Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SONG Yinping, MIAO Fengping, LIU Xianghong, JI Naiyun
- Responses of marine-derived Trichoderma fungi to seawater and their potential antagonistic behaviour
- Journal of Oceanology and Limnology, 37(2): 525-534
- http://dx.doi.org/10.1007/s00343-019-8059-3
Article History
- Received Mar. 25, 2018
- accepted in principle May. 24, 2018
- accepted for publication Jul. 2, 2018
2 University of Chinese Academy of Sciences, Beijing 100049, China
Trichoderma species are widespread as free-living fungi, mycoparasites, or opportunistic plant symbionts (Harman et al., 2004). As promising biocontrol agents in agriculture, those of terrestrial origin have been investigated for over 80 years and achieved great success especially to suppress phytopathogenic fungi (Papavizas, 1985). Following the pioneering work of Weindling(1932, 1934), a number of Trichoderma species with the biocontrol potential have been characterized, and some strains are officially registered or even commercially available as microbicides, such as T. harzianum T22 and KRLAG2, T. asperellum T34 and T211, T. atroviride I-1237, and T. virens G-41. Their interactions with pathogens and plants seem complex, and the production of antibiotic secondary metabolites is regarded as one of the key antagonistic mechanisms (Vinale et al., 2008). Up to now, a plethora of structurally diverse compounds have been isolated and identified therein, of which some are responsible for the ability of Trichoderma to attack or inhibit pathogenic fungi and other targets, such as bacteria and weeds (Ghisalberti and Sivasithamparam, 1991; Reino et al., 2008; Keswani et al., 2014).
Even though Trichoderma is normally considered as a terrestrial genus, halotolerant strains have been continuously discovered from marine sediments, invertebrates, and algae (Ji and Wang, 2016; Blunt et al., 2018). Marine isolates of Trichoderma species also produced multifarious new structures, such as polyketides (Sun et al., 2008; Song et al., 2010), terpenes (Miao et al., 2012), and peptides (Garo et al., 2003; Ren et al., 2009; Chen et al., 2013), but most bioassays were focused on pharmaceutical activity rather than ecological function toward phytopathogenic fungi and marine organisms. It is worth to mention that the secondary metabolism of marine-derived fungi appears sensitive to seawater concentration (Bugni and Ireland, 2004). However, little attention has been paid to the effects of seawater factors on the secondary metabolite production of marine-derived Trichoderma strains and their applications in agriculture and mariculture.
In order to gain a view of the survivability, metabolism, and antagonism of marine-derived Trichoderma fungi responding to environmental stresses, the effects of NaCl, NaBr, pH, and natural seawater (SW) on ten Trichoderma strains, including Trichoderma sp. dl8, T. pseudokoningii dl11, T. asperellum dl34 and dl48, T. harzianum dl36 and lyg12, T. longibrachiatum dl44 and cf11, and T. koningii dl49 and yt6, have been examined by analysis of the biomasses, production and NMR signals of lipophilic metabolites, and their bioactivities against some phytopathogenic fungi and marine organisms.
2 MATERIAL AND METHOD 2.1 Trichoderma strainsFollowing a previous procedure (Wang et al., 2006), ten marine-derived Trichoderma strains, including Trichoderma sp. dl8, T. pseudokoningii dl11, T. asperellum dl34 and dl48, T. harzianum dl36 and lyg12, T. longibrachiatum dl44 and cf11, T. koningii dl49 and yt6, were isolated from the inner tissue of the surface-sterilized marine macroalgae collected off the coasts of Dalian, Yantai, and Lianyungang of China. They were identified by morphological observation and by analysis of ITS regions for dl34 (GenBank accession number KR023953), dl36 (KX235318), dl44 (KX235319), dl48 (KX235320), and cf11 (JX089966) and 18S rDNA for dl8 (KX235317), dl11 (KX235313), dl49 (KX235314), lyg12 (KX235315), and yt6 (KX235316), which are preserved at the Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences.
2.2 FermentationFresh mycelia of ten Trichoderma strains were grown on the agar plates, and then they were cut into small segments and aseptically inoculated into Erlenmeyer flasks (1 L), each containing 150 mL freshwater (distilled water)-based media (glucose 20 g/L, peptone 5 g/L, yeast powder 2 g/L, K2HPO4 1 g/L, MgSO4 0.5 g/L, and pH 7.0) as the control. To examine the effects of marine environmental factors, the media with 30.0 g/L NaCl, 86.3 mg/L NaBr, pH 8.0, or 100% natural seawater (salinity 3.0% and pH 8.0) were also used respectively for all the strains. Static fermentations were performed at 28℃ for 30 days, with two replicates for each condition.
