Institute of Oceanology, Chinese Academy of Sciences
Article Information
- FAN Xin, CHENG Fangjin, YU Zhiming, SONG Xiuxian
- The environmental implication of diatom fossils in the surface sediment of the Changjiang River estuary (CRE) and its adjacent area
- Journal of Oceanology and Limnology, 37(2): 552-567
- http://dx.doi.org/10.1007/s00343-019-8037-9
Article History
- Received Feb. 14, 2018
- accepted in principle Mar. 30, 2018
- accepted for publication May. 21, 2018
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Environmental Monitoring Center of Qingdao, Qingdao 266003, China
Diatoms are important primary producers in aquatic ecosystems, and are the dominant planktonic algae in almost all high productivity waters and upwelling areas (Dawes, 1998; Abrantes et al., 2005). Diatom abundance and diversity provide abundant environmental information because of their sensitivity to environmental factors. Diatoms are generally preserved well in sediments for a long time because of their siliceous cell wall (Serieyssol et al., 2011). Therefore, analyzing the abundance and species changes of diatom fossils in sediments can help us understand the physical and chemical characteristics of the overlying water (Clarke et al., 2003; Hassan et al., 2009). Numerous studies have confirmed that diatom fossils preserved in sediment could be used to indicate and reconstruct environmental changes, such as water temperature, salinity, pH, eutrophication and even natural disasters, e.g. earthquakes and tsunamis (Clarke et al., 2003; Weckström et al., 2004; Romero et al., 2005; Hassan et al., 2009; Ren et al., 2014; Dura et al., 2016; Luoto et al., 2017). For example, Costa-Böddeker et al. (2017) used surface sediment diatom fossils such as Thalassionema nitzschioides and Thalassiosira cedarkeyensis to assess the ecological conditions in the Thi Vai estuary and Can Gio mangrove forest, Southern Vietnam. The distribution and abundance of Paralia sulcata fossils preserved in sediment can be used to indicate eutrophication in coastal waters (McQuoid and Nordberg, 2003; Liu et al., 2008, 2015a).
Estuaries and coastal areas are exposed to a variety of pressures, such as climate change, runoff and human activities (Wong et al., 2014). As the largest estuary in China, the Changjiang River estuary (CRE) is regarded as one of the most polluted areas affected by human activities (Liu et al., 2016a). In recent decades, with the influence of rapid economic development and human activities, the loading of nutrients from the Changjiang River (CR) has increased greatly, which has caused serious eutrophication in the CRE and its adjacent area (Gao and Song, 2005; Duan et al., 2008; Li et al., 2014). Frequent harmful algal blooms (HABs) and seasonal hypoxia resulting from eutrophication have exacerbated the ecological degradation (Zhang et al., 2007; Zhu et al., 2011; Li et al., 2014; Zhou et al., 2017). Meanwhile, the nutrient structure has dramatically changed in the CRE and its adjacent area because of intensified human activities, particularly after the construction of the Three Gorges Dam. The N:P ratio has increased whereas the Si:P and Si:N ratios have declined significantly, and P has been the potential limiting factor for phytoplankton growth (Duan et al., 2008; Chai et al., 2009; Liu et al., 2016b; Li et al., 2017). Changes in the nutrient structure, together with other environmental variations, have led to alteration of the phytoplankton community structure and diversity. For example, large diatoms have become less abundant (e.g. Podosira stelliger) and a shift in the dinoflagellate composition (e.g. Prorocentrum dentatum, Alexandrium spp.) has occurred (Zhou et al., 2008; Abate et al., 2017). As diatoms are the dominant species of phytoplankton in the CRE and its adjacent waters, changes in the composition of diatom assemblages and in diatom abundance can reflect the environmental variations (Lin et al., 2008). Therefore, diatoms can be used to explore how environmental factors in the CRE and its adjacent waters have affected the ecosystem both spatially and temporally. A lot of studies have established reliable data of how diatoms present in water bodies respond to environmental variables in the CRE and adjacent regions (Gao and Song, 2005; Lin et al., 2008; Song et al., 2008; Jiang et al., 2015; Song et al., 2017). In contrast, only a few investigations focused on diatoms preserved in sediments and their relationship with environmental variables have been published (Wang et al., 2001, 2016). Those studies mostly selected particular diatom taxa or taxa groups to indicate environmental changes, with insufficient taxonomic sampling to allow paleoenvironmental analyses in the CRE and its adjacent area.
In this study, the diatom fossils in surface sediment and their environmental implication were analyzed to achieve two main purposes: 1) to describe the geographical distribution feature of diatom fossils preserved in surface sediments, and to explore their potential as indicators of environmental variables; and 2) to find the most significant environmental variables that affect diatom distribution and composition independently. This work provides a basis for further paleoenvironmental study in the CRE and its adjacent waters.
2 MATERIAL AND METHOD 2.1 Study areaAs the largest river in China, the CR transports 9.28×1011m3 of freshwater and 4.86×108t of solid matter to the East China Sea (ECS) annually (Yang et al., 2006). These materials will affect the structure and composition of the coastal ecosystem. With the influence of the CR and other circulation systems, e.g. the Taiwan Warm Current, the Zhejiang Coastal Current and the Yellow Sea Coastal Current (Ning et al., 2011), unique hydrological features emerged in the CRE and its adjacent area, such as seasonal changes in direction of the Changjiang Diluted Water (CDW) and migration of the temperature and salinity fronts. The temperature and salinity of the CRE and its adjacent waters exhibit seasonal and regional differences. In summer, the CDW extends eastward at the surface, which results in high temperature and low salinity in the estuary area and low temperature and high salinity in the area outside of the estuary. In winter, low temperature and low salinity are present in the estuary, and high temperature and high salinity exist in the area outside the estuary (Tang and Wang, 2004).
