Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Yun, PENG Chengrong, WANG Jun, HUANG Shun, HU Yao, ZHANG Jinli, LI Dunhai
- Temperature and silicate are significant driving factors for the seasonal shift of dominant diatoms in a drinking water reservoir
- Journal of Oceanology and Limnology, 37(2): 568-579
- http://dx.doi.org/10.1007/s00343-019-8040-1
Article History
- Received Mar. 2, 2018
- accepted in principle May. 11, 2018
- accepted for publication Jul. 2, 2018
2 University of Chinese Academy of Sciences, Beijing 100049, China
In China, reservoirs were originally built for flood control, power generation and irrigation. However, in recent years, these reservoirs have been used to store water to meet the increasing demand of freshwater supply. With the intensification of pollution in rivers and lakes, the reservoirs play an increasingly important role in water supply (Han, 2010). Although the water quality in reservoirs is better than shallow lakes currently, most of reservoirs are suffering the pollution in industrialized and urbanized areas. Therefore, more and more attention has been paid to the prevention of water quality deterioration.
As a type of artificial structure, reservoirs are constructed by dams to adjust the water level. The frequently fluctuations of water level often resulting in aquatic organisms lacking sufficient time to complete their life cycle and maintaining their population. Therefore, the organisms in reservoirs are mainly dominated by r-strategists, such as phytoplankton (Han, 2010). As single-celled or simple colonial organisms with short regeneration times, phytoplankton respond more rapidly in terms of species composition and abundance to environmental changes than larger organisms (Yang et al., 2012). As the base of aquatic food webs, phytoplankton community structure and diversity are widely used to quantify water quality (Rakocevic-Nedovic and Hollert, 2005). Different from the shallow lakes where cyanobacteria often dominate, generally, reservoirs are usually dominated by diatoms (Class Bacillariophyceae) (Saros and Anderson, 2015). Especially in spring, diatoms are usually prevalent and some of them even form serious blooms, yet, also cause a series of ecological problems, such as low water transparency, bad odors, clogging or sedimentation in water treatment processes, and a decrease in the perceived aesthetic value of the water body (Kolmakov et al., 2002).
Diatoms are important indictors of environmental changes where individual species respond directly and sensitively to changes in chemical and physical variables such as nutrients, pH, light and temperature (Baier et al., 2004). Therefore, changes in physical and chemical parameters within aquatic ecosystems can markedly alter the dominant diatom species composition (Julius and Theriot, 2010). Some physical and chemical parameters, such as water temperature, nutrients concentration and light intensity, vary depend on the seasonal changes, may lead to a seasonal shift of dominant diatom species (Sommer et al., 1986). Numerous studies have explored the relationship between diatom distribution and physico-chemical characteristics. In most cases, the thermal regime (the indirect effect of temperature) and mixing in reservoirs caused changes in the underwater light conditions and nutrient concentrations, which was generally considered as the cause of the dynamics of algae (Hubble and Harper, 2002). However, few studies have focus on the direct effect of temperature on the dynamics of diatom species composition in subtropical reservoirs, and the main factors that drive the seasonal succession of diatoms are still poorly understand (Reynolds et al., 2000).
At present, a number of studies have focused on the driving factors to seasonal succession of the whole phytoplankton in eutrophic waters, there are few studies on the specific species of diatoms which are potential bloom species in drinking water reservoir. In this study, we investigated the temporal dynamics of species composition and abundance of diatom communities, as well as the temporal dynamics of physical and chemical factors, in a drinking water reservoir. Based on the obvious seasonal succession of dominant diatom species, we hypothesized that: (1) Temperature and inorganic nutrients are important factors for the succession of dominant diatom species, and silicate is one of the most important inorganic nutrients; (2) on temporal scales, the influence factors affect the growth of diatoms alone or in combination.
2 MATERIAL AND METHOD 2.1 Study areaThe Jinshahe reservoir is located at Hong'an County, Hubei Province, China (114°32'-114°35'E, 31°17'-31°23'N). It was built in 1965 and has a basin area of 108 km2 with a total storage capacity of 1.787×108 m3. The reservoir is of great importance for flood control and irrigation, and most importantly, it is the only drinking water source for Hong'an County and reserve water source for Wuhan city. It is one of the large reservoirs in the middle-lower reaches of Changjiang (Yangtze) River, and it is representative in terms of watershed characteristics, geomorphology and comprehensive functions. Since the completion of the reservoir, no fertilization and feeding for aquaculture has been implemented in order to alleviate pollution. However, in recent years, the water quality in this reservoir has been affected by industrial and agricultural activities.
2.2 Field sampling and measurementsThe investigation can be divided into three distinct periods. The first period was from September to December 2015 with monthly intervals, the second period was from January to February and June to August 2016 with two weeks intervals, and the third period was from March to May 2016 with ten days intervals. Samples were collected from five sampling sites (Fig. 1) in surface water (0-0.5 m). Water temperature (WT), Dissolved oxygen (DO), Specific Conductivity (SPC), pH and Oxidation-Reduction Potential (ORP) were measured in situ using a multiparameter probe (YSI-2000, USA). Water transparency was measured with a Secchi disc. Chemical oxygen consumption (COD), total phosphorus (TP) (mg/L), total nitrogen (TN) (mg/L) and dissolved inorganic nutrients (nitrate (mg/L), ammonia (mg/L), phosphate (mg/L) and silicate (mg/L)) were carried out following standard methods (APHA 1999). Samples for chlorophyll determination were filtered through a Whatman GF/C filter (biomass intercepted by 1.2 μm pore size). The filters were immersed with 90% acetone and extracted in the dark at 4℃ for 24 h. The extracting solution was measured using a spectrophotometer (TU-1810, DPC, China) and the concentration of chlorophyll a and c (Chl a and Chl c) were calculated by the standard method (Arar, 1997).
|
| Fig.1 Sampling sites in Jinshahe Reservoir |
One liter of water samples for phytoplankton cell count and species identification were preserved with Lugol's solution and were concentrated to a final volume of 30 mL after sedimentation for 48 h. All taxa of algal abundances were counted with a counting chamber (0.1 mL) at 400× magnification (at least 400 algal cells were counted and identified). In order to make an accurate identification of diatom species, six dominant diatom species from the raw water in Jinshahe Reservoir were isolated, purified and cultured. The cultured diatom cells were treated with sulfuric acid to remove organic matters. Cleaned frustule suspensions were settled onto microscopy glass slides and dried, then slides were mounted using Naphrax (refractive index, 1.703) and observed in light microscopy at 1 000× magnification (oil immersion lens) (Stockner and Benson, 1967).