2.3 ExtractionThe lipophilic metabolites in mycelia and broths of ten Trichoderma strains were extracted using ethyl acetate (EtOAc). Each culture was filtered to separate mycelia from broth, and the dried mycelia were weighted and then extracted with mixed CH2Cl2 and MeOH (1:1, v/v). The crude extract was further partitioned between EtOAc and H2O to give the EtOAc-soluble extract. On the other hand, the broth was directly extracted with EtOAc. The extracting and partitioning procedures were repeated for three times, and the EtOAc extracts of mycelia and broth were evaporated at 40℃ under reduced pressure and then weighted.
2.4 1H NMR determination10.0 mg sample of each extract was dissolved in 0.5 mL DMSO-d6 and then subjected to the 1H NMR determination by a Bruker Avance Ⅲ 500 NMR spectrometer (equipped with 5 mm probe) at 298 K. Chemical shifts (δH) in ×10-6 are referenced to tetramethylsilane (TMS), and signals in the upfield (δH (0.5–2.0)×10-6, US), midfield (δH (3.5–5.0)×10-6, MS), and downfield (δH (5.0–8.0)×10-6, DS) were integrated, respectively. The integral ratios of midfield or downfield signals to upfield ones of mycelium and broth extracts of ten Trichoderma strains under different conditions were calculated.
2.5 Statistical analysisThe data were processed using the Microsoft Excel 2010 software, and the error bars were obtained through the STDEV function. The bioassay results were calculated based on three independent experiments. Mycelial weights, weights of extracts, and integral ratios of signals in the 1H NMR spectra retained two effective repetitions.
2.6 BioassaysAntifungal activity against Fusarium oxysporum f. sp. cubense and F. oxysporum f. sp. cucumerinum was tested at 75 μg/disk using the disk diffusion method (Schulz et al., 1995), with amphotericin B (inhibition zone 12.0 mm for F. oxysporum f. sp. cubense and 15.0 mm for F. oxysporum f. sp. cucumerinum) and DMSO as positive and negative controls, respectively. Briefly, each fungus tested was incubated in PDA medium for four days, and then its spores were rinsed and suspended by 4 mL sterile 0.85% NaCl solution (including 0.25% Tween 20). Inoculum suspension was adjusted to match the 0.5 McFarland turbidity standard and further diluted to 5×105 CFU/mL. On the other hand, each extract was dissolved in DMSO to a concentration of 15 mg/mL as the stock solution, and then 5 μL was pipetted onto a sterile filter disk (5.0 mm diameter). Subsequently, eight disks were dispersedly placed onto a PDA plate, which was precoated uniformly with 200 μL suspension. Three parallel tests were set for each sample, and the diameters of inhibition zones were measured and recorded in millimeter after the incubation at 28℃ for 72 h.
The marine phytoplankton (microalgae) Prorocentrum donghaiense and Heterosigma akashiwo were used for antimicroalgal test, according to the procedure described previously (Chen et al., 1996). Firstly, each microalga was inoculated for four days using the sterilized f/2 medium at 20℃ in an incubator with the 14 h:10 h light-dark cycle (2 000 lux light). The culture at exponential growth phase was diluted to (2–3)×104 cells/mL and then added into 96- well microplate with 100 μL in each well. 1.5 μL sample solution (15 mg/mL) was pipetted into each well and mixed uniformly. After 12-h inoculation, 10 μL CCK (TransDetectTM cell counting kit) solution was added and further cultured for 2 h. K2CrO7 (inhibition rate 91.2% for P. donghaiense and 93.7% for H. akashiwo) and DMSO were taken as positive and negative controls, respectively, and three replicates were used for each treatment. Culture filtrate was used as a blank, which was treated synchronously. The OD values were recorded at 450 nm, and inhibition rates were calculated by the following equation.

Brine shrimp (Artemia salina) lethality assay followed the microplate method (Solis et al., 1993). Briefly, brine shrimp eggs were hatched in natural seawater for 48 h at 28℃ under natural light, and then some 15 brine shrimp larvae along with 196.7 μL seawater were placed into each well of a 96-well microplate. Subsequently, 3.3 μL sample solution (15 mg/mL) was pipetted into each well and treated for 24 h. K2CrO7 (lethality rate 100%) and DMSO were used as positive and negative controls, respectively, and three replicates were set. Died larvae were identified and recorded with the aid of an anatomical lens. For the samples with lethal rates exceeding 50%, their LC50 values were also determined by gradually decreasing the concentrations.