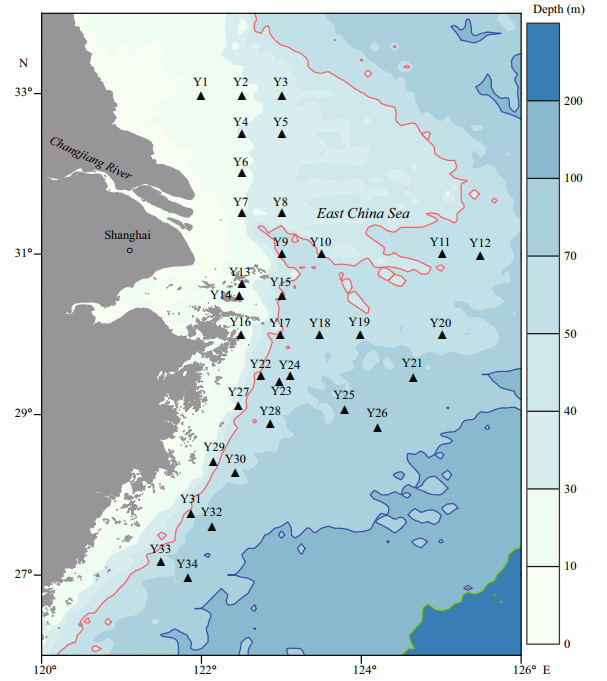
2.2 Sediment and water samplingSampling was carried in the CRE and its adjacent waters (27°-33°N, 121.0°-126°E). A total of 34 undisturbed sediment samples (Y1-Y34) were collected using a box corer (0.1 m2) in August and November 2015 (Fig. 1). The uppermost 3 cm of sediment was isolated for analysis of diatom fossils and measurement of sediment grain size and water content. Because of the complex hydrodynamic characteristics of the CRE and its adjacent waters, the deposition rate in the area varies greatly, with high deposition rates (> 3 cm/a) occurring adjacent to the subaqueous delta and generally decreasing southward along the inner shelf and eastward offshore (Huh and Su, 1999; Liu et al., 2006). Therefore, to obtain variable data that corresponded temporally with the samples from the uppermost 3 cm of surface sediment, water samples were collected from the surface, intermediate and bottom layers before sediment sampling at each sampling site seasonally throughout the whole year. Eight water environmental variables, including temperature, salinity, turbidity, dissolved oxygen, water depth, dissolved inorganic nitrogen, dissolved inorganic phosphate and dissolved inorganic silicate were measured.
|
| Fig.1 Map of the Changjiang River estuary and its adjacent waters showing the depth (m) (color) and sampling sites (triangles) |
The sediment samples were processed to extract diatom fossils following the method used by Battarbee et al. (2001). Approximately 10 g of freeze-dried sediment samples was treated with excess 10% HCl and 30% H2O2 to completely remove carbonates, metal salts, metal oxides and organic matter. After washing with ultra-pure water 3-4 times to attain a neutral pH, ammonium hydroxide was added to reduce the impact of clay. After chemical residues were removed by rinsing 3-4 times using ultra-pure water, zinc bromide (specific gravity, 2.4) was added to suspend the siliceous microfossils with centrifugation at 2 000 r/min for 10 min. Then, the suspended fossils were diluted to certain volumes, and aliquots were transferred to cover slides. After air-drying of the aliquots, permanently labeled slides were prepared with Naphrax (1-2 drops).
At least 300 diatom valves were counted on the slides of each sample under 1 000× magnification. Diatom taxa were identified according to Hasle and Syvertsen (1996), Guo and Qian (2003) and Algaebase (http://www.algaebase.org/). Because of slide and identification issues, some diatom taxa were identified only to the genus level instead of the species level. Only one silicoflagellate species (Dictyocha fibula) was identified, but it was not included in the follow-up analysis because of its low relative abundances.
The absolute abundance of diatom fossils (Dabs) was expressed as the number of valves per gram of dry sediment (valves/g DW) and was calculated as follows:
 (1)
(1)where Dabs is the absolute abundance of diatom fossils, N is the total number of diatom fossils in the sample, V is the total volume of diluted suspended diatom fossils, V1 is the volume of diluted suspended diatom fossils applied on the slides, and W is the weight of the dry sediment sample.
2.4 Measurements of grain size and water environmental variablesThe percentages of sand, silt and clay were measured in the surface sediment samples, grain size analysis was performed using a Malvern Mastersizer A3000 laser particle size analyzer (Malvern Ltd., United Kingdom). Approximately 50 g of freezedried sediment was treated with excess 1 mol/L HCl and 30% H2O2 to completely remove carbonate and organic matter. The sample was ultrasonically dispersed before analysis. Grain size was divided into three categories following Shepard (1954): clay (< 3.9 μm), silt (3.9-62.5 μm) and sand (> 62.5 μm).
Water environmental variables such as temperature, salinity, turbidity, dissolved oxygen and depth were measured using in situ instruments. Inorganic nutrient parameters were determined photometrically by a Skalar flow analyzer (Skalar Ltd., Netherlands) with a precision of < 5%-10%, and data quality was monitored via inter-calibrations. The detection limit was 0.14 μmol/L for nitrate, 0.07 μmol/L for phosphate and 0.18 μmol/L for silicate. The mean values of the variables in the different water layers in each season were calculated, then the mean annual values of the eight water variables were used in the subsequent analysis.
2.5 Data analysis 2.5.1 Data analysis of diatom fossilsIn this case, we defined dominant species to be those with accumulated relative abundances higher than 2%, following Di et al. (2013). The ShannonWeaver index (H′) and richness index (D) were calculated to reflect the spatial distribution and variation of species diversity of the diatom fossils and are calculated as follows (Shannon and Weaver, 1949; Margalef, 1968):
 (2)
(2)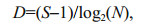 (3)
(3)where H' is the Shannon-Wiener index, D is the richness index, S is the number of species in the sample, N is the total number of diatom fossils in the sample, and Pi is the proportion of species i in the sample.
2.5.2 Ordination analysisWe selected taxa with relative abundances > 2% at a minimum of two sites for the ordination analysis because rare taxa contain little information and may be the result of exogenous input (Hassan et al., 2008). Prior to ordination analysis, the diatom taxa percentage data were square root transformed to decrease the weighting of dominant species (Bigler et al., 2006), such as P. sulcata, the relative abundance of which exceeded 50% at some sites. Additionally, eight water variables and four sediment variables were log(x+1) transformed to eliminate the impact of their units.
Detrended correspondence analysis was performed to determine whether a unimodal or linear model was more appropriate for the training set. If the axis length is greater than 4, the unimodal model is more appropriate, whereas if the axis length is less than 3, the linear model is preferred (Lepš and Šmilauer, 2003). In the present study, the axis length was 1.33, indicating that the linear model is preferable. Principal component analysis (PCA) and redundancy correspondence analysis (RDA) were applied to reveal the relationship between diatom fossils in the surface sediment and environmental variables in the CRE and its adjacent waters. Partial RDAs were used to identify the most significant environmental variables affecting the species composition and distribution of diatom fossils. The ordination analysis was carried out in the R software environment.
