Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Huixian, SHEN Chen, WANG Qiong, Richard B. ARONSON, CHEN Chen, XUE Junzeng
- Survivorship characteristics and adaptive mechanisms of phytoplankton assemblages in ballast water
- Journal of Oceanology and Limnology, 37(2): 580-588
- http://dx.doi.org/10.1007/s00343-019-7288-9
Article History
- Received Oct. 19, 2017
- accepted in principle Jan. 1, 2018
- accepted for publication May. 10, 2018
2 Ballast Water Detecting Lab, Shanghai Ocean University, Shanghai 201306, China;
3 Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901, USA
Marine shipping transports more than 90% of the cargo of the world's overseas trade (IMO, 2008). More than 92 000 vessels transit the world's oceans and other waterways. Ballast water is pumped in to maintain safe operating conditions throughout a voyage; however, it may pose serious ecological, economic and health problems due to the multitude of marine species carried in ships' ballast water. These include bacteria, microbes, small invertebrates, eggs, cysts and larvae of various species (IMO, 2004). The transfer of biota via ship's ballast water is a major vector for the introduction of non-indigenous aquatic species (Medcof, 1975; Carlton, 1985; Hallegraeff and Bolch, 1991; Pam et al., 2013). There are 4 500 species carried in the ballast water each day worldwide. So far about 500 species have been identified as being carried by ballast water (Carlton and Geller, 1993; Satir, 2008). Only a small proportion of species—about 11%—can be survive in the new environment, but when organisms successfully invade they can have significant, detrimental impacts on ecosystems, human health and economic well-being (Ruiz et al., 1997, 2000; Wang et al., 2013).
Approximately 12 billion tons of ballast water are carried by ships worldwide each year, with an average of 110 million plankton per cubic meter of ballast water (Mackenzie, 1999; Ruiz et al., 2000). Even though phytoplankton are small (0.7–200 μm) and ubiquitous, they can propagate rapidly and are adaptable to hostile environments. Although inimical conditions in ballast water tanks, such as darkness, physical collision with the ballast pump, the presence of harmful substances, and predation, may cause a decline in the abundance and diversity of phytoplankton (Carlton, 1985; Dickman and Zhang 1999; Gollasch et al., 2000; Klein et al., 2010), they are among the most abundant and diverse taxa successfully transported in ballast water (McCarthy and Crowder, 2000; Klein et al., 2010).
The abundance and diversity of phytoplankton in ballast water have been investigated previously (Gollasch et al., 2000; Burkholder et al., 2007; Sarinas et al., 2014). An incubation experiment over 20 d by Liebich et al. (2012), demonstrated regrowth of certain diatom taxa after treatment of the ballast water with UV radiation: Thalassiosira, Skeletonema, Chaetoceros, Pseudo-nitzschia, and Nitzschia. The results indicate that Bacillariophyta, Dinoflagellata, Chlorophyta and Cyanophyta, may cause harmful algal blooms. These are the main viable phytoplankton in ballast water (Carlton and Geller, 1993; Ruiz et al., 1997, 2000; Olenin, 2000; Burkholder, 2007; David et al., 2007; Steichen et al., 2015). The efficacy of ballast water treatment systems (Stan et al., 2013; Feng et al., 2014; Satir, 2014; Stehouwer et al., 2015) and risk assessments (Casas-Monroy et al., 2015; David et al., 2015) have received considerable scientific attention, and although the Bacillariophyta and Dinoflagellata in ballast water have been studied, the survival mechanisms of phytoplankton in the face of the stressful conditions in ballast water remain unclear (Klein et al., 2010). Casas-Monroy et al. (2016) presented the first estimation of propagule pressure for viable (i.e., alive and able to reproduce), nonindigenous dinoflagellates in ballast-water of commercial ships arriving at Pacific and Atlantic ports in Canada. The survival mechanisms of other species of phytoplankton need further study.
On September 8, 2017, the International Convention for the Management and Control of Ship Ballast Water and Sediment came into force. According to the Convention, vessels must be equipped with a ballastwater management system to treat ballast water to meet the discharge standards of the port. The State has the right to supervise and inspect the discharge of ballast water by arriving ships (IMO, 2004).
Shanghai Yangshan Deep-Water Port is a port for container ships in Hangzhou Bay, with berths up to 15 m deep that can handle today's largest container ships. In 2015, the port handled 36.54 million TEU (IMI, 2018). Since it began operations on December 10, 2005, Shanghai Yangshan Deep-Water Port has witnessed a continuous increase in container throughput each year. More than half the ships in Shanghai port discharge ballast water. The Fourth Phase Project of Shanghai Yangshan Deep-Water Port began at the end of 2014 and completed in 2017. With the increase in foreign ship-traffic expected to result from the Fourth Phase of construction, the discharge of ballast water and other human activities will increasingly influence the coastal ecosystems in and around the Port.