Marine mollusc Littorina brevicula larvae were used for the periwinkle-lethal assay. In spring, the adult L. brevicula was obtained from the coast of Yantai and cultured with Ulva pertusa in natural seawater. The released eggs were deposited on the vessel bottom, which were collected and further incubated for a week to afford the larvae tested. Then, 196 μL seawater containing about 15 larvae was gently pipetted into each well of a 96-well microplate, followed by the addition of 4 μL sample solution (0.5 mg/mL). Three replicates were used for each sample, and DMSO was taken as a negative control. Treated for 12 h, the larvae were checked through an anatomical lens, and the dead ones were counted for the calculation of lethal rates.
3 RESULT 3.1 Effect on biomassesPrior to examining metabolic responses, the survivability of ten marine-derived Trichoderma strains was investigated. All of them grew well under freshwater (control) and seawater conditions, but the latter increased the biomasses by 54%–125% cultured for 30 days (Fig. 1). T. asperellum dl34 with the biomass increased by 125% appeared the most sensitive to seawater, which along with Trichoderma sp. dl8, T. longibrachiatum dl44, and T. koningii yt6, was synchronously promoted by NaCl (30.0 g/L), NaBr (86.3 mg/L), and pH 8.0. Among these three factors, NaCl was predominant in the promotion of Trichoderma sp. dl8, T. harzianum lyg12, T. asperellum dl34 and dl48, whereas pH 8.0 exceeded the others to affect the growth of T. longibrachiatum dl44. NaBr outstood to increase the mycelium yields of T. pseudokoningii dl11 and T. longibrachiatum cf11, but they were inhibited by NaCl. Additionally, the proliferation of T. koningii dl49 only slightly (≤ 10%) responded to these three factors.
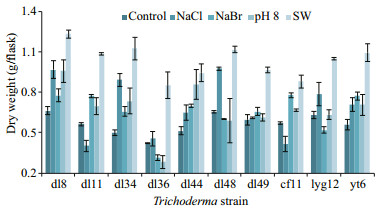
|
| Fig.1 Mycelial weights of ten Trichoderma strains under different conditions (mean±standard deviation) |
Except for Trichoderma sp. dl8 and T. asperellum dl48, the strains in seawater-based media afforded 22%–217% more lipophilic metabolites in mycelia than controls (Fig. 2). The yields were improved 113% for T. asperellum dl34 and reduced 45% for T. pseudokoningii dl11 by NaCl (30.0 g/L), though it appeared weak to affect the other strains. Moreover, NaBr (86.3 mg/L) increased the production of mycelial constituents by 63%–85% for T. pseudokoningii dl11, T. asperellum dl34, T. harzianum dl36, and T. longibrachiatum dl44 and >200% for T. longibrachiatum cf11 and T. koningii dl49. The positive effect of pH 8.0 on each strain was dwarfed by NaCl or NaBr. On the other hand, most of the strains gave less broth extracts than mycelium ones (Fig. 2). The lipophilic exudates of T. asperellum dl48, T. longibrachiatum cf11, T. harzianum lyg12, T. koningii dl49 and yt6 were improved over 30% under seawater condition. NaBr and/or pH 8.0 appeared effective to raise the production of exudates for most of the strains, which even exceeded seawater.

|
| Fig.2 Weights of mycelium and broth extracts of ten Trichoderma strains under different conditions (mean±standard deviation) |
To characterize these intra- and extracellular metabolites, their proton nuclear magnetic resonance (1H NMR) spectra were determined. The signals were divided into upfield (δH (0.5–2.0)×10-6, US), midfield (δH (3.5–5.0)×10-6, MS), and downfield (δH (5.0-8.0)×10-6, DS) ones, mainly corresponding to the protons of saturated (alkyl), heteroatom (oxygen, nitrogen, or halogen)-bearing, and unsaturated (vinyl or aryl) units, respectively. Their integral ratios (MS/ US and DS/US) preliminarily indicated the structural characteristics of lipophilic metabolites (Fig. 3). More heteroatom-bearing and unsaturated units were accumulated in mycelia of eight strains under seawater condition, especially in those of T. longibrachiatum dl44 and T. asperellum dl48. On the contrary, their yields in T. koningii yt6 were dramatically decreased by seawater. NaBr promoted the production of both heteroatom-bearing and unsaturated units in mycelia of Trichoderma sp. dl8, T. harzianum dl36, T. longibrachiatum dl44, T. asperellum dl34 and dl48. The MS/US of mycelial constituents was improved about three times for T. harzianum lyg12 under pH 8.0 condition, which also exceeded the others to promote the production of unsaturated units by Trichoderma sp. dl8, T. pseudokoningii dl11, T. longibrachiatum dl44, and T. koningii dl49. However, seawater was almost incapable of inducing the extracellular secretion of heteroatom-bearing and unsaturated units, and single factor (NaCl, NaBr, or pH 8.0) was also weak or negative to increase their exudation.