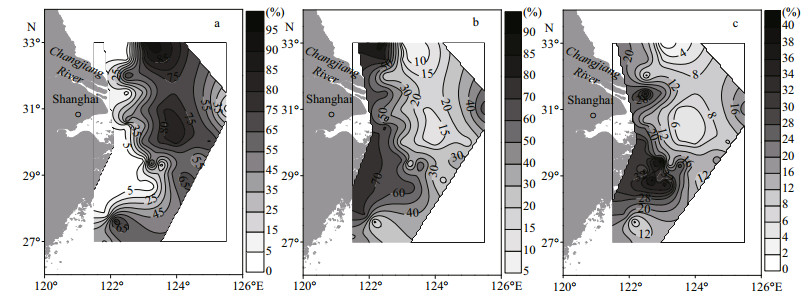
3 RESULT 3.1 Grain size composition of sedimentsIn the study area, the surface sediments were mainly composed of sand and silt, with lower percentages of clay (Fig. 2). This composition might result from the strong currents and complex hydrodynamic conditions in the CRE and its adjacent waters. The percentage of sand increased from inshore to offshore areas, ranging from 0-89.9% (Fig. 2a), whereas the percentages of silt and clay both decreased from inshore to offshore (Fig. 2b, c). Two muddy areas were apparent in the Zhejiang coastal area and the CRE, consistent with previous research findings (Wang et al., 2013). The silt percentage (9.4%-85.5%) was significantly higher than the clay percentage (0.7%-39.6%; P < 0.01). In general, the grains in offshore surface sediments were coarser than those in inshore surface sediments.
|
| Fig.2 Percentages of (a) sand, (b) silt and (c) clay in surface sediments |
A total of 114 taxa were identified, consisting of 72 centric diatoms, 41 pennate diatoms, and one silicoflagellate species (Dictyocha fibula). These taxa belonged to 16 families and 33 genera, of which Coscinodiscus (24 species), Nitzschia (6 species) and Diploneis (5 species) were the dominant genera. The diatom taxa were dominated by marine species. At these coastal sites and the sites near the CR mouth affected by the CDW and other fresh water input, many brackish species, e.g. Cyclotella stylorum and Actinoptychus splendens, were identified. In this case, 17 dominant species were selected, of which 15 were centric: Actinocyclus ehrenbergii, Actinocyclus octonarius var. ralfsii, Actinoptychus senarius, A. splendens, Coscinodiscus argus, Coscinodiscus centralis, Coscinodiscus curvatulus, Coscinodiscus decrescens, Coscinodiscus marginatus, Coscinodiscus radiatus, Cyclotella striata, C. stylorum, P. sulcata, P. stelliger and Thalassiosira excentrica. The remaining two, Pleurosigma spp. and Tryblioptychus cocconeiformis, were pennate (Fig. 3). The relative abundance of P. sulcata ranged from 4.9% to 56.1%, this species had the highest average abundance of 20.1% among the 17 dominant taxa. In addition, P. sulcata was the only species that appeared in all 34 samples. 37 of the 114 identified diatom taxa were present at relative abundance values > 2% in at least two samples and were included in the subsequent analyses (Table 1).

|
| Fig.3 Relative abundances of selected dominant genera and species in each sample |
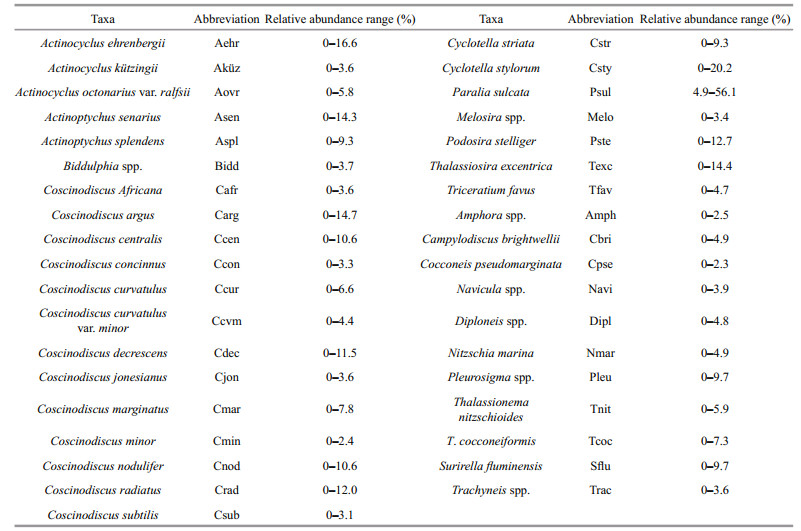 |
The Dabs values ranged from 243 valves/g DW (sample Y1) to 49 317 valves/g DW (sample Y30), with an average of 16 685 valves/g DW. Over the whole study area, Dabs did not obviously differ between inshore and offshore areas (Fig. 4a). The Shannon-Weaver index (H′) ranged from 3.0-5.0, with an average value of 4.0. The richness index (D) ranged from 2.8-5.3, average 4.1. Both parameters showed a downward trend from inshore to offshore areas, with the trend being more distinct in the Shannon-Weaver index (H′) (Fig. 4b, c). That is, compared to the surface sediments in offshore areas, the inshore surface sediments exhibited a higher diversity of diatom fossils.
|
| Fig.4 Spatial distribution of the absolute abundance of (a) diatom fossils, (b) the Shannon-Weaver index (H′) and (c) the richness index (D) |
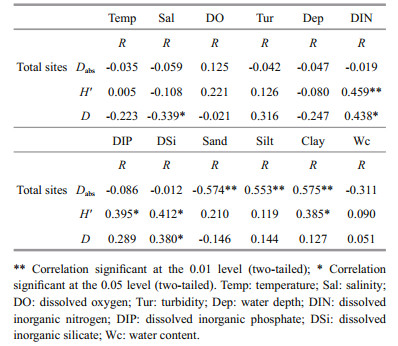
Correlation analysis between the 12 environmental variables (eight water variables and four sediment variables) and diatom fossils parameters was performed (Table 2). The results showed that Shannon-Weaver index (H′) and richness index (D) of diatom fossils were significantly positively correlated with dissolved inorganic nitrogen (DIN), dissolved inorganic phosphate (DIP) and dissolved inorganic silicate (DSi). No significant correlation was found between species diversity and sediment variables, but both indices were negatively correlated with sand and positively correlated with silt and clay. The result was different for Dabs, Dabs was significantly negatively correlated with sand (R=-0.574, P < 0.01) and significantly positively correlated with silt (R=0.553, P < 0.01) and clay (R=0.575, P < 0.01), which indicated that smaller sediment particles preserve diatom fossils better than larger ones.
