gene in this study is similar to data reported for disk abalone, H. discus (Kim et al., 2007), but different from that of bay scallop, Argopecten irradians, for which the highest expression was observed in hemocytes (Bao et al., 2009). In aquatic organisms, gills are the primary organ for respiration and osmoregulation (Havas and Rosseland, 1995), and present a large surface area in direct contact with the external environment. The upregulated expressions of Cu/Zn-SOD gene occurred in gill tissue of P. yessoensis, suggesting that Cu/ZnSOD is a constitutive protein that may also play a considerable role in gills.
5 CONCLUSIONThe variations in physiological and biochemical activities found in Yesso scallop, P. yessoensis, seem closely correlated with water temperature changes in summer. Oxygen consumption and ammonia-N excretion rates of P. yessoensis decreased significantly with an acute decrease in ambient water temperature and increased with a sudden rise in water temperature. Enzymatic activities, including SOD, CAT and ACP activities, can be induced by acute water temperature changes. However, lengthy exposure to thermal stress may lead to lipid peroxidation and cell damage. Moreover, the overexpression of the Cu/Zn-SOD gene in gills can also be triggered by thermal stress. These data provided valuable insights into the possible thermal mechanisms of scallop mass mortalities.
6 DATA AVAILABILITY STATEMENTThe datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThe authors would like to thank WANG Junwei, WANG Senlin, and ZHANG Yitao of the Chudao Fishery Group for obtaining experimental animals and valuable technical support.
Abele D, Burlando B, Viarengo A, Pörtner H O. 1998. Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpetNacella Concinna. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 120(2): 425-435.
DOI:10.1016/S0305-0491(98)10028-7 |
Bao X B, Liu W D, Jiang B, Su H, Li Y F, Shan Z G, Yu H N, He C B. 2011. Expression stability of reference genes for quantitative PCR in Japanese scallop Patinopecten yessoensis. Fisheries Science, 30(10): 603-608.
(in Chinese with English abstract) |
Bao Y B, Li L, Xu F, Zhang G F. 2009. Intracellular copper/zinc superoxide dismutase from bay scallop Argopecten irradians:its gene structure, mRNA expression and recombinant protein. Fish & Shellfish Immunology, 27(2): 210-220.
|
Bougrier S, Geairon P, Deslous-Paoli J M, Bacher C, Jonquières G. 1995. Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture, 134(1-2): 143-154.
DOI:10.1016/0044-8486(95)00036-2 |
Boveris A, Cadenas E, Stoppani A O M. 1976. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochemical Journal, 156(2): 435-444.
DOI:10.1042/bj1560435 |
Camus L, Grøsvik B E, Børseth J F, Jones M B, Depledge M H. 2000. Stability of lysosomal and cell membranes in haemocytes of the common mussel (Mytilus edulis):effect of low temperatures. Marine Environmental Research, 50(1-5): 325-329.
DOI:10.1016/S0141-1136(00)00056-8 |
Chen M Y, Yang H S, Delaporte M, Zhao S J. 2007. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture, 271(1-4): 479-487.
DOI:10.1016/j.aquaculture.2007.04.051 |
Cong M, Song L S, Wang L L, Zhao J M, Qiu L M, Li L, Zhang H. 2008. The enhanced immune protection of Zhikong scallop Chlamys farreri on the secondary encounter with Listonella anguillarum. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 151(2): 191-196.
DOI:10.1016/j.cbpb.2008.06.014 |
Corner E D S, Cowey C B. 1968. Biochemical studies on the production of marine zooplankton. Biological Reviews, 43(4): 393-426.
DOI:10.1111/brv.1968.43.issue-4 |
Fearman J, Moltschaniwskyj N A. 2010. Warmer temperatures reduce rates of gametogenesis in temperate mussels, Mytilus galloprovincialis. Aquaculture, 305(1-4): 20-25.
DOI:10.1016/j.aquaculture.2010.04.003 |
Góth L. 1991. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta, 196(2-3): 143-151.