As an offshore, narrow, deep-water port, the Yangshan Port is only weakly influenced by the tides, and the marine environment is relatively stable. Benefiting from its special geographical location, exotic organisms are able to survive, causing harm to native species. Studies of plankton in the ballast water of vessels arriving at the Port are urgently needed (Wang, 2014). Xue et al. (2011) investigated the zooplankton in the ballast water of the Yangshan Port. They identified exotic zooplankton species in the ballast water of 47.4% of the vessels, of which 44% replaced their ballast water in coastal ports. After being discharged into the Port, the zooplankton in the ballast water samples had a high survival rate. The zooplankton in the ballast water of vessels arriving at the Port influenced the local environment and were potential invaders. Until now, however, there has been no systematic study of phytoplankton in the ballast water discharged at the port. This paper summarizes the common features and adaptive mechanism of surviving phytoplankton, as well as the impacts on the ballast water phytoplankton assemblages of different water ages and sources. Such data can be used to formulate policies to regulate ballast water discharge and the introduction of exotic species.
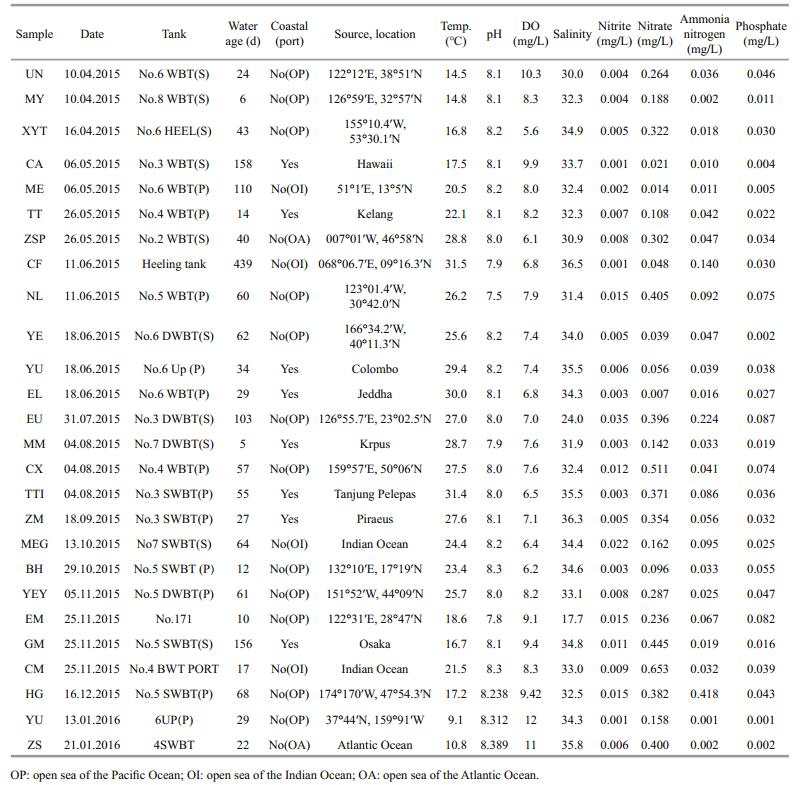
2 MATERIAL AND METHOD 2.1 Ballast water samplingFrom April 2015 to January 2016, ballast water samples were collected from 26 vessels in Yangshan Port. Information on the ballast water sampled is presented in Table 1. Eighteen vessels performed ballast water exchange on the high seas: 66.7% in the Pacific Ocean, 22.2% in the Indian Ocean, and 11.1% in the Atlantic Ocean. Eight vessels were ballasted from coastal waters.
|
Water samples were collected through the manholes of the ballast tanks. Water-quality parameters, including pH, dissolved oxygen (DO), salinity and temperature were measured by a portable multiparameter water analyzer (WTW-Multi 3430) device in situ immediately after sampling. Nutrient indexes, including nitrite, nitrate, ammonia nitrogen and phosphate were determined in the laboratory according to China's national standards: The Specification for Marine Monitoring Part 4: Seawater Analysis (GB 17378.4-2007) (2007a).