2.4 Statistical analysisRedundancy analysis (RDA) was used to describe the relationships between the diatom abundance and the environmental variables (CANOCO 4.5 program). RDA was chosen because the length of gradient of the first DCA (Detrended Correspondence Analysis) axis running on diatom abundance data was 0.611; therefore, we chose linear ordination techniques. Diatom densities data were lg(x+1) transformed. The significant driving environmental factors were selected by the automatic forward selection with 499 unrestricted Monte Carlo permutations, and the significance was evaluated by the probability values < 0.05. Bi-plot of relationship between environmental factors and diatom abundance and relationship between sampling units and phytoplankton abundance were presented using Cano Draw of CANOCO 4.5.
Generalized additive model (GAM) (Hastie and Tibshirani, 1990) was set up to model the relationship between the abundance of diatom species and driving factors using R version 3.2.5 with the 'mgcv' package. This technique allows the identification of different types of relationships between variables, including nonlinear ones. If GAM indicates that the smoother is a straight line, then the Generalized Least Squares model (GLS) is used. GLS allows estimation of unknown parameters in linear regression models even when assumptions are violated (Zuur et al., 2009). The GAM model was,
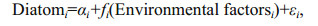
where i represents the six dominant diatom species, α and ε were intercept and error respectively, and fi was the smoothing function; environmental factors were water temperature or silicate. In fact, contrary to usual regression models, in GAM we do not have an equation, but a smoother, which can be evaluated by plotting (Zuur et al., 2009). The GLM models was,

where i represents the six dominant diatom species, α, β and ε were intercept, slop and error respectively; environmental factors were water temperature or silicate.
3 RESULT 3.1 Environmental parametersThe annual variations in environmental factors and chlorophyll of Jinshahe Reservoir were listed in Appendix Fig.S1. The lowest and the highest water temperatures occurred on 2 February and 31 July respectively. The concentration of silicate showed significant temporal variation. It dropped markedly from 16 December to 30 April to the lowest value, and then gradually increasing to its maximum in August. DO showed the opposite variation trend with water temperature. The concentration of nitrogen in various forms showed a downward trend from January to August in 2016. The average concentration of phosphorus was very low and the maximum (314.22) and minimum (19.84) of N:P value occurred in 16 December and 10 May respectively. The concentration of both Chl a and Chl c had their maximum and minimum in 16 September and 30 April respectively. Chl c showed high correlation with Chl a (R=0.66, P < 0.001), but they had no significant correlation with diatom abundance (Chl a, R=0.41, P=0.053; Chl c, R=0.19, P=0.37).
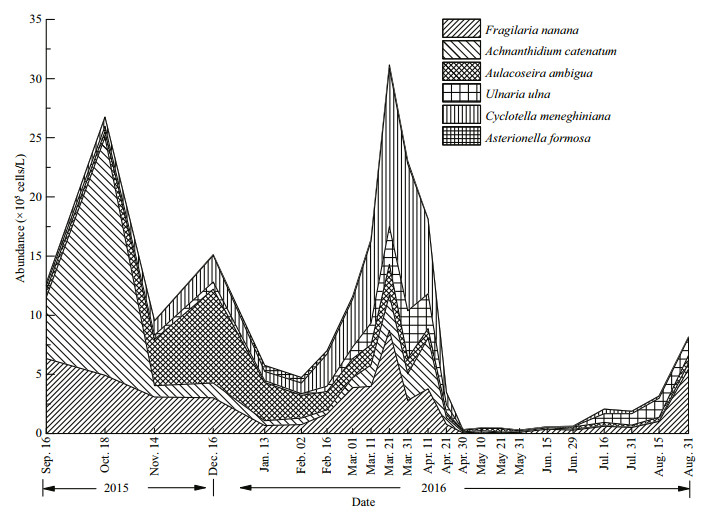
3.2 Seasonal succession of dominant diatom speciesFrom October 2015 to late April 2016, diatoms accounted for a large proportion of phytoplankton abundance, and the highest proportion was 85.3% at the end of March 2016 (Appendix Fig.S2). Six dominant diatom species, Fragilaria nanana Lange-Bertalot 1993, Achnanthidium catenatum (Bily and Marvan) Lange-Bertalot 1999, Aulacoseira ambigua (Grunow) Simonsen 1979, Ulnaria ulna (Nitzsch) Compère 2001, Cyclotella meneghiniana Küzing 1844 and Asterionella fOrmosa Hassall 1850 were identified, which accounted for 98.7% of the total abundance of diatoms and 46.8% of the total abundance of phytoplankton (Appendix Fig.S2). Obvious seasonal successions of the six diatom species were observed (Fig. 2). From the end of August to the middle of September, F. nanana was the dominant species in the diatom community, and then it was replaced by A. catenatum, which accounted for 75.2% of the total abundance of diatoms in 18 October. A. ambigua dominated from 14 November to 2 February and from 30 April to 21 May, while C. meneghiniana dominated from 2 February to 30 April. From the end of May to the end of June, F. nanana dominated again, and from 16 July to 15 August, the diatom community was dominated by U. ulna.
