Institute of Oceanology, Chinese Academy of Sciences
Article Information
- DING Huiping, GU Xiaohong, ZHANG Zhiming, HUO Bin, LI Dapeng, XIE Congxin
- Growth and feeding habits of invasive Pseudorasbora parva in the Chabalang Wetland (Lhasa, China) and its trophic impacts on native fish
- Journal of Oceanology and Limnology, 37(2): 628-639
- http://dx.doi.org/10.1007/s00343-019-8004-5
Article History
- Received Jan. 8, 2018
- accepted in principle Feb. 22, 2018
- accepted for publication May. 18, 2018
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China;
4 Institute of Hydroecology, MWR & CAS, Wuhan 430070, China;
5 Key Laboratory of Ecological Impacts of Hydraulic-Projects and Restoration of Aquatic Ecosystem, Ministry of Water Resources, Wuhan 430070, China
The Qinghai-Tibet Plateau is the highest and youngest plateau in the world with special geographical and ecosystem conditions. Indigenous fishes mainly belonging to the family Schizothoracinae and Sisoridae and Triplophysa species are endemic to Tibet, which are also very important fish germplasm resources (Wu and Wu, 1992). In recent years, along with overfishing and water conservancy projects, fish invasion has become another main reason for the decline of native fish stocks (Chen and Chen, 2010; Yang and Huang, 2011; Huo et al., 2014; Zhu et al., 2017).
Pseudorasbora parva, a small cyprinid species native to Eastern China, Japan, Korea, and the Heilong River Basin, is considered to be the most invasive and threatening species worldwide. It has invaded 32 countries from Central Asia to North Africa in less than 50 years (Gozlan et al., 2010). In Europe, it has been proven to be a highly invasive species, since its initial accidental introduction in 1960s, and has spread throughout Europe (Bianco, 1988; Wildekamp et al., 1997; Pollux and Korosi, 2006). Pseudorasbora parva was first introduced in China in the 1950s, as a result of translocation from its native range. Currently, it has invaded almost all the natural waters in China, including the upper reach of the Yellow River, inland waters of Yunnan, Inner Mongolia, Xinjiang, and the Qinghai-Tibet Plateau (Li, 1982; Chu and Chen, 1989; Fan et al., 2011).
However, reports of invasive P. parva are sporadic, and description of the population ecology, feeding habits, and relationship with native fishes is scarce, which may cause inadequate understanding of the invasion consequences. In this milieu, the present study intended to document the age, growth, and feeding habits of P. parva in the Chabalang Wetland (Lhasa, Tibet), which is one of the most important wetlands along the Lhasa River. Furthermore, the study aimed to clarify its food relationships with native fish in order to provide fundamental information for a better understanding of its effects on native species and to establish the corresponding risk assessment and management policies.
2 MATERIAL AND METHOD 2.1 Study site and sample collectionThe Chabalang Wetland is a shallow marsh (< 2.5 m) of 20 hm2 in Chabalang village (Qushui County, Lhasa), which belongs to the main agricultural area of Tibet, about 1.0 km north of the Lhasa River and 8.5 km from the confluence of the Yarlung Zangbo and Lhasa Rivers (29°22′30″–29°22′59″N, 90°49′20″–90°50′30″E; Fig. 1). There are ditches connecting the wetland to the Lhasa River. The average altitude is 3 600 m, annual precipitation is about 441.9 mm, and annual temperature is 7.18℃. Phragmites australis, Typha orientalis Presl, and Scirpus tabernaemontani are the main vegetation. The vulnerability of the wetland is moderate (Bai et al., 2012). Artificially transformed, the wetland is divided into three-interconnected parts: the east is an early-dug fishpond, north is primeval reed marsh, and south is now a native fish breeding research base.

|
| Fig.1 Map of Chabalang Wetland and fish sampling sites |
The fish samples were collected by backpack electrofishing at 9 different sampling sites once in January, April, July, and October 2009 and 2013. The fishing equipment set to a frequency of 60 Hz with 6 ms pulse duration and an output of 100 voltages. The voltage, pulse, and frequency were adjusted to maximize the capture probability without injuring the fishes. A micro-mesh gill net investigation was supplemented. After collection, the fishes were sorted according to Zhu (1989), Fishery Bureau in Tibet Municipality (1995), and Xie (2010), and were then preserved in 10% formaldehyde for laboratory analyses.