2.3 Species identificationFor phytoplankton, 10-L samples were collected directly from the manhole using a sample-collection bucket. The entire volume of water was filtered through a 10-μm plankton net. Organisms retained on the net were washed three or more times with ballast water filtered by plankton net. The organisms were concentrated to give a final volume of 50 mL in a 60-mL transparent polycarbonate bottle. All samples of phytoplankton were stored immediately after sampling at 4℃. These samples were transferred to the laboratory for analysis within 2 h.
We performed two operations for each sample. First, after the sample was transferred to the laboratory, the number of living organisms was counted using the fluorescence method. Subsamples of 1 mL from the concentrated sample were placed in microfuge tubes and vital-stained with 5 μL of 1-mmol/L FDA and 10 μL of 250-μmol/L CMFDA, for final concentrations of 5 and 2.5 μmol/L, respectively. Stained subsamples were incubated in the dark at room temperature for 10 min and then loaded onto 1-mL Sedgewick-Rafter counting chambers. Numbers of viable and nonviable cells were counted at 100× magnification using compound epifluorescence microscopes with standard blue-light excitation and green-band-pass emission filter cubes. To maintain consistency among sites and samples, chambers were only analyzed 20 min after incubation, regardless of whether or not background fluorescence was seen at that time. The numbers of moving and living cells with fluorescence were recorded as live cells.
Species were identified after the sample was fixed in Lugol's solution. Nine subsamples of 0.1 mL were analyzed under 100× magnification magnification using a compound microscope. Micro-diatoms were observed and identified by transmission electron microscopy (TEM). Phytoplankton were identifieds primarily according to Jin et al.(1965, 1982, 1991), Yang and Dong (2006), Guo and Qian (2003), and Round et al. (1990). Freshwater species were identified using Hu and Wei (2006).These protocols are in compliance with China's national standards: The Specification for Marine Monitoring Part 7: Ecological Survey for Offshore Pollution and Biological Monitoring (GB 17378.7-2007) (2007b).
2.4 StatisticsThe data were analyzed using SigmaStat (v3.5 SPSS). Graphical analysis was performed using Graph Pad Prism 5 mapping software.
3 RESULT 3.1 Abiotic conditionTwenty-six container ships were sampled. The temperatures of the ballast water samples from tanks varied according to sampling season from 9.1℃ (YU) in winter to 31.5℃ (CF) in summer (Table 1). Salinity ranged between 17.5 and 36.5, whilst the ranges of pH and dissolved-oxygen (DO) were 7.5–8.4 units and 5.6–12.0 mg/L, respectively. Nitrite, nitrate, ammonia nitrogen and phosphate were analyzed. Nitrite ranged from 0.001 to 0.035 mg/L, with a mean value of 0.008 04±0.000 6 SD mg/L. Nitrate varied between 0.007 and 0.653 mg/L, with a mean value of 0.244 88±0.030 16 mg/L. Ammonia-nitrogen ranged from 0.001 to 0.418 mg/L (mean 0.062 77± 0.007 61 mg/L) and phosphate ranged from 0.01 to 0.087 mg/L (mean 0.033 92±0.000 62 mg/L).
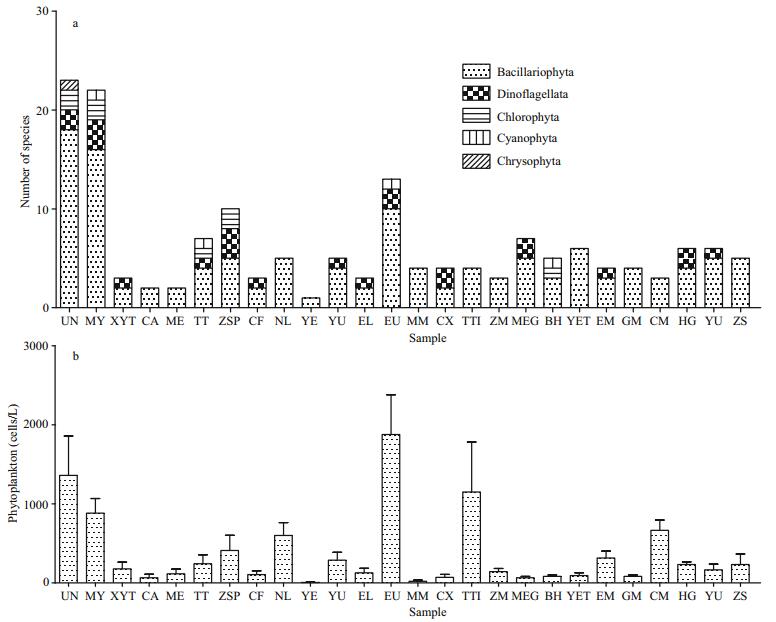
3.2 Phytoplankton species composition and community structureIn total 84 species of phytoplankton were identified, belonging to 43 genera and 5 phyla. Phytoplankton species were found in all the ballast water samples. Species richness ranged between 1 (sample YE; water age 62 d) and 23 (sample UN; water age 24 d). Bacillariophyta, which included 30 genera and 63 species, were the dominant algae in the ballast water, accounting for 75.0% of the phytoplankton diversity (Appendix 1). Dinoflagellata, which included 5 genera and 11 species, accounted for 13.0% of species richness. Chlorophyta (5 genera and 5 species) and Cyanophyta (3 genera and 3 species) accounted for 5.9% and 3.5% of the total number of species, respectively. Chrysophyta (Isochrysis sp.) were only found in UN, which ballasted from the open Pacific. The range of phytoplankton densities was (5.55±9.62) to (1.878±0.872)×103 cells/L (mean 410.1 cells/L; Fig. 1).