|
| Fig.2 The temporal dynamics of abundance of six dominant diatoms in Jinshahe Reservoir from September 2015 to August 2016 |
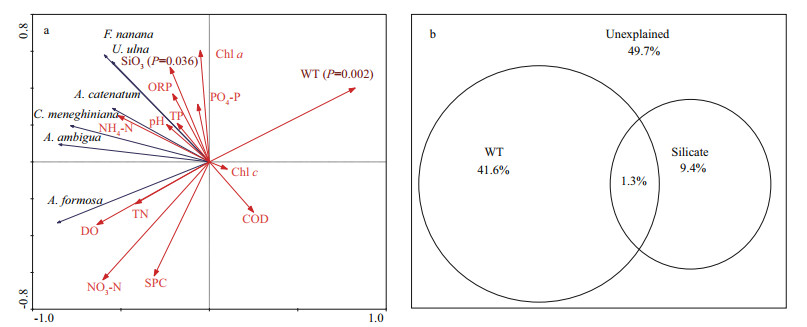
As expected, only water temperature and silicate explained significant proportions (P < 0.05) of the variations of the diatom communities (Fig. 3a). They explained 51% of the diatom variance, while it was 77% for all variables. Water temperature was the most important variable and explained 41.6% of the diatom variance, followed by silicate explained 9.4%. The interaction between water temperature and silicate was weak, which explained only 1.3% of the diatom variance (Fig. 3b).
|
| Fig.3 Ordination biplot of the Redundancy analysis (RDA) for dominant diatoms abundance and environmental variables (a); variance partitioning of diatom composition explained by water temperature and silicate (b) In a: Monte Carlo permutation tests identified that water temperature (P=0.002) and silicate (P=0.036) explained significant proportions. Blue vectors: dominant diatoms, red vectors: environmental variables; WT: water temperature; TN: total nitrogen; NO3-N: nitrate; NH4-N: ammonia; TP: total phosphorus; PO4-P: phosphate; SiO3: silicate; ORP: oxidation-reduction potential; SPC: specific conductivity; COD: chemical oxygen consumption; Chl a: chlorophyll a; Chl c: chlorophyll c. |
The abundances of all diatom species decreased rapidly at the end of March (Fig. 4), at the same time, the concentration of silicate dropped below 1.0 mg/L. After 29 June, most diatoms increased in abundances, especially U. ulna, and the concentration of silicate rose above 1.0 mg/L again. Therefore, during the year, from 31 March to 29 June was the period that silicate limited the growth of diatoms, while in the remaining months, silicate satisfied the growth of diatoms, and the key factor that controlling the succession of diatoms was water temperature.
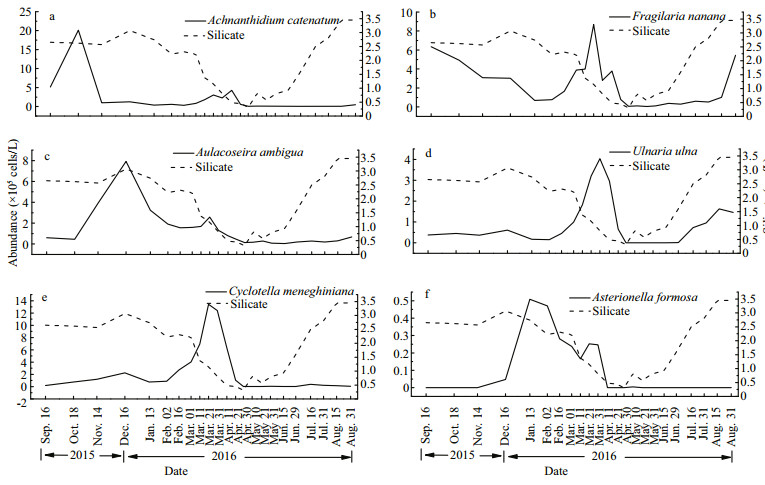
|
| Fig.4 Temporal dynamics of abundance in the six dominant diatom species with silicate a. Achnanthidium catenatum; b. Fragilaria nanana; c. Aulacoseira ambigua; d. Ulnaria ulna; e. Cyclotella meneghiniana; f. Asterionella formosa. Solid line: diatoms, dashed line: silicate. |
The abundance of A. catenatum increased with the water temperature dropped from 28.0℃ to 22.7℃, and reached its maximum at 22.7℃, however, as the temperature continued to decline, its abundance declined sharply. After 2 February, the rise in temperature promoted the growth of A. catenatum until it was limited by silicate, while after the threshold time of silicate limitation, the rise in temperature would inhibit the growth of diatoms (Fig. 5a). The growth of F. nanana was limited by the low temperature in severe winter and was promoted by the rising temperature from 2 February to 31 March. From 29 June to 31 July, it was limited by high temperature until the temperature started to drop (Fig. 5b). A. ambigua grew rapidly with the decline of temperature, and it reached the maximum abundance at temperature of 9.5℃ on 16 December. Its subsequent trend was the same with A. catenatum (Fig. 5c). The abundance of U. ulna increased rapidly as the temperature increased in spring. After the period of silicate limitation, its abundance increased again with the rising temperature (Fig. 5d). From 16 September to 29 June, the abundance variation of C. meneghiniana had the same trend with that of U. ulna, but it did not recover its proliferation at high temperatures in summer (Fig. 5e). A. formosa is a cold adapted species, the lower the temperature, the higher its abundance appeared (Fig. 5f).