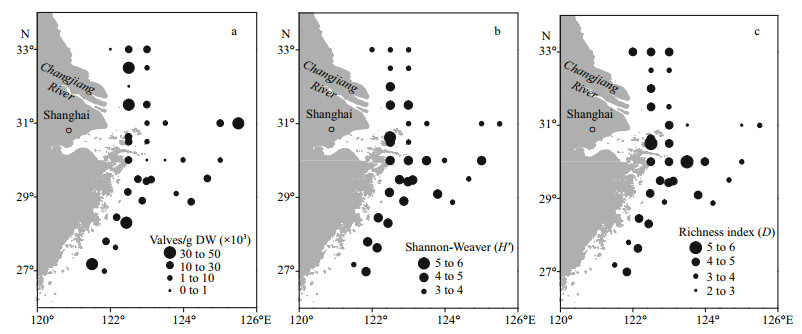
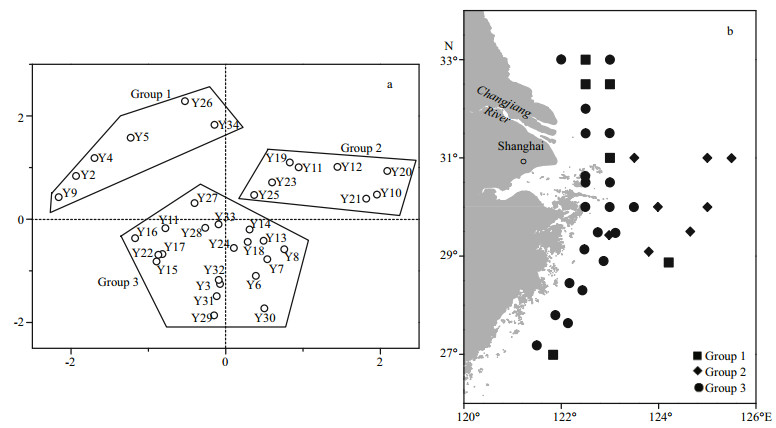
3.4 Geographical distribution of diatom taxaThe PCA results showed that the diatom taxa could be divided into three groups (Fig. 5a). The spatial distribution of Group 2 clearly differed from the distributions of Group 1 and Group 3 (Fig. 5b). Most of the coastal sites belonged to Group 1 (6 sites) and Group 3 (20 sites), whereas the remote sites belonged to Group 2 (8 sites).
|
| Fig.5 PCA classification based on diatom composition at the sampling sites (a); spatial distribution of diatom fossils (b) |
In all three groups, P. sulcata was the dominant species, but the average relative abundance of P. sulcata was significantly higher in Group 2 (39.5%) than in Group 1 (18.3%) and Group 3 (12.9%) (P < 0.01). The distribution pattern of T. excentrica was similar to that of P. sulcata, with the highest average relative abundances (9.3%) in Group 2. In contrast, C. stylorum had the lowest average relative abundance in Group 2 (1.4%), with significantly higher abundances in the other two groups because of the influence of fresh water (Group 1: 5.7%, Group 3: 9.9%) (P < 0.01). The average relative abundance of Coscinodiscus spp. in Group 1 (30.4%) was significantly higher than that in Group 2 (13.7%) or Group 3 (18.2%) (P < 0.01).
3.5 RDA resultsThe RDA based on 37 diatom taxa and constrained by 12 environmental variables showed that the species-environment correlations of RDA axis 1 (0.91) and axis 2 (0.86) were high, indicating a strong relationship between the diatom taxa and environmental variables. In the RDA ordination diagram, the environmental variables are plotted as vectors, the diatom taxa are plotted as triangles (Fig. 6a). The diatom taxa are positioned according to their relationship with the environmental variables. As RDA axis 1 is highly negatively correlated with salinity, water depth and temperature and positively correlated with DIN and DSi (Fig. 6a), diatom taxa that prefer high temperature and salinity, such as P. sulcata, C. radiatus and P. stelliger, are mostly distributed along the left half of RDA axis 1, and the relative abundances of these diatom taxa in the offshore samples are higher. In contrast, the diatom species that are distributed in the right half of RDA axis 1, such as A. ehrenbergii, T. excentrica and C. stylorum, are consistently dominant in coastal waters characterized by low temperature and low salinity. The three groups delineated by PCA can also be distinguished in Fig. 6b. Group 1 and Group 3 were mainly positioned in the right half of RDA axis 1, these sites were characterized by high nutrients. Group 2 were positioned in the left half of RDA axis 1, and were positively correlated with salinity, temperature and water depth.

|
| Fig.6 RDA results of (a) 37 diatom taxa and (b) 34 surface sediment samples divided by PCA, changing with the environmental variables Abbreviations of diatom taxa corresponding to those listed in Table 1. |
The RDA results indicated that in this case, the 12 environmental variables explained a total of 42.43% of the variation in diatom taxa composition (Fig. 7a). According to the associated Monte Carlo permutation tests (P=0.008), the explanation was accepted by the ordination analysis. Partial RDAs revealed that, of the 12 environmental variables, salinity, DIN, temperature and water depth affected the composition and distribution of diatom taxa independently (P < 0.05). The result can be used to establish transfer functions for paleoenvironmental reconstruction in the CRE and its adjacent waters. The total explained variance was composed of contributions of salinity (15.4%), DIN (12.7%), temperature (11.2%), water depth (9.3%), water content (8.2%), turbidity (7.4%), DSi (7.0%), DIP (4.6%), sand (6.3%), clay (3.9%), silt (2.9%), dissolved oxygen (2.5%) and the total interaction between the various environmental variables (8.7%) (Fig. 7b).

|
| Fig.7 Pie charts of (a) the explained and unexplained portions of the total variance and (b) the contributions of each environmental variable and their total interaction * Correlation significant at the 0.05 level (two-tailed). |
Because of the special geographical location and complex hydrological conditions of the CRE, the water variables were variable in space and time, which were expected to affect the growth and distribution of phytoplankton. Phytoplankton diversity and abundance in seawater were consistently higher in coastal waters than offshore areas of the CRE and the ECS (Song et al., 2008; Liu et al., 2015b). The environment is not stable in these coastal and estuarine regions, because of increased nutrient levels caused by the influence of the CDW and coastal currents; thus, it is difficult for any one species to gain a strong advantage, making the environment more suitable for more brackish diatoms (Song et al., 2008; Wang et al., 2016). In this case, the pattern observed in diatom fossils, of species diversity being higher in coastal areas than in offshore sites, is in accordance with the results of previous in situ surveys of seawater. However, the Dabs values did not differ significantly between inshore and offshore areas (Fig. 4a), which was not consistent with previous investigations of seawater (Song et al., 2008; Liu et al., 2015b). This difference may have two explanations: 1) as the CRE and its adjacent area are hydrodynamically complex, diatoms were easily dissolved and ingested during sedimentation; and 2) the sediment composition may affect the preservation of diatom fossils. Crosta and Koç (2007) found, by comparison with the living diatom assemblage in waters, that the sedimentary environment played an important role in preservation of diatom fossils in sediment.
Sediment composition was an important factor affecting the preservation of diatom, diatom fossils were better preserved in silt and clay than in sandy sediments (Cunningham et al., 2005; Di et al., 2013; Liu et al., 2015a). This pattern is reflected in this research, in that Dabs values were significantly higher in fine grained area than in in sites containing coarse grained sediment (Table 2). Water content represents the capacity for sediment resuspension (Defersha and Melesse, 2012); a higher water content indicates higher resuspension, which may increase the probability of diatom cracking. This phenomenon was also reflected in the present study: although water content did not significantly affect the abundance of diatom fossils, there was a negative correlation between these two parameters, indicating that a higher water content was harmful to the preservation of diatom fossils. Notably, the sediment composition and water content did not significantly affect the diversity of diatom fossils, potentially because sediment variables mainly play a major role in the preservation of all diatoms, with no discrimination, and have a weaker influence on diatom growth, especially on the planktonic species considered herein.