|
| Fig.1 Composition of phytoplankton (a) and density of phytoplankton (b) in each ballast water sample |
Potentially harmful phytoplankton taxa were wellrepresented: Ceratium furca, Ce. marcroceros, Leptocylindrus danicus, Coscinodiscus radiatus, Co. granii, Prorocentrum micans, Melosira sulcata, Meuniera membranacea, and Skeletonema costatum were detected in water from the ballast tanks (Appendix 1). Skeletonema costatum was observed in UN, ZM, CM, HG, ZS, with densities ranging between 83 and 1 617 cells/L. Species causing harmful algal blooms (HABs) were mostly diatoms and were detected primarily in samples from the Western European route samples (MY). Most of these HAB-causing algae are adaptable species.
3.4 Abiotic control of phytoplankton densityThe density of phytoplankton was negatively correlated with the salinity of the ballast water (Pearson correlation: R=-0.41, P=0.037). Phytoplankton was positively correlated with nitrite and phosphate (R=0.444, P=0.023 and R=0.429, P=0.029, respectively). Species richness of phytoplankton was negatively correlated with temperature (R=-0.44, P=0.037).
3.4.1 Water ageWe detected no significant statistical relationships between water age and phytoplankton; however, the number of phytoplankton decreased with water age. Two species of Bacillariophyta and one species of Dinoflagellata were found in sample CF, which was 439-d old. Chlorophyta and Cyanophyta were detected in samples of ages 0–40 and 0–103 d, respectively. In Sample UN, which was 24-d old, Isochrysis sp. (Chrysophyta) was detected, although it was not detected in any of the other samples.
3.4.2 Ballast water sourceThe composition of phytoplankton from the samples of different ballast sources were compared, and both Bacillariophyta and Dinoflagellata were detected in the samples of the Pacific Ocean, the Indian Ocean, the Atlantic Ocean and the coastal water sources. The species-richness values of ballast water sourced in the Indian Ocean and ports (coastal) were lower than in the samples from the Pacific Ocean and the Atlantic Ocean. Only Bacillariophyta and Dinoflagellata were detected in the samples from the Indian Ocean. Cyanophyta were found only in the samples from the Pacific Ocean and the coastal water. Chrysophyta were detected only in the samples from the Pacific Ocean (Fig. 1). The density and species richness of phytoplankton in the ballast water samples from the Pacific Ocean were higher than those from other ballast-water sources (Fig. 2).

|
| Fig.2 Phytoplankton densities from different water sources Error bars indicate standard errors. |
The composition and abundance of phytoplankton in ballast water is affected by the age of the water, the type of ship, the source of the ballast water, the season, and the management system applied. Choosing the correct management system requires determining a priori which plankton are living in the ballast water in order to prevent undesirable releases. It is, therefore, necessary to have a full understanding of the attributes and survival of phytoplankton in the ballast water; such information can streamline the assessment process and reduce the cost of ballast-water management for each vessel.
The ballast tank is a closed, dark environment; therefore, the age of the water, the lack of natural light and nutrient levels all potentially affect the survival of phytoplankton in ballast water (Gollasch et al., 2000; Liu et al., 2011). Some phytoplankton can survive post-voyage in the harsh environment of the ballast tank in the absence of human disturbance. Bacillariophyta, the outer cell walls of which consist of silica, are adaptable to the harsh environment of the tank and form resting spores. The spores survive well in the ballast water after long voyages (Sicko-Goad et al., 1989; McQuoid and Hobson, 1996). Bacillariophyta is the most common taxon in ballast water, and they can be cultured in one-year-old ballast water (Chu et al., 1997). Most of the phytoplankton from the open-ocean water samples were cosmopolitan and euryhaline. Water temperatures in Yangshan Port varied from 11.0℃ to 27.10℃, whilst the salinity ranged between 17.4 and 21.1 (Xue et al., 2016). Samples EU and EM, ballasted from the Pacific Ocean, had water conditions similar to the water from Yangshan Port.