|
| Fig.5 Temporal dynamics of abundance in the six dominant diatom species with water temperature a. Achnanthidium catenatum; b. Fragilaria nanana; c. Aulacoseira ambigua; d. Ulnaria ulna; e. Cyclotella meneghiniana; f. Asterionella formosa. Solid line: diatoms, dashed line: water temperature. |
Since the cross-validation between water temperature and silicate was weak, the relationship between the two factors and diatom communities were fitted using GAM dividedly. According to the ordinal controls of water temperature and silicate on the growth of diatoms, water temperature and diatoms were fitted with GAM when silicate met the growth needs of diatoms. Contrarily, the silicate and diatoms were fitted with GAM when the temperature favored the growth of diatoms.
Except for A. ambigua, the smoothers obtained by fitting water temperature and all diatoms with GAM were curves (Fig. 6). Based on the AIC (Akaike Information Criterion), GAM models of the six diatom species included both smoothers and intercepts. The abundance curve of spring-autumn species (A. catenatum and C. meneghiniana) showed a narrow single peak with the change of water temperature. The abundance curve of warm adapted species (U. ulna) presented an obvious peak at 14.4℃ and a small peak at 34℃. A wide peak of the abundance of eurythermal species (F. nanana) was observed. Cold adapted species (A. ambigua and A. formosa) showed significant negative correlations with water temperature.
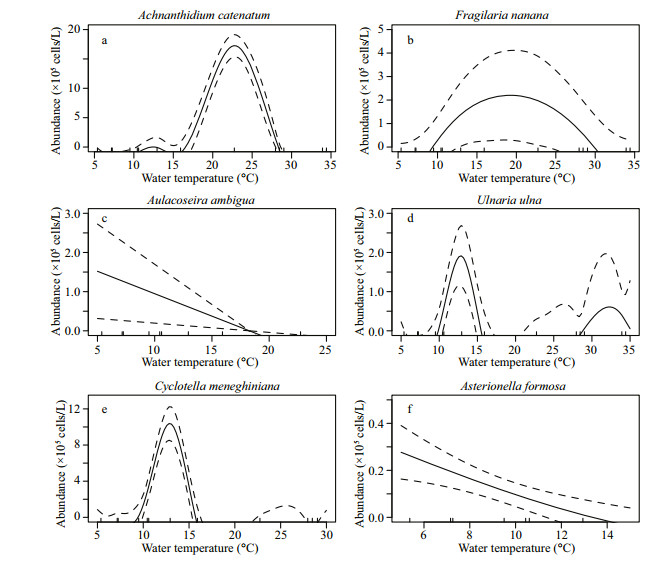
|
| Fig.6 Smoothing curves obtained by applying the GAM model based on diatom abundance and water temperature a. Achnanthidium catenatum; b. Fragilaria nanana; c. Aulacoseira ambigua; d. Ulnaria ulna; e. Cyclotella meneghiniana; f. Asterionella formosa. Dotted lines are 95% point-wise confidence bands. |
A straight line relating silicate and diatoms was obtained by applying the GAM on lg (abundance+1)transformed (Fig. 7). Besides significant (P < 0.001) intercept, the relationship of silicate between diatom abundance was also significant (P < 0.05). GLS was used to highlight significant slope (P < 0.05) and intercept (P < 0.001) between the abundance of diatoms and silicate. Based on the transformation of response variables, the relationship between the abundance of diatoms and silicate obtained by GLS can be suggested by the following model:
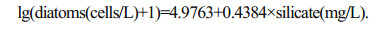

|
| Fig.7 Straight line obtained by applying the GAM model based on the abundance of diatom and silicate Dotted lines are 95% point-wise confidence bands. |
This equation indicated that the abundance of diatoms increased logarithmically with the increasing silicate concentration. The abundances of diatoms were 3.47×105 cells/L and 2.07×105 cells/L at the beginning and the end of silicate restriction period respectively, and the corresponding silicate concentrations obtained from above equation were 1.29 mg/L and 0.77 mg/L, respectively, which should be the predicted thresholds for limiting the growth of diatoms. According to actual observations, the silicate threshold for limiting the growth of diatoms was 1.0 mg/L, which was within the predicted values.
4 DISCUSSION 4.1 Seasonal succession of dominant diatom species in drinking water reservoirUnder the condition of silicate satisfaction, water temperature is the main factor affecting the succession of diatoms. The area of the middle and lower reaches of Changjiang River belong to a typical subtropical monsoon climate with four distinct seasons, unusually hot summers and cold winters. The hottest period is from July to August and the coldest period is from the end of December to early February. The apparent seasonal variation of temperature leads to changes in diatom community structure. As a typical large drinking water reservoir in the middle and lower reaches of the Changjiang River, the phytoplankton community in Jinshahe Reservoir was dominated by diatoms. The dominant diatom species adapted to different temperatures and showed seasonal succession. In fact, this phenomenon is very common in subtropical drinking water reservoir in China, such as Shahe Reservoir and Hengshan Reservoir in Jiangsu Province (Zhu et al., 2016), Tangpu Reservoir in Zhejiang Province (Shi et al., 2013), and Daoguanhe Reservoir in Hubei Province (Lü et al., 2012). Furthermore, these reservoirs have the similar dominant diatom species and the similar seasonal succession pattern with Jinshahe Reservoir. Namely, A. catenatum dominates in spring or autumn, Ulnaria dominates in summer, A. formosa and A. ambigua dominate in winter. This succession pattern does not exist in freshwater lakes, especially in eutrophic lake, owing to Stephanodiscus hantzschii and Cyclotella atomus usually dominate in diatoms in eutrophic lake (Yao et al., 2011; Wei et al., 2015). Therefore, this succession pattern in these dominant diatom species is commonly exist in most of drinking water reservoirs in subtropical region, especially in the area of the middle and lower reaches of Changjiang River.