In this case, sites with Group 1 and Group 3 are mainly near the CRE and in the coastal area west of 123°E (Fig. 5b), which are primarily impacted by the CDW, East China Sea Coastal Current, North Jiangsu Coastal Current and human activities (Liu et al., 2007). Therefore, this area is characterized by low salinity and high nutrients. Group 2 sites are in areas with depths greater than 50 m (Fig. 4b) that are influenced by the Taiwan Warm Current and the Yellow Sea Warm Current (YSWC), with high temperature and salinity all year round (Liu et al., 2007). C. stylorum and A. ehrenbergii were the dominant species in Group 1 and Group 3, their relative abundances showed an obvious downward trend from coastal sites to offshore areas (Fig. 8a, b), consistent with previous studies (Wang et al., 1984, 2001, 2016). C. stylorum prefers living in brackish water and will be the dominant species in areas with salinity below 31 (McQuoid and Hobson, 2001; Mirabdullayev et al., 2004). A. ehrenbergii is a coastal species usually found in low-salinity water and brackish water (Wang et al., 1984). P. sulcata and P. stelliger were the two most dominant species in Group 2, their relative abundances were much higher in offshore than in inshore areas (Fig. 8c, d; P < 0.01), with average relative abundances of 39.5% and 6.4%, respectively. McQuoid and Hobson (1998) found that whether in phytoplankton or sediment communities, the abundance of P. sulcata was higher in a saltwater environment; warmer conditions were also conductive to its growth (Zong, 1997). Because of its high abundance in response to the YSWC, this species has been regarded as an indicator of the YSWC (Wang et al., 1985; Liu et al., 2015a). In this case, P. sulcata was significantly positively correlated with salinity and temperature, which is consistent with previous studies. P. stelliger was always abundant in the area outside the ECS shelf (Wang et al., 1985), and this species has been used to indicate changes in temperature and salinity under the influence of currents (Zong and Tooley, 1999).

|
| Fig.8 Geographic distribution of four selected indicative diatom species |
Temperature, salinity, nutrients and water depth control the boundaries of the living spaces of different diatom species (Battarbee et al., 2001; Schröder et al., 2015; Nazarova et al., 2017). Juggins (1992) and Hassan et al. (2007) reported that salinity is the most important variable affecting the composition and abundance of diatom in estuarine regions. This finding was reflected in our study: salinity had the highest explanation of 15.38% (Fig. 7b). In the CRE and its adjacent area, salinity has a wide range of 19-33.5 caused by the CDW input in summer and intrusion from the ECS (Chen, 2009). Salinity is the major environmental variable used in classifying phytoplankton communities. Wang (2002) divided phytoplankton into five classes according to their adaptability to salinity in the CRE and its adjacent waters: a freshwater class inside the river mouth; a true estuarine class; a coastal class; a euryhaline marine class and a high-salinity class. In this case, the coastal-offshore difference of diatom taxa composition could be a salinity effect, as brackish species such as C. stylorum were dominant in coastal areas with low salinity (Fig. 8a), but diatoms favoring high salinity such as P. sulcata dramatically increased in sites east of 123°E characterized by high salinity. The variance explained by DIN is second only to salinity, demonstrating its importance for the distribution of diatom fossils. As the basic element used to synthesize proteins, nucleic acids and chlorophyll, nitrogen is a necessary element for the growth and development of diatoms (Geider et al., 1998). DIN limitation will affect the adaptability of diatom cells to external stress. For example, the cell repair rates and photosynthetic capacity of Thalassiosira pseudonana and Skeletonema costatuma decreased under nitrogen deficiency (Loebl et al., 2010; Li and Gao, 2014). Human activity is the main reason for increase in the nitrogen flux; the nitrogen output to CR showed 1.6-fold increase during the ten-year period from 1980 to 1990 (Bao et al., 2006). The large runoff input increases the N:P and N:Si ratios, and diatom growth is affected by phosphate and silica limitation in the coastal areas of the CRE. In the contrast, nitrogen could be the limiting factor in offshore areas (Pu et al., 2001; Wang, 2006). In the present study, temperature also independently influenced the composition of diatom taxa. Based on the tolerance to temperature, diatoms can be divided into warm-water species, cold-water species and species with a wide temperature range. Montagnes and Franklin (2001) reported that the growth rates of five diatoms, including Cyclotella cryptica and Ditylum brightwellii, increased with increasing temperature, but their cell sizes decreased with increasing temperature. Weckström et al. (1997) demonstrated that temperature is an important factor affecting the composition of diatoms in lake surface sediments, and a diatom-temperature transform model was established to reconstruct paleotemperature variability. The temperature of the CRE and its adjacent waters exhibit seasonal and regional differences. In summer, the water temperature is higher in the estuary area than in the nearby open sea; in winter, the situation is opposite, with water temperature being higher in open seas (Tang and Wang, 2004). In our analysis, diatom taxa were distributed based on their adaption to temperature (Fig. 6a). Water depth is a key factor determining the lifestyles of diatoms. Sayer et al. (1999) found that the abundance changes of floating species, benthic species and epiphytic diatoms can be used to reflect changes in the water level in paleoecological studies. Meanwhile, water depth corresponds to the sinking time of diatoms: the longer the sinking period, the more readily will diatoms be affected by hydrodynamic conditions, and be dissolved or be ingested (Crosta and Koç, 2007).
In the present study, the relative abundances of four representative diatom fossils: A. ehrenbergii, C. stylorum, P.sulcata and P. stelliger were significantly influenced by salinity, DIN, temperature and water depth (Fig. 6a). Therefore, we can infer that in paleoecological studies, variations in the distribution and abundance of A. ehrenbergii, C. stylorum, P. sulcata and P. stelliger preserved in sediment can be used to distinguish coastal and offshore waters.
5 CONCLUSIONThe diatom fossils in 34 surface sediments from the CRE and its adjacent area were analyzed. The absolute abundances of diatom fossils were significantly correlated with the grain size composition of the sediment, with diatoms being better preserved in finer-grained sediments. The diversity of diatom fossils was significantly correlated with nutrient concentrations (DIN, DIP and DSi). In this case, salinity, DIN, temperature and water depth could independently affect the composition of diatom fossils (P < 0.05), meaning that the four environmental parameters can be used for paleoenvironmental reconstruction in further analysis. Three groups were distinguished based on the distribution of relative abundances of diatom fossils. Four representative dominant species, A. ehrenbergii, C. stylorum, P. sulcata and P. stelliger can be used to distinguish coastal and offshore waters.
6 DATA AVAILABILITY STATEMENTSupporting data are included in the manuscript as figures and tables; any additional data may be obtained from X. Song (e-mail: songxx@qdio.ac.cn).