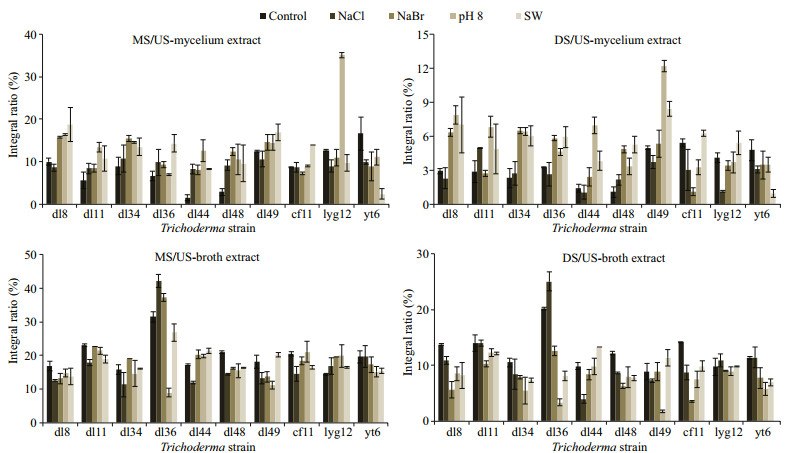
|
| Fig.3 Integral ratios of midfield ((3.5–5.0)×10-6, MS) or downfield signals ((5.0–8.0)×10-6, DS) to upfield ones ((0.5–2.0)×10-6, US) in the 1H NMR spectra of mycelium and broth extracts (mean±standard deviation) |
The lipophilic metabolites of ten Trichoderma strains cultured under different conditions were evaluated for antifungal activity against two plant pathogens, Fusarium oxysporum f. sp. cubense and F. oxysporum f. sp. cucumerinum. Only several exhibited inhibitory activity against these two pathogenic fungi at 75 μg/disk, as shown in Tables 1 and 2. The results were ranked by comparison with those of positive control (weak ≤1/3, moderate >1/3 and ≤2/3, and strong >2/3 of the inhibition zone of amphotericin B). Both F. oxysporum f. sp. cubense and F. oxysporum f. sp. cucumerinum could be inhibited by the lipophilic metabolites of Trichoderma sp. dl8, T. harzianum dl36, and T. asperellum dl48, but only the latter responded to those of T. pseudokoningii dl11, T. asperellum dl34, T. koningii dl49 and yt6. Among them, T. harzianum dl36 represented the most potent strain, which in freshwater-based media produced intra- and extracellular metabolites with strong inhibitory activity against F. oxysporum f. sp. cucumerinum. This inhibition was slightly affected by NaCl (30.0 g/L) but reduced or even removed by the other factors tested. Additionally, all the other active extracts are moderate to inhibit F. oxysporum f. sp. cubense and/or F. oxysporum f. sp. cucumerinum.

|
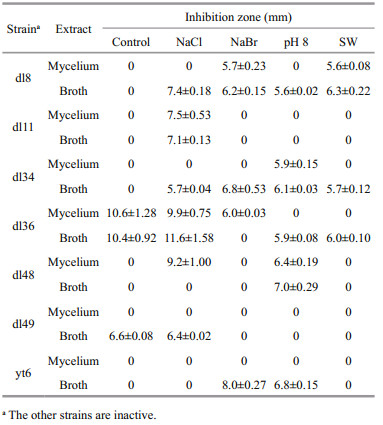
|
Most mycelium extracts of ten Trichoderma strains cultured in seawater-based media exhibited more or less inhibition of the marine phytoplankton Prorocentrum donghaiense and Heterosigma akashiwo at 220 μg/mL, but it was interesting that NaCl (30.0 g/L) often prevented the production of inhibitory metabolites (Fig. 4). The mycelial constituents of T. longibrachiatum dl44 under freshwater condition could promote the proliferation of P. donghaiense, which was remarkably accelerated by NaCl. Moreover, the proliferation of H. akashiwo greatly profited from the mycelial constituents of T. longibrachiatum dl44 under all the conditions. On the other hand, some half of the strains positively responded to seawater in producing extracellular exudates with inhibition of P. donghaiense or H. akashiwo. Seawater condition effectively stimulated T. harzianum lyg12 to secrete inhibitory metabolites, while NaCl significantly reduced the inhibitory activity of T. longibrachiatum dl44 exudates.