4.2 Effect of water temperature on diatom communitiesWater temperature directly controls the growth and proliferation of diatoms. The direct effect depends on the adaption of enzyme activity to temperatures. The seasonal succession among different diatom species may be directly related to the optimum temperature for the growth of specific diatoms being different (Cullen and MacIntyre, 2016). According to the GAM, U. ulna peaked at 14.4℃ and 34℃, the low abundance between the two temperatures was resulted from the restriction of silicate, which indicated that U. ulna can adapt a wide and warm temperature range. U. ulna also has been found in considerable amounts in summer at Decazeville (Morin et al., 2007). F. nanana had a wide peak in the GAM model. Prior studies suggested that Fragilaria genus is a eurythermal genus capable of surviving over a wide range of temperatures (Chaffin et al., 2012; Snitko et al., 2015). A. catenatum and C. meneghiniana peaked at 22℃ and 13℃ in the GAM model respectively. A. catenatum has been found as the absolutely dominant species in spring bloom in Tangpu reservoir (Ma et al., 2016) and Jinshahe Reservoir (Zhang et al., 2015) in China, which indicates that the temperature in spring or autumn is suitable for the growth of A. catenatum. C. meneghiniana is always considered as a eutrophic species and can adapt to a wide temperature range. In this study, it dominating only in early spring may be related to the restrictions of silicate. More remarkable, the presence of this eutrophic indicator species is an alert of deterioration of water quality. A. formosa and A. ambigua showed negative correlations with water temperature in GAM model. A. formosa has always been reported to prefer low temperatures and usually dominates during the cold season in well-mixed reservoirs (Bertrand et al., 2003). The blooms of A. ambigua in a reservoir in north central Texas were found at temperature < 15℃ (Grover and Chrzanowski, 2006), which indicated that it can adapt to a low temperature range. Therefore, there was the following succession pattern: from summer to spring, the warm adapted species U. ulna was replaced by spring-autumn species A. catenatum, and then the latter was replaced by cold adapted species A. ambigua and A. formosa and early spring species C. meneghiniana. Eurythermal species F. nanana had a wider growth period. Water temperature has been considered as the key factor of driving seasonal succession of diatoms in various water bodies, such as soda lake (Kocer and Şen, 2012), freshwater lake (Wei et al., 2015), stream (Maraslioglu et al., 2016), as well as reservoir (Sonmez, 2011).
The thermal regime caused by temperature changes is commonly considered as an important indirect effect of temperature on seasonal shift of algae composition (Winder and Schindler, 2004). In spring, the water column is well mixed and the high availability of nutrients enables diatom communities to develop biomass peaks. As the temperature rises in summer, thermal stratification forms, and some warmadapted diatom species may grow well. But other taxonomic groups, such as cyanobacteria, are more common in eutrophic waters (Znachor et al., 2013). In autumn, as the water temperature decreases, the water column is mixed again, and the diatom community may become abundant again (Sommer et al., 1986). In winter, some hypothermophilous diatom species usually dominate in oligotrophic reservoirs. Temperature may also alter the cell size (Peter and Sommer, 2012; Polovina and Woodworth, 2012), ecological stoichiometry (Xiu and Chai, 2012) and biogeochemical components (Wohlers-Zöllner et al., 2012) of diatoms. Compared to smaller diatoms, larger diatoms (U. ulna) may have different response under high temperature conditions, and smaller surface area to volume ratio may be advantageous for diatoms at high temperatures (Yun et al., 2010).
4.3 The limitation of silicate to diatom communitiesSilicon (Si) is an essential element of diatoms. Without Si, diatoms will not form valves and their cell cycle will not be completed (Koester et al., 2016). In Jinshahe Reservoir, the concentration of dissolved silicate began to decline in mid-December and reached the minimum value (0.3 mg/L, namely, 3.9 μm/L) at the end of April and then increased gradually. The decline in silicate concentration may be caused by uptake and utilization. In this reservoir, diatoms constituted the majority of phytoplankton in autumn, winter and spring, and the uptake of silicate by diatoms for reproduction would decrease the concentration of silicate in the water (Downing et al., 2016). The increase in silicate concentration in May might be related to rainfall. From May 2016, the rainfall in the middle and lower reaches of Changjiang River has increased significantly. The total rainfall from May to June was 1 268.4 mm, which was more than six times (192.1 mm) over the last three months (China Meteorological Data Service Center, CMDC). The increase in silicate concentration could be explained by an intensive inflow during the rains from the shoreside regions and inflow streams. Heavy rains also caused a dramatic increase in nutrients in Dongping Lake, China (Tian et al., 2013). From April to July, silicate deficiency caused all diatoms hardly to be detected. During silicate limitation, phytoplankton cells would be disfigured, malformed, and discolored (Yang et al., 2004), especially for diatoms, the valve formation and the metabolic process would be inhibited, consequently, the reproductive capacity and abundance of phytoplankton decreased.
In 2010, there was a community shift from the dominance of large fast-growing diatoms to smaller diatoms and other smaller algae species, when the silicate concentration reached 2 μm/L, which was generally considered to inhibit the growth of diatoms (Eliasen et al., 2017). This concentration is lower than ours, it may be that different diatom species have various thresholds of silicate concentration. The different requirement of silicate for various species may be related to cell size and molecular mechanisms of silicate metabolism. Larger diatoms have a higher half-saturation constant for Si uptake (Shi et al., 2015), and they need to synthesize larger cell walls during proliferation, and therefore they require more silicate. However, the smaller diatoms have a higher surface to volume ratio and survive better in Sidepleted environment. There is also Si competition between different diatom species. Asterionella formosa seemed to be a better competitor for silica, which achieved the highest biomass during the decrease of dissolved reactive silicate (DRSi) concentrations, while Fragilaria crotonensis was outcompeted by it (Tolotti et al., 2012).