Potentially harmful phytoplankton taxa were wellrepresented: Ceratium furca, Ce. marcroceros, Leptocylindrus danicus, Coscinodiscus radiatus, Co. granii, Prorocentrum micans, Melosira sulcata, Meuniera membranacea, Skeletonema costatum were detected in water from the ballast tanks (Appendix 1). These species can form HABs (Jin et al., 1991). HABs caused by S. costatum occur frequently in China, including near Yangshan Port (Wang et al., 2013). Thus, ballast water arriving at the Yangshan Port has the potential to generate HABs in Shanghai and surrounding waters. Most HAB-forming algae species were detected in samples from the Western European route samples, and most of them were diatoms. Most of these HABs-causing algae are also adaptable species. Discharge of these species threatens the ecosystems at the destination-ports (Xue et al., 2011). There are 16 species of HAB-organisms known in Chinese seas that have been introduced through ballast water, including Chaetoceros concavicornis, Cyclindrotheca closterium, and Melosiar cancellate (Liu, 2005).
In addition to the ecological threat, alien HABorganisms can have an impact on fishery production and human health in China. Therefore, rapid and reliable methods are urgently required to accurately detect and monitor the discharge of harmful algae. PCR-based methods have proven reliable for the identifying single species of harmful algae, particularly microalgae (Handy et al., 2008; Yuan et al., 2012; Antonella and Luca, 2013). Several PCRbased DNA microarrays have been developed to detect harmful microalgae (Galluzzi et al., 2011; McCoy et al., 2013). Chen et al. (2016) recently developed a multiplex PCR-based DNA microarray method capable of detecting harmful algal species. Future work should combine more accurate classification and identification methods to study harmful algae in ballast water.
No ballast-water sediment was sampled in this study. In a harsh environment of the ballast tank, harmful dinoflagellate species may survive in the sediments as resting spores (John et al., 2000; Hamer et al., 2001; Fahnenstiel et al., 2009), and study has been successful germination of spores at rest stages under laboratory conditions (Casas-Monroy et al., 2011). As can be seen from these studies, environmental conditions, including temperature, salinity, nutrient concentrations, and abrupt changes in light are the major causes of formation of resting stages in most diatom species of diatoms. Sediments should be sampled in future studies to explore more fully how phytoplankton survive in ballast tanks.
5 CONCLUSIONPhytoplankton diversity and abundance were determined in ballast-water from 26 vessels in the Yangshan Port. A total of 84 species of phytoplankton were detected, among which diatom species were abundant, accounting for 75.0% of the total number of identifications, including 30 genera and 63 species. Of these, 6 species of harmful phytoplankton were detected: Leptocylindrus danicus, Coscinodiscus radiatus, Co. granii, Melosira sulcata, Meuniera membranacea and Skeletonema costatum, of which Meuniera membranacea has a density of up to 983 cells/L. In addition, there are three harmful algae that are dinoflagellates. Ballast water arriving at the Yangshan Port has the potential to generate HABs in Shanghai and surrounding waters. Most HABforming algae species were detected in samples from the Western European route samples, and most of them were diatoms. Most of these HABs-causing algae are also adaptable species. And the speciesrichness values of ballast water sourced in the Indian Ocean and ports (coastal) were lower than in the samples from the Pacific Ocean and the Atlantic Ocean. Therefore, the investigation and analysis of the phytoplankton in ballast water aims to provide suggestions, as well as statistical and theoretical support, for future research, in order to enhance the efficacy of inspections of vessels arriving at the Yangshan Port and other ports.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGEMENTWe are very grateful to the Shanghai Entry-Exit Inspection and Quarantine Bureau, as well as to the officers and crew of all the vessels that were boarded and sampled. The comments and suggestions of the reviewers are greatly appreciated.
Electronic supplementary materialSupplementary material (Appendix 1) is available in the online version of this article at https://doi.org/10.1007/s00343-019-7288-9.
Antonella P, Luca G. 2013. The quantitative real-time PCR applications in the monitoring of marine harmful algal bloom (HAB) species. Environmental Science and Pollution Research, 20(10): 6851-6862.