DOI:10.1016/0009-8981(91)90067-M |
Guo X M, Luo Y S. 2016. Scallops and scallop culture in China. In: Shumway S E, Parsons G J eds. Scallops: Biology, Ecology, Aquaculture, and Fisheries. Elsevier, Amsterdam, Netherlands. p.937-953.
|
Havas M, Rosseland B O. 1995. Response of zooplankton, benthos, and fish to acidification:an overview. Water, Air, and Soil Pollution, 85(1): 51-62.
|
Hermes-Lima M, Storey J M, Storey K B. 1998. Antioxidant defenses and metabolic depression.The hypothesis of preparation for oxidative stress in land snails. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 120(3): 437-448.
DOI:10.1016/S0305-0491(98)10053-6 |
Hermes-Lima M, Zenteno-Savín T. 2002. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 133(4): 537-556.
|
Ikeda T. 1974. Nutritional ecology of marine zooplankton. Memoirs of the Faculty of Fisheries, Hokkaido University, 22(1): 1-97.
|
Jiang B, Bao X B, Zhang M, Gao X G, Li S L, Liu W D. 2011. Cu/Zn superoxide dismutase and its transcription expression analysis in Mizuhopecten yessoensis. Biotechnology Bulletin, (12): 150-156.
(in Chinese with English abstract) |
Kehrer J P. 1993. Free radicals as mediators of tissue injury and disease. Critical Reviews in Toxicology, 23(1): 21-48.
DOI:10.3109/10408449309104073 |
Kim K Y, Lee S Y, Cho Y S, Bang I C, Kim K H, Kim D S, Nam Y Y. 2007. Molecular characterization and mRNA expression during metal exposure and thermal stress of copper/zinc- and manganese-superoxide dismutases in disk abalone, Haliotis discus discus. Fish & Shellfish Immunology, 23(5): 1 043-1 059.
|
Kosaka Y. 2016. Scallop fisheries and aquaculture in Japan. In: Shumway S E, Parsons G J eds. Scallops: Biology, Ecology, Aquaculture, and Fisheries. Elsevier, Amsterdam, Netherlands. p.891-937.
|
Lan S F. 1990. Hydrologic analysis of the death of scallops in sea area of Changshan Island in summer. Marine Sciences, 14(2): 60-61.
(in Chinese with English abstract) |
Le Moullac G, Haffner P. 2000. Environmental factors affecting immune responses in Crustacea. Aquaculture, 191(1-3): 121-131.
DOI:10.1016/S0044-8486(00)00422-1 |
Lesser M P, Bailey M A, Merselis D G, Morrison J R. 2010. Physiological response of the blue mussel Mytilus edulis to differences in food and temperature in the Gulf of Maine. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 156(4): 541-551.
|
Lurman G J, Bock C H, Pörtner H O. 2007. An examination of the metabolic processes underpinning critical swimming in Atlantic cod (Gadus morhua L.) using in vivo 31P-NMR spectroscopy. Journal of Experimental Biology, 210(21): 3 749-3 756.
DOI:10.1242/jeb.008763 |
Matés J M, Sánchez-Jiménez F. 1999. Antioxidant enzymes and their implications in pathophysiologic processes. Frontiers in Bioscience, 4: D339-D345.
DOI:10.2741/A432 |
Mayzaud P, Conover R J. 1988. O:N atomic ratio as a tool to describe zooplankton metabolism. Marine EcologyProgress Series, 45: 289-302.
DOI:10.3354/meps045289 |
Mayzaud P. 1973. Respiration and nitrogen excretion of zooplankton. Ⅱ. Studies of the metabolic characteristics of starved animals. Marine Biology, 21(1): 19-28.
DOI:10.1007/BF00351188 |
Ni D J, Song L S, Gao Q, Wu L T, Yu Y D, Zhao J M, Qiu L M, Zhang H, Shi F F. 2007. The cDNA cloning and mRNA expression of cytoplasmic Cu, Zn superoxide dismutase(SOD) gene in scallop Chlamys farreri. Fish & Shellfish Immunology, 23(5): 1 032-1 042.
|
Pilditch C A, Grant J. 1999. Effect of temperature fluctuations and food supply on the growth and metabolism of juvenile sea scallops (Placopecten magellanicus). Marine Biology, 134(2): 235-248.