7 ACKNOWLEDGEMENTWe thank editor and three reviewers for their helpful comments on an earlier draft of this paper.
Abate R, Gao Y H, Chen C P, Liang J R, Mu W H, Kifile D, Chen Y H. 2017. Decadal variations in diatoms and dinoflagellates on the inner shelf of the East China Sea, China. Chin. J. Oceanol. Limn., 35(6): 1374-1386.
DOI:10.1007/s00343-017-6029-1 |
Abrantes F, Gil I, Lopes C, Castro M. 2005. Quantitative diatom analyses-a faster cleaning procedure. Deep Sea.Res. Part. Ⅰ. Oceanogr. Res. Pap., 52(1): 189-198.
DOI:10.1016/j.dsr.2004.05.012 |
Bao X, Watanabe M, Wang Q X, Hayashi S, Liu J Y. 2006. Nitrogen budgets of agricultural fields of the Changjiang River basin from 1980 to 1990. Sci. Total. Environ., 363(1-3): 136-148.
DOI:10.1016/j.scitotenv.2005.06.029 |
Battarbee R W, Jones V J, Flower R J, Cameron N G, Bennion H, Carvalho L, Juggins S. 2001. Diatoms. In: Smol J P, Birks H J B, Last W M eds. Tracking Environmental Change Using Lake Sediments. Volume 3: Terrestrial, Algal, and Siliceous Indicators. Kluwer Academic Publishers, Dordrecht. p.155-202.
|
Bigler C, Heiri O, Krskova R, Lotter A F, Sturm M. 2006. Distribution of diatoms, chironomids and cladocera in surface sediments of thirty mountain lakes in southeastern Switzerland. Aquat. Sci., 68(2): 154-171.
DOI:10.1007/s00027-006-0813-x |
Chai C, Yu Z M, Shen Z L, Song X X, Cao X H, Yao Y. 2009. Nutrient characteristics in the Yangtze River estuary and the adjacent East China Sea before and after impoundment of the Three Gorges Dam. Sci. Total. Environ., 407(16): 4687-4695.
DOI:10.1016/j.scitotenv.2009.05.011 |
Chen C T A. 2009. Chemical and physical fronts in the Bohai, Yellow and East China seas. J. Mar. Syst., 78(3): 394-410.
DOI:10.1016/j.jmarsys.2008.11.016 |
Clarke A, Juggins S, Conley D. 2003. A 150-year reconstruction of the history of coastal eutrophication in Roskilde Fjord, Denmark. Mar. Pollut. Bull., 46(12): 1615-1618.
DOI:10.1016/S0025-326X(03)00375-8 |
Costa-Böddeker S, Thuyên L X, Schwarz A, Huy HĐ, Schwalb A. 2017. Diatom assemblages in surface sediments along nutrient and salinity gradients of Thi Vai estuary and Can Gio mangrove forest, Southern Vietnam. Estuar. Coast., 40(2): 479-492.
DOI:10.1007/s12237-016-0170-5 |
Crosta X, Koç N. 2007. Diatoms: from micropaleontology to isotope geochemistry. In: Hillaire-Marcel C, De Vernal A eds. Proxies in Late Cenozoic Paleoceanography. Elsevier, Amsterdam. p.327-369.
|
Cunningham L, Snape I, Stark J S, Riddle M J. 2005. Benthic diatom community response to environmental variables and metal concentrations in a contaminated bay adjacent to Casey Station, Antarctica. Mar. Pollut. Bull., 50(3): 264-275.
DOI:10.1016/j.marpolbul.2004.10.012 |
Dawes C J. 1998. Marine Botany. 2nd edn. John Wiley and Sons, Inc., New York. 484p.
|
Defersha M B, Melesse A M. 2012. Effect of rainfall intensity, slope and antecedent moisture content on sediment concentration and sediment enrichment ratio. CATENA, 90: 47-52.
DOI:10.1016/j.catena.2011.11.002 |
Di B P, Liu D Y, Wang Y J, Dong Z J, Li X, Shi Y J. 2013. Diatom and silicoflagellate assemblages in modern surface sediments associated with human activity:a case study in Sishili Bay, China. Ecol. Indic., 24: 23-30.
DOI:10.1016/j.ecolind.2012.05.020 |
Duan S W, Liang T, Zhang S, Wang L J, Zhang X M, Chen X B. 2008. Seasonal changes in nitrogen and phosphorus transport in the lower Changjiang River before the construction of the Three Gorges Dam. Estuarine Coast.Shelf Sci., 79(2): 239-250.
DOI:10.1016/j.ecss.2008.04.002 |
Dura T, Hemphill-Haley E, Sawai Y, Horton B P. 2016. The application of diatoms to reconstruct the history of subduction zone earthquakes and tsunamis. Earth-Sci.Rev., 152: 181-197.
DOI:10.1016/j.earscirev.2015.11.017 |
Gao X L, Song J M. 2005. Phytoplankton distributions and their relationship with the environment in the Changjiang estuary, China. Mar. Pollut. Bull., 50(3): 327-335.
DOI:10.1016/j.marpolbul.2004.11.004 |
Geider R J, MacIntyre H L, Graziano L M, McKay R M L. 1998. Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur. J. Phycol., 33(4): 315-332.
DOI:10.1080/09670269810001736813 |
Guo Y J, Qian S B. 2003. Marine Bacillariophyta Centricae Flora China Sea. Science Press, Beijing.
(in Chinese)
|
Hasle G R, Syvertsen E E. 1996. Marine diatoms. In: Tomas C R ed. Identifying Marine Diatoms and Dinoflagellates.Academic Press, San Diego. p.5-386.
|
Hassan G S, Espinosa M A, Isla F I. 2007. Dead diatom assemblages in surface sediments from a low impacted estuary:the Quequén Salado River, Argentina. Hydrobiologia, 579(1): 257-270.
DOI:10.1007/s10750-006-0407-6 |
Hassan G S, Espinosa M A, Isla F I. 2008. Fidelity of dead diatom assemblages in estuarine sediments:how much environmental information is preserved?. PALAIOS, 23(1-2): 112-120.
|
Hassan G S, Espinosa M A, Isla F I. 2009. Diatom-based inference model for paleosalinity reconstructions in estuaries along the northeastern coast of Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol., 275(1-4): 77-91.
DOI:10.1016/j.palaeo.2009.02.020 |
Huh C A, Su C C. 1999. Sedimentation dynamics in the East China Sea elucidated from 210Pb, 137Cs and 239, 240Pu. Mar.Geol., 160(1-2): 183-196.
DOI:10.1016/S0025-3227(99)00020-1 |
Jiang Z B, Chen J F, Zhou F, Shou L, Chen Q Z, Tao B Y, Yan X J, Wang K. 2015. Controlling factors of summer phytoplankton community in the Changjiang (Yangtze River) estuary and adjacent East China Sea shelf. Cont.Shelf. Res., 101: 71-84.