DOI:10.1007/s11356-012-1377-z |
Burkholder J M, Hallegraeff G M, Melia G, Cohen A, Bowers H A, Oldach D W, Parrow M W, Sullivan M J, Zimba P V, Allen E H, Kinder C A, Mallin M A. 2007. Phytoplankton and bacterial assemblages in ballast water of U.S. military ships as a function of port of origin, voyage time, and ocean exchange practices. Harmful Algae, 6(4): 486-518.
DOI:10.1016/j.hal.2006.11.006 |
Carlton J T, Geller J B. 1993. Ecological roulette: the global transport of nonindigenous marine organisms. Science, 261(5117): 78-82.
DOI:10.1126/science.261.5117.78 |
Carlton J T. 1985. Transoceanic and interoceanic dispersal of coastal marine organisms: the biology of ballast water. Oceanography and Marine Biology, 23(4): 313-371.
|
Casas-Monroy O, Linley R D, Adams J K, Chan F T, Drake D A R, Bailey S A. 2015. Relative invasion risk for plankton across marine and freshwater systems: examining efficacy of proposed international ballast water discharge standards. PLoS One, 10(3): 6851-6862.
|
Casas-Monroy O, Parenteau M, Drake D A R, Roy S, Rochon A. 2016. Absolute estimates of the propagule pressure of viable dinofagellates across Canadian coasts: the variable infuence of ballast water exchange. Marine Biology, 163: 174.
DOI:10.1007/s00227-016-2946-3 |
Casas-Monroy O, Roy S, Andre R. 2011. Ballast sedimentmediated transport of non-indigenous species of dinoflagellates on the East Coast of Canada. Aquatic Invasions, 6(3): 231-248.
DOI:10.3391/ai |
Chen X F, Zhou Q J, Duan W J, Zhou C X, Duan L J, Zhang H L, Sun A L, Yan X J, Chen J. 2016. Development and evaluation of a DNA microarray assay for the simultaneous detection of nine harmful algal species in ship ballast and seaport waters. Chinese Journal of Oceanology and Limnology, 34(1): 86-101.
DOI:10.1007/s00343-015-4265-9 |
Chu K H, Tam P F, Fung C H, Chen Q C. 1997. A biological survey of ballast water in container ships entering Hong Kong. Hydrobiologia, 352(1-3): 201-206.
|
David M, Gollasch S, Cabrini M, Perkovič M, Bošnjak D, Virgilio D. 2007. Results from the first ballast water sampling study in the Mediterranean Sea—the Port of Koper study. Marine Pollution Bulletin, 54(1): 53-65.
DOI:10.1016/j.marpolbul.2006.08.041 |
David M, Gollasch S, Leppäkoski E, Hewitt C. 2015. Risk assessment in ballast water management. In: David M, Gollasch S eds. Global Maritime Transport and Ballast water Management. Springer, Netherland. p.133-169. https://link.springer.com/chapter/10.1007%2F978-94-017-9367-4_7
|
Dickman M, Zhang F Z. 1999. Mid-ocean exchange of container vessel ballast water. 2: effects of vessel type in the transport of diatoms and dinoflagellates from Manzanillo, Mexico, to Hong Kong, China. Marine Ecology Progress, 176: 253-262.
DOI:10.3354/meps176253 |
Fahnenstiel G, Hong Y, Millie D, Doblin M A, Johengen T, Reid D. 2009. Marine dinoflagellate cysts in the ballast tank sediments of ships entering the Laurentian Great Lakes. Transport Engineering in Australia, 30(6): 353-355.
|
Feng D L, Xu S H, Liu G. 2015. Application of immobilized TiO 2 photocatalysis to improve the inactivation of heterosigma akashiwo in ballast water by intense pulsed light. Chemosphere, 125: 102-107.
DOI:10.1016/j.chemosphere.2014.11.060 |
Galluzzi L, Cegna A, Casabianca S, Penna A, Saunders N, Magnani M. 2011. Development of an oligonucleotide microarray for the detection and monitoring of marine dinoflagellates. Journal of Microbiological Methods, 84(2): 234-242.
DOI:10.1016/j.mimet.2010.11.024 |
Gollasch S, Lenz J, Dammer M, Andres H G. 2000. Survival of tropical ballast water organisms during a cruise from the Indian Ocean to the North Sea. Journal of Plankton Research, 22(5): 923-937.
DOI:10.1093/plankt/22.5.923 |
Guo Y G, Qian S D. 2003. China Seaweed. Science Press, Beijing, China.