2.2 Laboratory analysesIn the laboratory, the body weight (BW) and standard length (SL) of each individual were measured to the nearest 0.01 g and 0.01 mm, respectively. Eight to ten well-developed scales were removed from above the lateral line on the left side of the fish, just behind the dorsal-fin origin for age determination. After dissection, the gut contents were removed, weighed (0.1 mg), and preserved in 10% formaldehyde. Sex was determined by visual examination of gonads morphology. The scales were immersed in 4%–5% sodium hydroxide solution for about 5 h, and the extraneous matters were brushed off using a soft brush. Age were estimated by counting the number of annual rings on scales. All scales were analyzed thrice by the same reader after a considerable time interval (≥3 weeks).
The relationship between BW and SL was estimated by the power regression analysis described by BW=aSLb (a and b are constants) (Le Cren, 1951). The observed length-at-age data was fitted to the von Bertalanffy growth function (VBGF): Lt=L∞ (1– e-k(t–t0)), where Lt is the length at age t, L∞ is the asymptotic length, k is the growth coefficient, and t0 is the theoretical age at length 0.
2.3 Feeding habitsFor dietary analysis, the gut contents were diluted with distilled water and carefully transferred to a counting chamber for microscopic determination. The macro-organisms were identified and counted using a dissecting microscope (Leica EZ4D) and weighed after absorbing excess water with blotting paper. Small organisms were identified and counted under 10× and 40× microscopes (Olympus, CX21) using counting chambers (0.1 and 1 mL). The weight was calculated according to mass and volume conversion (Liu et al., 2006).
The contribution of each prey type to the diet was evaluated using the percent of occurrence (O%), number (N%), and weight (W%), and the index of relative importance IRIi=Oi% (Ni%+Wi%) (Pinkas et al., 1971). To compare with that of other studies, the values of IRI were expressed in percent (IRI%) (Cortés, 1997). Dominance index was IPi%=100 × Oi%Wi%/∑1n(Oi%Wi%) also calculated (Mohan and Sankaran, 1988).
The Amundsen graphical method (Amundsen et al., 1996), which is applied to the data set of prey taxa identified at genus level, was used to describe the feeding strategy of P. parva. In mathematical terms, the prey-specific abundance was summarized as Pi%=100×(∑Si/∑Sti), where, Pi is the prey-specific abundance of prey i, Si is the gut content (W%) comprised of prey i, and Sti is the total gut content of only those fish with prey i in their guts. Through the position of prey types in the two-dimensional plot (Pi against O%, Fig. 3b), information regarding prey importance, feeding strategy, and niche width contribution can be inferred.

|