DOI:10.1016/j.csr.2015.04.009 |
Juggins S. 1992. Diatoms in the Thames estuary, England:ecology, palaeoecology, and salinity transfer function. Bibl. Diatomol., 25: 1-216.
|
Lepš J, Šmilauer P. 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, New York. 1936p.
|
Li G, Gao K S. 2014. Effects of solar UV radiation on photosynthetic performance of the diatom Skeletonema costatum grown under nitrate limited condition. ALGAE, 29(1): 27-34.
DOI:10.4490/algae.2014.29.1.027 |
Li H M, Tang H J, Shi X Y, Zhang C S, Wang X L. 2014. Increased nutrient loads from the Changjiang (Yangtze)River have led to increased harmful algal blooms. Harmful Algae, 39: 92-101.
DOI:10.1016/j.hal.2014.07.002 |
Li L, Shen X, Jiang M. 2017. Change characteristics of DSi and nutrition structure at the Yangtze River estuary after Three Gorges Project impounding and their ecological effect. Arch. Environ. Prot., 43(2): 74-79.
DOI:10.1515/aep-2017-0012 |
Lin F Z, Wu Y L, Yu H C, Xian W W. 2008. Phytoplankton community structure in the Changjiang estuary and its adjacent waters in 2004. Oceanol. Limnol. Sin, 39(4): 401-410.
(in Chinese with English abstract) |
Liu D Y, Liu L X, Di B P, Wang Y J, Wang Y N. 2015a. Paleoenvironmental analyses of surface sediments from the Bohai Sea, China, using diatoms and silicoflagellates. Mar. Micropaleontol., 114: 46-54.
DOI:10.1016/j.marmicro.2014.11.002 |
Liu D Y, Sun J, Zhang J, Liu G S. 2008. Response of the diatom flora in Jiaozhou Bay, China to environmental changes during the last century. Mar. Micropaleontol., 66(3-4): 279-290.
DOI:10.1016/j.marmicro.2007.10.007 |
Liu H J, Fu W C, Sun J. 2015b. Seasonal variations of netzphytoplankton community in East China Sea continental shelf from 2009-2011. Haiyang Xuebao, 37(10): 106-122.
(in Chinese with English abstract) |
Liu J P, Li A C, Xu K H, Velozzi D M, Yang Z S, Milliman J D, DeMaster D J. 2006. Sedimentary features of the Yangtze River-derived along-shelf clinoform deposit in the East China Sea. Cont. Shelf Res., 26(17-18): 2141-2156.
DOI:10.1016/j.csr.2006.07.013 |
Liu J P, Xu K H, Li A C, Milliman J D, Velozzi D M, Xiao S B, Yang Z S. 2007. Flux and fate of Yangtze River sediment delivered to the East China Sea. Geomorphology, 85(3-4): 208-224.
DOI:10.1016/j.geomorph.2006.03.023 |
Liu R M, Men C, Liu Y Y, Yu W W, Xu F, Shen Z Y. 2016a. Spatial distribution and pollution evaluation of heavy metals in Yangtze estuary sediment. Mar. Pollut. Bull., 110(1): 564-571.
DOI:10.1016/j.marpolbul.2016.05.060 |
Liu S M, Qi X H, Li X N, Ye H R, Wu Y, Ren J L, Zhang J, Xu W Y. 2016b. Nutrient dynamics from the Changjiang (Yangtze River) estuary to the East China Sea. J. Marine.Syst., 154: 15-27.
DOI:10.1016/j.jmarsys.2015.05.010 |
Loebl M, Cockshutt A M, Campbell D A, Finkel Z V. 2010. Physiological basis for high resistance to photoinhibition under nitrogen depletion in Emiliania huxleyi. Limnol.Oceanogr., 55(5): 2150-2160.
DOI:10.4319/lo.2010.55.5.2150 |
Luoto T P, Rantala M V, Tammelin M H. 2017. Tracking the limnoecological history of Lake Hiidenvesi (Southern Finland) using the paleolimnological approach. Water Air Soil Poll., 228(12): 461.
DOI:10.1007/s11270-017-3622-z |
Margalef R. 1968. Perspective in Ecological Theory. University of Chicago Press, Chicago. 480p.
|
McQuoid M R, Hobson L A. 1998. Assessment of palaeoenvironmental conditions on southern Vancouver Island, British Columbia, Canada, using the marine tychoplankter Paralia sulcata. Diatom. Res., 13(2): 311-321.
DOI:10.1080/0269249X.1998.9705453 |
McQuoid M R, Hobson L A. 2001. A Holocene record of diatom and silicoflagellate microfossils in sediments of Saanich Inlet, ODP Leg 169S. Mar. Geol., 174(1-4): 111-123.
DOI:10.1016/S0025-3227(00)00145-6 |
McQuoid M R, Nordberg K. 2003. The diatom Paralia sulcata as an environmental indicator species in coastal sediments. Estuar. Coast. Shelf Sci., 56(2): 339-354.
DOI:10.1016/S0272-7714(02)00187-7 |
Mirabdullayev I M, Joldasova I M, Mustafaeva Z A, Kazakhbaev S, Lyubimova S A, Tashmukhamedov B A. 2004. Succession of the ecosystems of the Aral Sea during its transition from oligohaline to polyhaline water body. J.Marine Syst., 47(1-4): 101-107.
DOI:10.1016/j.jmarsys.2003.12.012 |
Montagnes D J S, Franklin M. 2001. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content:reconsidering some paradigms. Limnol.Oceanogr., 46(8): 2008-2018.
DOI:10.4319/lo.2001.46.8.2008 |
Nazarova L, Bleibtreu A, Hoff U, Dirksen V, Diekmann B. 2017. Changes in temperature and water depth of a small mountain lake during the past 3000 years in Central Kamchatka reflected by a chironomid record. Quatern.Int., 447: 46-58.
DOI:10.1016/j.quaint.2016.10.008 |
Ning X, Lin C, Su J, Liu C, Hao Q, Le F. 2011. Long-term changes of dissolved oxygen, hypoxia, and the responses of the ecosystems in the East China Sea from 1975 to 1995. J. Oceanogr., 67(1): 59-75.
DOI:10.1007/s10872-011-0006-7 |
Pu X M, Wu Y L, Zhang Y S. 2001. Nutrient limitation of phytoplankton in the Changjiang estuary Ⅱ. Condition of nutrient limitation in spring. Acta Oceanol. Sin., 23(3): 57-65.
(in Chinese with English abstract) |
Ren J, Gersonde R, Esper O, Sancetta C. 2014. Diatom distributions in northern North Pacific surface sediments and their relationship to modern environmental variables. Palaeogeogr. Palaeoclimatol. Palaeoecol., 402: 81-103.
DOI:10.1016/j.palaeo.2014.03.008 |
Romero O E, Armand L K, Crosta X, Pichon J J. 2005. The biogeography of major diatom taxa in Southern Ocean surface sediments:3. Tropical/Subtropical species. Palaeogeogr. Palaeoclimatol. Palaeoecol., 223(1-2): 49-65.