|
| Fig.4 Inhibition rates of mycelium and broth extracts against P. donghaiense and H. akashiwo at 220 μg/mL (mean±standard deviation) |
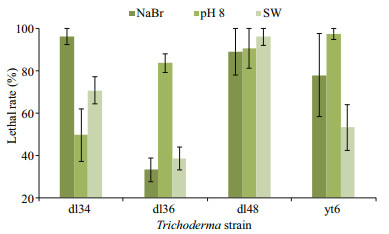
All the mycelium and broth extracts of ten Trichoderma strains cultured in seawater-based media exhibited over 70% lethal rates against the marine zooplankton Artemia salina at 250 μg/mL, but several were less toxic under other conditions (Fig. 5). The extracellular exudates, except for those of T. koningii dl49, under NaBr (86.3 mg/L), pH 8.0, and seawater conditions were extremely lethal to A. salina, with LC50 values ranging from 5.4 to 57.8 μg/mL. However, those under freshwater and NaCl (30.0 g/L) conditions were less toxic. Due to the high toxicity of the exudates of T. harzianum dl36, T. koningii yt6, T. asperellum dl34 and dl48, they were further assessed for the lethal effect on the mollusc Littorina brevicula (Fig. 6) that feeds on marine macroalgae (Xue, 1992). T. asperellum dl48 produced exudates with significant lethality against L. brevicula larvae under three conditions, but the high lethality of T. asperellum dl34 relied on NaBr. Moreover, pH 8.0 increased the toxicity of T. harzianum dl36 and T. koningii yt6 exudates.
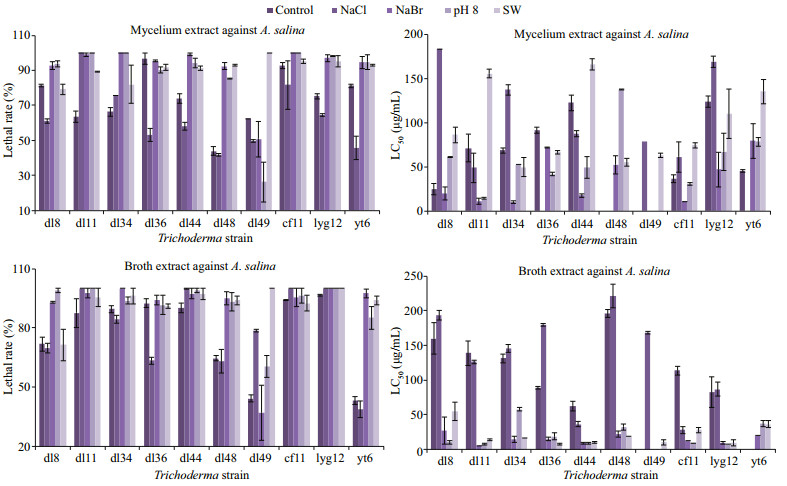
|
| Fig.5 Lethal rates at 250 μg/mL and LC50 values of mycelium and broth extracts against A. salina (mean±standard deviation) |
|
| Fig.6 Lethal rates of broth extracts against L. brevicula at 10 μg/mL (mean±standard deviation) |
Fungi in the marine ecosystem have been divided into obligate and facultative ones according to the necessity of seawater during their growth and sporulation (Bugni and Ireland, 2004). The survival in both freshwater- and seawater-based media suggests ten Trichoderma strains tested to be facultative marine fungi rather than obligate ones. The larger biomasses under seawater condition imply that these strains prefer to propagate in marine environment, though they are possibly terrigenous. In contrast, the true terrestrial-derived Trichoderma strains tend to be inhibited by high-level salt and pH (Lejeune et al., 1995; Yin et al., 2013). Trichoderma fungi tested adapt well to seawater, but the influences of seawater factors on their growth vary with species and strains. Although the effects of halides (30.0 g/L NaCl and 86.3 mg/L NaBr) and/or pH (8.0) of seawater are not always positive, each strain enjoys at least one of them. There are other constituents in seawater, such as metal ions (Cacciola et al., 2015), which may also contribute more or less to the growth. Based on the low or negative effects of NaCl, NaBr, and pH 8.0 on T. harzianum dl36 and T. koningii dl49, their extra biomasses are deduced to be even dominated by the other factors. The competition for space and nutrients is one of the key antagonistic mechanisms of Trichoderma species (Vinale et al., 2008), which undoubtedly profits from the rapid proliferation to some extent. Thus, marine isolates of Trichoderma seem suitable for the biocontrol in agriculture at the saline lands of costal zones.