DOI:10.1007/s002270050542 |
Power A, Sheehan D. 1996. Seasonal variation in the antioxidant defence systems of gill and digestive gland of the blue mussel, Mytilus edulis. Comparative Biochemistry and Physiology Part C:Pharmacology, Toxicology and Endocrinology, 114(2): 99-103.
DOI:10.1016/0742-8413(96)00024-2 |
Regoli F, Principato G. 1995. Glutathione, glutathionedependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions:implications for the use of biochemical biomarkers. Aquatic Toxicology, 31(2): 143-164.
DOI:10.1016/0166-445X(94)00064-W |
Sadok S, Uglow R F, Haswell S J. 1999. Some aspects of nitrogen metabolism in Mytilus edulis:effects of aerial exposure. Marine Biology, 135(2): 297-305.
DOI:10.1007/s002270050627 |
Saucedo P E, Ocampo L, Monteforte M, Bervera H. 2004. Effect of temperature on oxygen consumption and ammonia excretion in the Calafia mother-of-pearl oyster, Pinctada mazatlanica (Hanley, 1856). Aquaculture, 229(1-4): 377-387.
DOI:10.1016/S0044-8486(03)00327-2 |
Somero G N. 2002. Thermal physiology and vertical zonation of intertidal animals:optima, limits, and costs of living. Integrative and Comparative Biology, 42(4): 780-789.
DOI:10.1093/icb/42.4.780 |
Tian X L, Dong S L, Wang F, Wu L X. 2004. The effects of temperature changes on the oxygen consumption of juvenile Chinese shrimp Fenneropenaeus chinensis Osbeck. Journal of Experimental Marine Biology and Ecology, 310(1): 59-72.
DOI:10.1016/j.jembe.2004.04.002 |
Van Der Have T M. 2002. A proximate model for thermal tolerance in ectotherms. Oikos, 98(1): 141-155.
DOI:10.1034/j.1600-0706.2002.980115.x |
Vosloo A, Laas A, Vosloo D. 2013a. Differential responses of juvenile and adult South African abalone (Haliotis midae Linnaeus) to low and high oxygen levels. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 164(1): 192-199.
|
Vosloo D, Van Rensburg L, Vosloo A. 2013b. Oxidative stress in abalone:The role of temperature, oxygen and L-proline supplementation. Aquaculture, 416-417: 265-271.
DOI:10.1016/j.aquaculture.2013.09.031 |
Vosloo D, Vosloo A. 2010. Response of cold-acclimated, farmed South African abalone (Haliotis midae) to shortterm and long-term changes in temperature. Journal of Thermal Biology, 35(7): 317-323.
DOI:10.1016/j.jtherbio.2010.06.006 |
Wang F Y, Yang H S, Gao F, Liu G B. 2008. Effects of acute temperature or salinity stress on the immune response in sea cucumber, Apostichopus japonicus. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 151(4): 491-498.
|
Wang X Q, Wang L L, Zhang H, Ji Q Z, Song L S, Qiu L M, Zhou Z, Wang M Q, Wang L L. 2012. Immune response and energy metabolism of Chlamys farreri under Vibrio anguillarum challenge and high temperature exposure. Fish & Shellfish Immunology, 33(4): 1 016-1 026.
|
Wei K Q, Yang J X. 2015. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish & Shellfish Immunology, 43(2): 510-519.
|
Wootton E C, Dyrynda E A, Ratcliffe N A. 2003. Bivalve immunity:comparisons between the marine mussel(Mytilus edulis), the edible cockle (Cerastoderma edule)and the razor-shell (Ensis siliqua). Fish & Shellfish Immunology, 15(3): 195-210.
|
Xiao J, Ford S E, Yang H S, Zhang G F, Zhang F S, Guo X M. 2005. Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture, 250(3-4): 602-615.
DOI:10.1016/j.aquaculture.2005.05.002 |
Zhang Z H, Li X X, Vandepeer M, Zhao W. 2006. Effects of water temperature and air exposure on the lysosomal membrane stability of hemocytes in pacific oysters, Crassostrea gigas (Thunberg). Aquaculture, 256(1-4): 502-509.
DOI:10.1016/j.aquaculture.2006.02.003 |
 2019, Vol. 37
2019, Vol. 37