DOI:10.1016/j.palaeo.2005.03.027 |
Sayer C, Roberts N, Sadler J, David C, Wade P M. 1999. Biodiversity changes in a shallow lake ecosystem:a multi-proxy palaeolimnological analysis. J. Biogeogr., 26(1): 97-114.
DOI:10.1111/jbi.1999.26.issue-1 |
Schröder M, Sondermann M, Sures B, Hering D. 2015. Effects of salinity gradients on benthic invertebrate and diatom communities in a German lowland river. Ecol. Indic., 57: 236-248.
DOI:10.1016/j.ecolind.2015.04.038 |
Serieyssol K, Chatelard S, Cubizolle H. 2011. Diatom fossils in mires:a protocol for extraction, preparation and analysis in palaeoenvironmental studies. Mires Peat, 7(12): 12.
|
Shannon C E, Weaver W. 1949. The Mathematical Theory of Communication. University of Illinois Press, Urbana. 117p.
|
Shepard F P. 1954. Nomenclature based on sand-silt-clay ratios. J. Sediment. Petrol., 24(3): 151-158.
|
Song S Q, Li Z, Li C W, Yu Z M. 2017. The response of spring phytoplankton assemblage to diluted water and upwelling in the eutrophic Changjiang (Yangtze River) estuary. Acta Oceanol. Sin., 36(12): 101-110.
DOI:10.1007/s13131-017-1094-z |
Song S Q, Sun J, Luan Q S, Shen Z L. 2008. Size-fractionated phytoplankton biomass in autumn of the Changjiang(Yangtze) River estuary and its adjacent waters after the Three Gorges Dam construction. Chin. J. Oceanol.Limnol., 26(3): 268-275.
DOI:10.1007/s00343-008-0268-0 |
Tang X H, Wang F. 2004. Analyses on hydrographic structure in the Changjiang River estuary adjacent waters in Summer and Winter. Stud. Mar. Sin., 46: 42-66.
(in Chinese with English abstract) |
Wang B D. 2006. Cultural eutrophication in the Changjiang(Yangtze River) plume:history and perspective. Estuar.Coast. Shelf Sci., 69(3-4): 471-477.
DOI:10.1016/j.ecss.2006.05.010 |
Wang G Q, Shi X F, Liu Y G, Fang X S, Yang G. 2013. Seasonal and spatial variation in suspended sediment characteristics off the Changjiang estuary. In: Li M Z, Sherwood C R, Hill P R eds. Sediments, Morphology and Sedimentary Processes on Continental Shelves: Advances in Technologies, Research and Applications. WileyBlackwell, Hoboken, NJ. p.351-368.
|
Wang J H. 2002. Phytoplankton Communities in three distinct ecotypes of the Changjiang estuary. J. Ocean Univ.Qingdao, 32(3): 422-428.
(in Chinese with English abstract) |
Wang K F, Jiang H, Zhang Y L, Wang Y J, Xu J S. 1985. Environmental discussion on distribution of the diatom in the surface sediments of the Huanghai Sea. Oceanol.Limnol. Sin., 16(5): 400-407.
(in Chinese with English abstract) |
Wang K F, Jiang H, Zhi C Y, Tao M H, Wang H G. 2001. Study on the relationship between diatom assemblage in surface sediments and the environment in the East China Sea. Acta Micropalaeontol. Sin., 18(4): 379-384.
(in Chinese with English abstract) |
Wang K F, Zhang Y L, Jiang H, Sun Y H. 1984. Analysis of fossil algal assemblages from the late late Pleistocene sediments in the continental shelf of the Dong Hai and their paleoecological environment. Acta Ecol. Sin., 4(3): 224-230.
(in Chinese with English abstract) |
Wang Y N, Liu D Y, Di B P, Shi Y J, Wang Y J. 2016. Distribution of diatoms and silicoflagellates in surface sediments of the Yellow Sea and offshore from the Changjiang River, China. Chin. J. Oceanol. Limn., 34(1): 44-58.
DOI:10.1007/s00343-015-4237-0 |
Weckström J, Korhola A, Blom T. 1997. Diatoms as quantitative indicators of pH and water temperature in subarctic Fennoscandian lakes. Hydrobiologia, 347(1-3): 171-184.
|
Weckström K, Juggins S, Korhola A. 2004. Quantifying background nutrient concentrations in coastal waters:a case study from an urban embayment of the Baltic Sea. Ambio, 33(6): 324-327.
DOI:10.1579/0044-7447-33.6.324 |
Wong P P, Losada I J, Gattuso J P, Hinkel J, Khattabi A, McInnes K L, Saito Y, Sallenger A. 2014. Coastal systems and low-lying areas. In: Field C B, Barros V R, Dokken D J, Mach K J, Mastrandrea M D, Bilir T E, Chatterjee M, Ebi K L, Estrada Y O, Genova R C, Girma B, Kissel E S, Levy A N, MacCracken S, Mastrandrea P R, White L L eds. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects.Contribution of Working Group Ⅱ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United. p.361-409.
|
Yang Z, Wang H, Saito Y, Milliman J D, Xu K, Qiao S, Shi G. 2006. Dam impacts on the Changjiang (Yangtze) River sediment discharge to the sea:the past 55 years and after the Three Gorges Dam. Water Resour. Res., 42(4): W04407.
|
Zhang J, Liu S M, Ren J L, Wu Y, Zhang G L. 2007. Nutrient gradients from the eutrophic Changjiang (Yangtze River) estuary to the oligotrophic Kuroshio waters and reevaluation of budgets for the East China Sea Shelf. Prog. Oceanogr., 74: 449-478.
DOI:10.1016/j.pocean.2007.04.019 |
Zhou M J, Shen Z L, Yu R C. 2008. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res., 28(12): 1483-1489.
DOI:10.1016/j.csr.2007.02.009 |
Zhou Z X, Yu R C, Zhou M J. 2017. Resolving the complex relationship between harmful algal blooms and environmental factors in the coastal waters adjacent to the Changjiang River estuary. Harmful Algae, 62: 60-72.
DOI:10.1016/j.hal.2016.12.006 |
Zhu Z Y, Zhang J, Ying Wu, Zhang Y Y, Lin J, Liu S M. 2011. Hypoxia off the Changjiang (Yangtze River) estuary:oxygen depletion and organic matter decomposition. Mar. Chem., 125(1-4): 108-116.
DOI:10.1016/j.marchem.2011.03.005 |
Zong Y Q, Tooley M J. 1999. Evidence of mid-Holocene storm-surge deposits from Morecambe Bay, northwest England:a biostratigraphical approach. Quat. Int., 55(1): 43-50.
DOI:10.1016/S1040-6182(98)00022-6 |
Zong Y Q. 1997. Implications of Paralia sulcata abundance in Scottish isolation basins. Diatom. Res., 12(1): 125-150.
DOI:10.1080/0269249X.1997.9705407 |
 2019, Vol. 37
2019, Vol. 37