Fungal lipophilic metabolites are mainly produced through secondary metabolism, and those of Trichoderma origin exhibit high structural diversity and various biological activities (Reino et al., 2008). Marine environment has added the diversity to some extent (Blunt et al., 2018), but its roles need to be further cleared. It is notable that the effects of NaCl (30.0 g/L), NaBr (86.3 mg/L), pH 8.0, and seawater on the yields of lipophilic metabolites of Trichoderma strains tested are not always parallel to those on biomasses. Most of them feature positive metabolic responses to seawater, producing more intra- and extracellular lipophilic metabolites. Although NaCl and pH 8.0 outstand to influence several strains, they are less common than NaBr to effectively promote the production of lipophilic metabolites. Single factor tested often exceeds seawater to increase the yields, which suggests that other components in seawater may play a negative role. Seawater tends to increase the production of heteroatom-bearing and unsaturated units in mycelia, which often profits from NaBr and/ or pH 8.0. Trichoderma species feature the ability to produce halogenated compounds in seawater-based media (Garo et al., 2003; Yamazaki et al., 2015), which probably contribute to the large portions of heteroatom-bearing units. Those can also be improved by polyols and amino compounds that have been indicated to respond to the high osmotic pressure (Bugni and Ireland, 2004). Alkaline condition (pH 8.0) has been evidenced to benefit the enzyme activity of some fungal dehydrogenases (Liu et al., 2006), which may help these Trichoderma strains to produce unsaturated units. It has been demonstrated that metal ions are able to induce the gene expression of Trichoderma (Puglisi et al., 2012; Cacciola et al., 2015), which likely promote the production of heteroatom-bearing units in T. harzianum dl36 and T. longibrachiatum cf11 in view of the weak or negative contribution of NaCl, NaBr, and pH 8.0. Despite the improved production of heteroatom-bearing and unsaturated units in mycelia under the seawater condition, their exudation seems restricted, which may be partially caused by the high osmotic pressure of seawater.
The bioactivities of lipophilic metabolites of Trichoderma strains tested also vary with culture conditions, as is the case with other marine-derived fungi (Masuma et al., 2001; Tarman et al., 2011). Many strains play a fungicidal role depending on NaCl (30.0 g/L), NaBr (86.3 mg/L), pH 8.0, or seawater condition, and their effects differ slightly from each other. T. harzianum represents one of the most effective fungicides in agriculture (Ghisalberti and Sivasithamparam, 1991), but marine-derived strains remain less explored. Although T. harzianum dl36 and lyg12 belong to the same species, only the former is active to plant pathogens tested. Especially, its activities rely more greatly on culture conditions and exhibit higher strain-specific property than the others. The majority of extracellular exudates are more active to inhibit the proliferation of marine phytoplankton tested than mycelial constituents, which demonstrates the differences between intracellular metabolites and extracellular exudates. For most mycelial constituents produced in seawater, their higher inhibition of marine phytoplankton than those produced in freshwater is deduced to correlate with the larger portions of heteroatom-bearing and unsaturated units (Fig. 3). On the other hand, NaBr, pH 8.0, and seawater, rather than NaCl, permit the production of highly marine animal-toxic exudates, but it is not the common case with intracellular metabolites. The differences in toxicity also suggest varied components of exudates responding to seawater factors, which are not obviously reflected by 1H NMR (Fig. 3). Moreover, the extra heteroatom-bearing and unsaturated units in mycelial constituents produced under the seawater condition appear not highly toxic to marine animals. All Trichoderma strains tested were isolated from marine macroalgae, which live in the ocean faced with pathogenic fungi (Kubanek et al., 2003), feeding herbivores (Xue, 1992), and phytoplankton competition for space and nutrients (Nan and Dong, 2004). Their interactions with macroalgae remain little understood, but the algicolous Trichoderma strains that are antagonistic to pathogenic fungi, phytoplankton, and marine animals probably contribute to the survival of host macroalgae. Marine phytoplankton, represented by P. donghaiense and H. akashiwo (Tyrrell et al., 2002; Lu et al., 2005), have often resulted in harmful algal blooms that damage marine fisheries, and some Trichoderma strains may be developed as the effective agents to control them.