The nutrients nitrogen (N) and phosphorus (P) are also important for the growth of algae, and P is usually the key limiting nutrient in most oligo-mesotrophic lakes and reservoirs (Dzialowski et al., 2005). In Jinshahe Reservoir, the average ratio of TN to TP was 109.3, which was much higher than the Redfield Ratio of 16 (Parsons et al., 1984) and the empirical threshold for P limitation (Hecky and Kilham, 1988). In this reservoir, the concentration of dissolved phosphate remained below 0.006 mg/L during the whole year and it had no significant variation on temporal scale. These indicate that P was the limiting factor in Jinshahe Reservoir. However, the growth of diatoms was not affected by P limitation, and the variation of diatom abundances had no significant correlation (R=0.18, P=0.42) with the concentration of phosphate. Nevertheless, diatom abundances were positively correlated with the concentration of silicate which varied obviously with seasonal variation. We can conclude that Si, rather than P, is the key nutrient that determines the reproduction and decline of diatoms.
4.4 The ordinal controls of water temperature and silicate on the growth of diatomsThe ordinal controls of water temperature and silicate on the growth of diatoms resulted in different dominant diatom species on temporal scale. Environmental variables change with seasons, which in turn influence algal biomass and species composition. In fact, in all lakes or reservoirs, the response of algal communities is most evident and striking when environment-mediated ecological thresholds are passed (Smol and Douglas, 2007; Cao et al., 2016). In the case of silicate satisfaction, the abundance of A. catenatum increased rapidly when the appropriate temperature threshold approached in autumn, and it decreased rapidly when the temperature fell below its growth threshold. In spring, the abundance of A. catenatum increased again until it encountered the silicate limitation threshold. The similar phenomenon was also found in other diatom species. Silicate and water temperature also affect the occurrence time of diatom blooms. In 14 April of 2014, a bloom of A. catenatum occurred in this reservoir, and its abundance reached 3.28×108 cells/L (Zhang et al., 2015). However, the A. catenatum bloom was limited by silicate limitation in the spring of 2016. F. nanana also appeared the same phenomenon. Water temperature was also recognized that it could shift the timing of diatom blooms. Researchers had simulated the effect of climate warming on the timing of the phytoplankton spring maximum, and most of them concluded that the rise in water temperature would result in a more intense and earlier spring diatom bloom (Straile, 2002; Huber et al., 2008; Frenken et al., 2016). Therefore, we believe that water temperature and silicate all play important roles in limiting diatom growth in Jinshahe Reservoir, and they act in turn in different time phases.
5 CONCLUSIONThe six diatom species F. nanana, A. catenatum, A. ambigua, U. ulna, C. meneghiniana and A. formosa usually dominate in subtropical drinking water reservoirs in different seasons. Water temperature and silicate were the main environmental factors that drive this seasonal shift. These two main driving factors varied seasonally, further influencing the structure and seasonal succession of dominant diatom communities. Diatoms grow well only when the two controlling factors simultaneously satisfy the growth conditions; as limiting factors, the two factors played their respective limiting roles in turn on temporal scales.
The water quality of drinking water resource is directly related to human health, and its protection should be highly valued. It is less likely to occur cyanobacterial blooms in oligotrophic reservoirs as in eutrophic lakes, but diatoms blooms are reported frequently in recent years in drinking water resource. Therefore, it is important to make out the appropriate growth conditions of the bloom-forming diatom species, aiming at providing scientific basis for the early warning of blooms and reservoir management.
6 DATA AVAILABILITY STATEMENTThe datasets analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGEMENTWe would like to thank the Jinshahe Reservoir Management Office for its cooperation in sampling. We are grateful to ZHANG Fengge, LI Lu, ZHOU Weicheng, GAO Xiang and LI Shuangshuang for their assistance during the field survey.
Electronic supplementary materialSupplementary material (Appendix Figs.S1-S2) is available in the online version of this article at https://doi.org/10.1007/s00343-019-8040-1.
Arar E J. 1997. Method 446.0: In vitro Determination of Chlorophylls a, b, c1+c2 and Pheopigments in Marine and Freshwater Algae by Visible Spectrophotometry. EPA, Cincinnati, Ohio.
|
Baier J, Lücke A, Negendank J F W, Schleser G H, Zolitschka B. 2004. Diatom and geochemical evidence of mid-to late Holocene climatic changes at Lake Holzmaar, West-Eifel(Germany). Quaternary International, 113(1): 81-96.
DOI:10.1016/S1040-6182(03)00081-8 |
Bertrand C, Fayolle S, Franquet E, Cazaubon A. 2003. Responses of the planktonic diatom Asterionella formosa Hassall to abiotic environmental factors in a reservoir complex (south-eastern France). Hydrobiologia, 501(1-3): 45-58.
|
Cao X F, Wang J, Liao J Q, Sun J H, Huang Y. 2016. The threshold responses of phytoplankton community to nutrient gradient in a shallow eutrophic Chinese lake. Ecological Indicators, 61: 258-267.
DOI:10.1016/j.ecolind.2015.09.025 |
Chaffin J D, Mishra S, Kuhaneck R M, Heckathorn S A, Bridgeman T B. 2012. Environmental controls on growth and lipid content for the freshwater diatom, Fragilaria capucina: a candidate for biofuel production. Journal of Applied Phycology, 24(5): 1045-1051.
DOI:10.1007/s10811-011-9732-x |
Cullen J J, MacIntyre H L. 2016. On the use of the serial dilution culture method to enumerate viable phytoplankton in natural communities of plankton subjected to ballast water treatment. Journal of Applied Phycology, 28(1): 279-298.