(in Chinese)
|
Hallegraeff G M, Bolch D J. 1991. Transport of toxic dinoflagellate cysts via ships' ballast water. Marine Pollution Bulletin, 22(1): 27-30.
DOI:10.1016/0025-326X(91)90441-T |
Hamer J P, Lucas I A N, McCollln T A. 2001. Harmful dinoflagellate resting cysts in ships' ballast tank sediments: potential for introduction into English and Welsh waters. Phycologia, 40(3): 246-255.
DOI:10.2216/i0031-8884-40-3-246.1 |
Handy S M, Demir E, Hutchins D A, Portune K J, Whereat E B, Hare C E, Rose J M, Warner M, Farestad M, Cary S C, Coyne K J. 2008. Using quantitative real-time PCR to study competition and community dynamics among Delaware Inland Bays harmful algae in field and laboratory studies. Harmful Algae, 7(5): 599-613.
DOI:10.1016/j.hal.2007.12.018 |
Hu H J, Wei Y X. 2006. The Freshwater Algae of China-Systematics, Taxonomy and Ecology. Science Press, Beijing, China.
(in Chinese)
|
IMI (International Maritime Information Website). 2018. Shanghai port's news. APL launches premium Shanghai-LA service to help airfreight shippers. 2018-03-0708: 33: 58 View: 39, http://www.simic.net.cn/news_list.php?lan=en&id=368&flag=cnports&pname=shanghai.
|
IMO (International Maritime Organization). 2004. International Convention for the Controland Management of Ships' Ballast Water and Sediments. London, England: International Maritime Organization.
|
IMO. 2008. IMO. http://www.imo.org/en/About/Pages/Default.aspx. Accessed on 2008-04-24.
|
Jin D X, Chen J H, Huang K G. 1965. Chinese Marine Planktonic Diatoms. Shanghai Science and Technology Press, Shanghai, China.
(in Chinese)
|
Jin D X, Cheng Z, Lin J. 1982. China Ocean Bottom Handle Diatoms. Ocean Press, Beijing, China.
(in Chinese)
|
Jin D X, Cheng Z, Liu S C. 1991. China Marine Benthic Diatoms. Ocean Press, Beijing, China.
(in Chinese)
|
John P H, McCollin T A, Lucas I A N. 2000. Dinoflagellate cysts in ballast tank sediments: between tank variability. Marine Pollution Bulletin, 40(9): 731-733.
DOI:10.1016/S0025-326X(99)00198-8 |
Klein G, Macintosh K, Kaczmarska I, Ehrman J M. 2010. Diatom survivorship in ballast water during trans-pacific crossings. Biological Invasions, 12(5): 1031-1044.
DOI:10.1007/s10530-009-9520-6 |
Liebich V, Stehouwer P P, Veldhuis M. 2012. Re-growth of potential invasive phytoplankton following UV-based ballast water treatment. Aquatic Invasions, 7(1): 29-36.
DOI:10.3391/ai |
Liu K. 2005. Science and technology in Foreign countries. Marine Invasion, (5): 26-28.
|
Liu Y, Wang S, Wang Q, Li Y, Wang H X, Guan Y Y. 2011. Notice of retraction investigation of plankton in ballast water of two Chinese domestic voyages. In: Proceedings of 20115th International Conference on Bioinformatics and Biomedical Engineering. IEEE, Wuhan, China. p.78-82.
|
Mackenzie M. 1999. Alien invaders. New Scientist, 162(2183): 18-19.
|
McCarthy H P, Crowder L B. 2000. An overlooked scale of global transport: phytoplankton species richness in ships' ballast water. Biological Invasions, 2(4): 321-322.
DOI:10.1023/A:1011418432256 |
McCoy G R, Touzet N, Fleming G T A, Raine R. 2013. An evaluation of the applicability of microarrays for monitoring toxic algae in Irish coastal waters. Environmental Science and Pollution Research, 20(10): 6751-6764.
DOI:10.1007/s11356-012-1294-1 |
McQuoid M R, Hobson L A. 1996. Diatom resting stages. Journal of Phycology, 32: 889-902.
DOI:10.1111/j.0022-3646.1996.00889.x |
Medcof J C. 1975. Living marine animals in a ships' ballast water. Proceedings of the National Shellfisheries Association, 65: 11-12.
|
Olenin S, Gollasch S, Jonušas S, Rimkutė I. 2000. En-route investigations of plankton in ballast water on a ship's voyage from the Baltic Sea to the open Atlantic coast of Europe. International Review of Hydrobiology, 85(5-6): 577-596.