5 CONCLUSIONOverall, ten marine isolates of Trichoderma grow positively responding to seawater, which suggests they are more adaptable to marine environment. High yields of lipophilic metabolites often match the large biomasses, but heteroatom-bearing and unsaturated units tend to be accumulated in mycelia in response to the seawater condition. Single factor, NaCl, NaBr, or pH of seawater, may play a positive or negative role in the growth of mycelia and production of lipophilic metabolites, and one of them often exceeds seawater to increase the metabolism. Seawater factors can promote many strains to produce bioactive metabolites, but the effects of NaCl are often weak or negative. The larger portions of intracellular heteroatom-bearing and unsaturated units produced in seawater may be responsible for the inhibition of marine phytoplankton rather than toxicity to marine animals, and the varied toxicities to marine animals further signify the divergences of extracellular exudates produced under different conditions. Although four pairs of the strains are regarded as the same species, respectively, from a mycological perspective, those in each pair feature different chemical and bioactive profiles. Considering the wide distribution of Trichoderma fungi in marine environment, the results may greatly contribute to further understanding and mining the structural diversity and biological activity of their secondary metabolites.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Blunt J W, Carroll A R, Copp B R, Davis R A, Keyzers R A, Prinsep M R. 2018. Marine natural products. Nat. Prod.Rep., 35(1): 8-53.
DOI:10.1039/C7NP00052A |
Bugni T S, Ireland C M. 2004. Marine-derived fungi:a chemically and biologically diverse group of microorganisms. Nat. Prod. Rep., 21(1): 143-163.
DOI:10.1039/b301926h |
Cacciola S O, Puglisi I, Faedda R, Sanzaro V, Pane A, Lo Piero A R, Evoli M, Petrone G. 2015. Cadmium induces cadmium-tolerant gene expression in the filamentous fungus Trichoderma harzianum. Mol. Biol. Rep., 42(11): 1559-1 570.
DOI:10.1007/s11033-015-3924-4 |
Chen C Y, Imamura N, Nishijima M, Adachi K, Sakai M, Sano H. 1996. Halymecins, new antimicroalgal substances produced by fungi isolated from marine algae. J. Antibiot., 49(10): 998-1 005.
DOI:10.7164/antibiotics.49.998 |
Chen L, Zhong P, Pan J R, Zhou K J, Huang K, Fang Z X, Zhang Q Q. 2013. Asperelines G and H, two new peptaibols from the marine-derived fungus Trichoderma asperellum. Heterocycles, 87(3): 645-655.
DOI:10.3987/COM-12-12644 |
Garo E, Starks C M, Jensen P R, Fenical W, Lobkovsky E, Clardy J. 2003. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod., 66(3): 423-426.
|
Ghisalberti E L, Sivasithamparam K. 1991. Antifungal antibiotics produced by Trichoderma spp. Soil Biol.Biochem., 23(11): 1 011-1 020.
DOI:10.1016/0038-0717(91)90036-J |
Harman G E, Howell C R, Viterbo A, Chet I, Lorito M. 2004. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol., 2(1): 43-56.
DOI:10.1038/nrmicro797 |
Ji N Y, Wang B G. 2016. Mycochemistry of marine algicolous fungi. Fungal Divers., 80(1): 301-342.
|
Keswani C, Mishra S, Sarma B K, Singh S P, Singh H B. 2014. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol.Biotechnol., 98(2): 533-544.
|
Kubanek J, Jensen P R, Keifer P A, Sullards M C, Collins D O, Fenical W. 2003. Seaweed resistance to microbial attack:a targeted chemical defense against marine fungi. Proc.Natl. Acad. Sci. USA, 100(12): 6 916-6 921.
DOI:10.1073/pnas.1131855100 |
Lejeune R, Nielsen J, Baron G V. 1995. Influence of pH on the morphology of Trichoderma reeseiqm 9414 in submerged culture. Biotechnol. Lett., 17(3): 341-344.
DOI:10.1007/BF01190650 |
Liu B N, Qiao C S, Jia S R. 2006. Studies on the method for the measurement of fungal biomass. Pharm. Biotechnol., 13(1): 40-44.
(in Chinese with English abstract) |
Lu D D, Goebel J, Qi Y Z, Zou J Z, Han X T, Gao Y H, Li Y G. 2005. Morphological and genetic study of Prorocentrum donghaiense Lu from the East China Sea, and comparison with some related Prorocentrum species. Harmful Algae, 4(3): 493-505.
DOI:10.1016/j.hal.2004.08.015 |
Masuma R, Yamaguchi Y, Noumi M, Ōmura S, Namikoshi M. 2001. Effect of sea water concentration on hyphal growth and antimicrobial metabolite production in marine fungi. Mycoscience, 42(5): 455-459.