DOI:10.1007/s10811-015-0601-x |
Downing J A, Cherrier C T, Fulweiler R W. 2016. Low ratios of silica to dissolved nitrogen supplied to rivers arise from agriculture not reservoirs. Ecology Letters, 19(12): 1414-1418.
DOI:10.1111/ele.2016.19.issue-12 |
Dzialowski A R, Wang S H, Lim N C, Spotts W W, Huggins D G. 2005. Nutrient limitation of phytoplankton growth in central plains reservoirs, USA. Journal of Plankton Research, 27(6): 587-595.
DOI:10.1093/plankt/fbi034 |
Eliasen S K, Hátún H, Larsen K M H, Jacobsen S. 2017. Faroe shelf bloom phenology-the importance of ocean-toshelf silicate fluxes. Continental Shelf Research, 143: 43-53.
DOI:10.1016/j.csr.2017.06.004 |
Frenken T, Velthuis M, de Senerpont Domis L N, Stephan S, Aben R, Kosten S, van Donk E, van de Waal D B. 2016. Warming accelerates termination of a phytoplankton spring bloom by fungal parasites. Global Change Biology, 22(1): 299-309.
DOI:10.1111/gcb.13095 |
Grover J P, Chrzanowski T H. 2006. Seasonal dynamics of phytoplankton in two warm temperate reservoirs: association of taxonomic composition with temperature. Journal of Plankton Research, 28(1): 1-17.
DOI:10.1093/plankt/fbi095 |
Han B P. 2010. Reservoir ecology and limnology in China: a retrospective comment. Journal of Lake Sciences, 22(2): 151-160.
(in Chinese with English abstract) |
Hastie T J, Tibshirani R J. 1990. Generalized Additive Models. Chapman and Hall, London.
|
Hecky R E, Kilham P. 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnology and Oceanography, 33(4part 2): 796-822.
|
Hubble D S, Harper D M. 2002. Phytoplankton community structure and succession in the water column of Lake Naivasha, Kenya: a shallow tropical lake. Hydrobiologia, 488(1-3): 89-98.
|
Huber V, Adrian R, Gerten D. 2008. Phytoplankton response to climate warming modified by trophic state. Limnology and Oceanography, 53(1): 1-13.
DOI:10.4319/lo.2008.53.1.0001 |
Julius M L, Theriot E C. 2010.The diatoms: a primer. In: Smol J P, Stoermer E F eds. The Diatoms: Applications for the Environmental and Earth Sciences. 2nd edn. Cambridge University Press, Cambridge. p.8-22.
|
Kocer M A T, Şen B. 2012. The seasonal succession of diatoms in phytoplankton of a soda lake (Lake Hazar, Turkey). Turkish Journal of Botany, 36(6): 738-746.
|
Koester J, Brownlee C B, Taylor A R. 2016. Algal calcification and silicification. In: Encyclopedia of Life Sciences. John Wiley & Sons, Ltd. Chichester.
|
Kolmakov V I, Gaevskii N A, Ivanova E A, Dubovskaya O P, Gribovskaya I V, Kravchuk E S. 2002. Comparative analysis of ecophysiological characteristics of Stephanodiscus hantzschii Grun. in the periods of its bloom in recreational water bodies. Russian Journal of Ecology, 33(2): 97-103.
DOI:10.1023/A:1014448707663 |
Lü G J, Xiong B X, Chen P. 2012. Community structure and diversity of phytoplankton of four reservoirs in middle China. Journal of Fishery Sciences of China, 19(1): 690-699.
(in Chinese with English abstract) |
Lv H, Yang J, Liu L M, Yu X Q, Yu Z, Chiang P. 2014. Temperature and nutrients are significant drivers of seasonal shift in phytoplankton community from a drinking water reservoir, subtropical China. Environmental Science and Pollution Research, 21(9): 5917-5928.
DOI:10.1007/s11356-014-2534-3 |
Ma P M, Shi L D, Zhang J F, Hu J X, Zhao X F. 2016. Succession of phytoplankton assemblages and its influencing factors in Tangpu Reservoir, Zhejiang Province. Environmental Science, 37(12): 4560-4569.
(in Chinese with English abstract) |
Maraslioglu F, Soylu E N, Aksoy A. 2016. Seasonal succession of the phytoplankton community and evaluation of water quality using trophic diatom index in a stream. Oxidation Communications, 39(1-Ⅱ): 459-465.
|
Morin S, Vivas-Nogues M, Duong T T, Thi T, Boudou A, Coste M, Delmas F. 2007. Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (RiouMort, France). Fundamental and Applied Limnology/Archiv für Hydrobiologie, 168(2): 179-187.
DOI:10.1127/1863-9135/2007/0168-0179 |
Parsons T R, Takahashi M, Hargrave B. 1984. Biological Oceanographic Processes. 3rd edn. Pergamon Press, New York. 131p.
|
Peter K H, Sommer U. 2012. Phytoplankton cell size: intraand interspecific effects of warming and grazing. PLoS One, 7(11): e49632.
DOI:10.1371/journal.pone.0049632 |
Polovina J J, Woodworth P A. 2012. Declines in phytoplankton cell size in the subtropical oceans estimated from satellite remotely-sensed temperature and chlorophyll, 1998-2007. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 77-80: 82-88.
DOI:10.1016/j.dsr2.2012.04.006 |
Rakocevic-Nedovic J, Hollert H. 2005. Phytoplankton community and chlorophyll a as trophic state indices of Lake Skadar (Montenegro, Balkan) (7 pp). Environmental Science and Pollution Research, 12(3): 146-152.
DOI:10.1065/espr2005.04.241 |
Reynolds C, Dokulil M, Padisak J. 2000. Understanding the assembly of phytoplankton in relation to the trophic spectrum: where are we now?. Hydrobiologia, 424(1-3): 141-152.
|
Saros J E, Anderson N J. 2015. The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biological Reviews, 90(2): 522-541.