DOI:10.1002/(ISSN)1522-2632 |
Pam E D, Li K X, Wall A, Yang Z, Wang J. 2013. A subjective approach for ballast water risk estimation. Ocean Engineering, 61: 66-76.
DOI:10.1016/j.oceaneng.2012.12.045 |
Round F E, Crawford R M, Mann D G. 1990. The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
|
Ruiz G M, Carlton J T, Grosholz E D, Hines A H. 1997. Global invasions of marine and estuarine habitats by nonindigenous species: mechanisms, extent, and consequences. American Zoologist, 37(6): 621-632.
DOI:10.1093/icb/37.6.621 |
Ruiz G M, Rawlings T K, Dobbs F C, Drake L A, Mullady T, Huq A, Colwell R R. 2000. Global spread of microorganisms by ships. Nature, 408(6808): 49-50.
DOI:10.1038/35040695 |
Sarinas B G S, Gellada L D, Magramo M M, Baria LO, Tirazona D B, Sorio L R D, Tornalejo J A. 2014. Plankton diversity in ballast water of an inter-island passengercargo ship calling the Philippine ports. Asian Journal of Biodiversity, 5(1): 78-91.
|
Satir T. 2008. Ship's ballast water and marine pollution. In: Coskun H G, Cigizoglu H K, Maktav M D eds. Integration of Information for Environmental Security. Springer, Netherlands. p.453-463.
|
Satir T. 2014. Ballast water treatment systems: design, regulations, and selection under the choice varying priorities. Environmental Science and Pollution Research, 21(18): 10686-10695.
DOI:10.1007/s11356-014-3087-1 |
Sicko-Goad L, Stoermer E F, Kociolek J P. 1989. Diatom resting cell rejuvenation and formation: time course, species records and distribution. Journal of Plankton Research, 11(2): 375-389.
DOI:10.1093/plankt/11.2.375 |
Stan L C, Sabau A, Buzbuchi N. 2013. Ballast water treating by advanced oxidation technology. Annals of the University Dunarea De Jos of Galati: Fascicle II, 36(2): 287-291.
|
Stehouwer P P, Buma A, Peperzak L. 2015. A comparison of six different ballast water treatment systems based on UV radiation, electrochlorination and chlorine dioxide. Environmental Technology, 36(13-16): 2094-2104.
|
Steichen J L, Denby A, Windham R, Brinkmeyer R, Quigg A. 2015. A Tale of Two Ports: Dinoflagellate and Diatom Communities Found in the High Ship Traffic Region of Galveston Bay, Texas (USA). Journal of Coastal Research, 31(2): 407-416.
|
The General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and the National Standardization Administration of China. 2007a. GB 17378.4-200. The Specification for Marine Monitoring—Part 4: Seawater Analysis. China Standard Press, Beijing, China. (in Chinese)
|
The General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and the National Standardization Administration of China. 2007b. GB 17378.7-200. The Specification for Marine Monitoring—Part 7: Ecological Survey for Offshore Pollution and Biological Monitoring. China Standard Press, Beijing, China. (in Chinese)
|
Wang C C, Niu Z G, Zhang Y. 2013. Health risk assessment of inhalation exposure of irrigation workers and the public to trihalomethanes from reclaimed water in landscape irrigation in Tianjin, north China. Journal of Hazardous Materials, 262: 179-188.
DOI:10.1016/j.jhazmat.2013.08.044 |
Wang G. 2014. The international shipping situation and the development Suggestions of Yangshan deep-water port. Transportation Enterprise Management, 29(8): 11-14.
(in Chinese) |
Xue J Z, Liu Y, Wang J H, Xing X L, Xu L P, Feng D L, Wu H X. 2011. A biological survey of zooplankton taken from ballast water of the international navigation ships entering the Shanghai Yangshan Deep-water Port in China. Acta Oceanologica Sinica, 33(1): 138-145.
(in Chinese with English abstract) |
Xue J Z, Xiao N Y, Wang Q, Wu H X. 2016. Seasonal variation of bacterial community diversity in Yangshan Por. Acta Ecologica Sinica, 36(23): 7758-7767.
(in Chinese with English abstract) |
Yang S M, Dong S. 2006. Common Planktonic Diatoms in China Sea Map. China Ocean University Press, Qingdao, China.
(in Chinese)
|
Yuan J, Mi T Z, Zhen Y, Yu Z G. 2012. Development of a rapid detection and quantification method of Karenia mikimotoi by real-time quantitative PCR. Harmful Algae, 17: 83-91.
DOI:10.1016/j.hal.2012.03.004 |
 2019, Vol. 37
2019, Vol. 37