DOI:10.1007/BF02464342 |
Miao F P, Liang X R, Yin X L, Wang G, Ji N Y. 2012. Absolute configurations of unique harziane deterpenes from Trichoderma species. Org. Lett., 14(15): 3 815-3 817.
DOI:10.1021/ol3014717 |
Nan C R, Dong S L. 2004. Progress on the competition between macroalgae and microalgae. Mar. Sci., 28(11): 64-66.
(in Chinese with English abstract) |
Papavizas G C. 1985. Trichoderma and Gliocladium:biology, ecology, and potential for biocontrol. Ann. Rev.Phytopathol., 23(1): 23-54.
DOI:10.1146/annurev.py.23.090185.000323 |
Puglisi I, Faedda R, Sanzaro V, Lo Piero A R, Petrone G, Cacciola S O. 2012. Identification of differentially expressed genes in response to mercury Ⅰ and Ⅱ stress in Trichoderma harzianum. Gene, 506(2): 325-330.
|
Reino J L, Guerrero R F, Hernández-Galán R, Collado I G. 2008. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev., 7(1): 89-123.
|
Ren J W, Xue C M, Tian L, Xu M J, Chen J, Deng Z W, Proksch P, Lin W H. 2009. Asperelines A-F, peptaibols from the marine-derived fungus Trichoderma asperellum. J. Nat.Prod., 72(6): 1 036-1 044.
DOI:10.1021/np900190w |
Schulz B, Sucker J, Aust H J, Krohn K, Ludewig K, Jones P G, Döring D. 1995. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res., 99(8): 1 007-1 015.
DOI:10.1016/S0953-7562(09)80766-1 |
Solis P N, Wright C W, Anderson M M, Gupta M P, Phillipson J D. 1993. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med., 59(3): 250-252.
DOI:10.1055/s-2006-959661 |
Song F H, Dai H Q, Tong Y J, Ren B, Chen C X, Sun N, Liu X Y, Bian J, Liu M, Gao H, Liu H W, Chen X P, Zhang L X. 2010. Trichodermaketones A-D and 7-O-methylkoninginin D from the marine fungus Trichoderma koningii. J. Nat.Prod., 73(5): 806-810.
DOI:10.1021/np900642p |
Sun Y, Tian L, Huang J, Ma H Y, Zheng Z, Lv A L, Yasukawa K, Pei Y H. 2008. Trichodermatides A-D, novel polyketides from the marine-derived fungus Trichoderma reesei. Org. Lett., 10(3): 393-396.
DOI:10.1021/ol702674f |
Tarman K, Lindequist U, Wende K, Porzel A, Arnold N, Wessjohann L A. 2011. Isolation of a new natural product and cytotoxic and antimicrobial activities of extracts from fungi of Indonesian marine habitats. Mar. Drugs, 9(3): 294-306.
DOI:10.3390/md9030294 |
Tyrrell J V, Connell L B, Scholin C A. 2002. Monitoring for Heterosigma akashiwo using a sandwich hybridization assay. Harmful Algae, 1(2): 205-214.
DOI:10.1016/S1568-9883(02)00012-4 |
Vinale F, Sivasithamparam K, Ghisalberti E L, Marra R, Woo S L, Lorito M. 2008. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem., 40(1): 1-10.
DOI:10.1016/j.soilbio.2007.07.002 |
Wang S, Li X M, Teuscher F, Diesel A, Ebel R, Proksch P, Wang B G. 2006. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod., 69(11): 1 622-1 625.
DOI:10.1021/np060248n |
Weindling R. 1932. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology, 22(10): 837-845.
|
Weindling R. 1934. Studies on lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctinia solani and other soil fungi. Phytopathology, 24(11): 1 153-1 179.
|
Xue Q Z. 1992. Study on population dynamics of Littorina brevicula on rocky shore in Qingdao, China. Oceanol. Limnol. Sin., 23(4): 438-444.
(in Chinese with English abstract) |
Yamazaki H, Rotinsulu H, Narita R, Takahashi R, Namikoshi M. 2015. Induced production of halogenated epidithiodiketopiperazines by a marine-derived Trichoderma cf. brevicompactum with sodium halides. J.Nat. Prod., 78(10): 2 319-2 321.
|
Yin D C, Deng X, Chet I, Song R Q. 2013. Responses of Trichoderma harzianum T28 under drought and saltalkali stress. J. Anhui Agri. Sci., 41(30): 11 997-12 000.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37