DOI:10.1111/brv.2015.90.issue-2 |
Shi L D, Zhu W J, Zhang J F, Chen H, Liang L, Ma P M. 2013. Seasonal succession of phytoplankton community structures and analysis of spring water bloom in subtropical reservoir-as Tangpu Resevior in Zhejiang province. Journal of Hydroecology, 34(2): 32-39.
(in Chinese with English abstract) |
Shi P L, Shen H, Wang W J, Chen W J, Xie P. 2015. The relationship between light intensity and nutrient uptake kinetics in six freshwater diatoms. Journal of Environmental Sciences, 34: 28-36.
DOI:10.1016/j.jes.2015.03.003 |
Smol J P, Douglas M S. 2007. From controversy to consensus: making the case for recent climate change in the Arctic using lake sediments. Frontiers in Ecology and the Environment, 5(9): 466-474.
DOI:10.1890/060162 |
Snitko L V, Rogozin A G, Timoshkin O A. 2015. Thermoindicator properties of phytoplankton species (by the example of waterbodies of the Southern Urals). Inland Water Biology, 8(2): 147-156.
DOI:10.1134/S1995082915020121 |
Sommer U, Gliwicz Z M, Lampert W, Duncan A. 1986. The PEG-model of seasonal succession of planktonic events in fresh waters. Archiv für Hydrobiologie, 106(4): 433-471.
|
Sonmez F. 2011. The seasonal variations of planktonic and epilithic diatoms in Kalecik reservoir (Elazig, Turkey). Journal of Animal and Veterinary Advances, 10(24): 3231-3235.
|
Stockner J G, Benson W W. 1967. The succession of diatom assemblages in the recent sediments of lake Washington. Limnology and Oceanography, 12(3): 513-532.
DOI:10.4319/lo.1967.12.3.0513 |
Straile D. 2002. North Atlantic Oscillation synchronizes foodweb interactions in central European lakes. Proceedings of the Royal Society B: Biological Sciences, 269(1489): 391-395.
DOI:10.1098/rspb.2001.1907 |
Tian C, Lu X T, Pei H Y, Hu W R, Xie J. 2013. Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environmental Monitoring and Assessment, 185(3): 2627-2645.
DOI:10.1007/s10661-012-2736-4 |
Tolotti M, Thies H, Nickus U, Psenner R. 2012. Temperature modulated effects of nutrients on phytoplankton changes in a mountain lake. Hydrobiologia, 698(1): 61-75.
DOI:10.1007/s10750-012-1146-5 |
Wei J L, Cao P F, Hu W R, Xie J, Pei H Y, Ren Y, Feng Y W. 2015. Seasonal succession of planktonic diatom community and its relationship with environmental factors in Nansi Lake. Research of Environmental Sciences, 28(8): 1209-1218.
(in Chinese with English abstract) |
Winder M, Schindler D E. 2004. Climatic effects on the phenology of lake processes. Global Change Biology, 10(11): 1844-1856.
DOI:10.1111/gcb.2004.10.issue-11 |
Wohlers-Zöllner J, Biermann A, Engel A, Dörge P, Lewandowska A M, von Scheibner M, Riebesell U. 2012. Effects of rising temperature on pelagic biogeochemistry in mesocosm systems: a comparative analysis of the AQUASHIFT Kiel experiments. Marine Biology, 159(11): 2503-2518.
DOI:10.1007/s00227-012-1958-x |
Xiu P, Chai F. 2012. Spatial and temporal variability in phytoplankton carbon, chlorophyll, and nitrogen in the North Pacific. Journal of Geophysical Research: Oceans, 117(C11).
|
Yang D F, Wang F, Gao Z H, Cui W L, Huo S X. 2004. Ecological phenomena of phytoplankton in Jiaozhou Bay. Marine Sciences, 28(6): 71-74.
(in Chinese with English abstract) |
Yang J, Yu X Q, Liu L M, Zhang W J, Guo P Y. 2012. Algae community and trophic state of subtropical reservoirs in southeast Fujian, China. Environmental Science and Pollution Research, 19(5): 1432-1442.
DOI:10.1007/s11356-011-0683-1 |
Yao M, Li Y L, Yang X D, Liu Q. 2011. Three-year changes in planktonic diatom communities in a eutrophic lake in Nanjing, Jiangsu province, China. Journal of Freshwater Ecology, 26(1): 133-141.
|
Yun M S, Lee S H, Chung I K. 2010. Photosynthetic activity of benthic diatoms in response to different temperatures. Journal of Applied Phycology, 22(5): 559-562.
DOI:10.1007/s10811-009-9493-y |
Zhang Y, Ma X F, Guo F F, Li J Z, Xiong B X. 2015. Community structures of phytoplankton and their relationships with environmental factors in the Jinshahe Reservoir, Hubei Province. Journal of Lake Sciences, 27(5): 902-910.
(in Chinese with English abstract) DOI:10.18307/2015.0517 |
Zhu G W, Jin Y W, Ren J, Xia M F, Xu H, Zhu M Y, Fei G S, Chen W M. 2016. Characteristics of diatom blooms in a reservoir-water supply area and the countermeasures in Taihu basin, China. Journal of Lake Sciences, 28(1): 9-21.
(in Chinese with English abstract) DOI:10.18307/2016.0102 |
Znachor P, Visocká V, Nedoma J, Rychtecký P. 2013. Spatial heterogeneity of diatom silicification and growth in a eutrophic reservoir. Freshwater Biology, 58(9): 1889-1902.
DOI:10.1111/fwb.2013.58.issue-9 |
Zuur A F, Ieno E N, Walker N J, Saveliev A A, Smith G M. 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York. 574p.
|
 2019, Vol. 37
2019, Vol. 37