| Fig.3 Graphic representation of diet composition in P. parva a. diagram representing the prey taxa. a: Chironomidae larvae; b: Nitzschia; c: Synedra; d: Navicula: Scenedesmus; e: Fragilaria: Nauplius: rotifer eggs; f: Cyclotella; g: Ankistrodesmus: Diatoma; h: Cyclopoida; i: Cymbella: Diploneis; j: unidentified arthropod: Alona: Mougeotia; k: Nematode: Chyolorus: Brachionus: Tetraedron: Epithemia; l: Rhopalodia: Monostyla; m: Oscollatoria; n: Dictyosphaerium: Lepadella: Cosmarium; o: Chironomidaea pupae; p: Pinnularia: Chodatella: Achnanthes: Rotaria; q: Oedogonium: Colurella: Chlorella: Caloneis: Gomphonema: Tintinnopsis: r: plant debris: Pediastrum: Tetrastrum: Neidiun: Dinobryon: Merismopedia: Keratella; s: Ulothrix: Araneae: Amphora; t: Trachelomonas: Water Bear: Notholca: Cymatopleura: Chlorococcum: Lyngbya: u: Peridinium: Treubaria: Graptoleberis: Golenkinia: Arcella: Euglena: Philodina: Difflugia: Actinastrum; v: Coelastrum: unidentified Bdelloidea: Chroococcus: Glenodinium: Phacus: Staurastrum: Cyclopyxis: Lecane; w: Microspora; x: Ceriodaphnia: Kirchneriella: Stauroneis: Trichocerca; y: Spirogyra; z: Zygnema; 1. fish scales, Cladophora, Surirella, Ascomorpha; 2. Closterium; 3. other preys; b. explanatory diagram for interpretation of feeding strategy, prey importance and niche width contribution (adapted from Amundsen et al., 1996). BPC and WPC represent betweenphenotype component and within-phenotype component, respectively. |
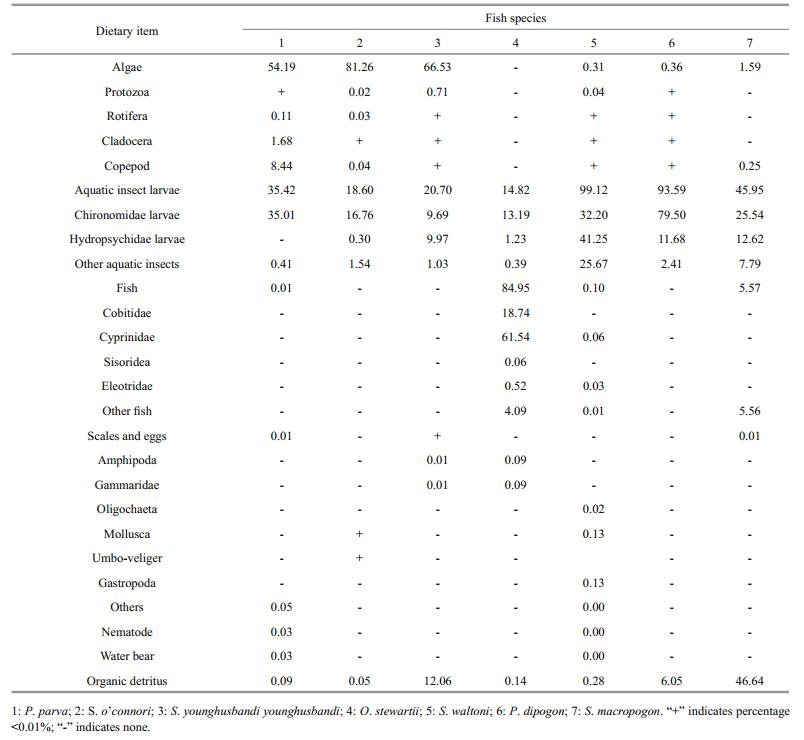
Food relationships between P. parva and native Schizothorax o'connori, Schizopygopsis younghusbandi younghusbandi, Oxygymnocypris stewartii, Schizothorax waltoni, Ptychobarbus dipogon, and Schizothorax macropogon (Yang et al., 2011; Huo et al., 2014; Ma et al., 2014; Zhou, 2014; Yang, 2015; Liu, 2016) were determined by the diet items in terms of weight percent (W%). Diet items of all the fishes were classified as 21 dietary taxa (Table 3). Trophic level of these dietary taxa was assigned according to Liu (1999), while that of forage fishes was calculated based on their diet items.
|
Hill's diversity number (Hill, 1973), which is expressed as N0 (the number of prey taxa), N1= eH′ (the number of abundant prey taxa), and N2=1/∑1nPi2 (the number of very abundant prey taxa), was used to discuss diet spectrum. Where, H′=-∑1n(PilnPi) is the Shannon-Wiener Index (Shannon, 1948), Pi is the proportion of prey i, and n is the number of dietary taxa. Levin's standardized niche breath index Ba=1/(n-1)(1/Pi2-1) (Krebs, 1989) was applied to calculate the niche breath. Diet overlaps of P. parva and native fishes were discussed using simplified Morisita's index C=2×∑1nPxiPyi/(∑1nPxi2+∑1nPyi2) (Hall et al., 1990), where, Pxi and Pyi are the proportions of the common prey i of fish x and y, respectively, and C > 0.6 indicates significance. Trophic levels T=1+∑1n(tiPi) (Odum and Heald, 1975) were used to analyze their position in the food chain, where, ti is the trophic level of prey i. Data and images were analyzed using Microsoft Excel 2007 and Origin 8.5.1. Statistical analysis was carried out using the SPSS version 18.0 software at a significance level of 0.05.
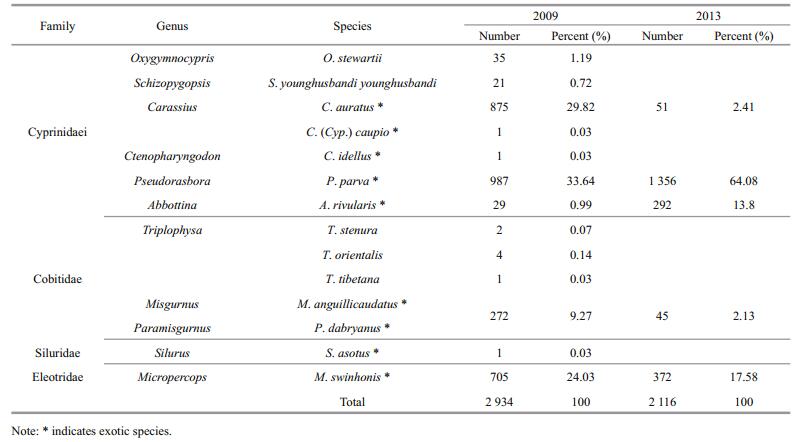
3 RESULT 3.1 Fish compositionIn total, 2 934 fish were sampled in 2009, with 8 exotic fish species (n=2 871) accounting for 97.85%. The remaining were native species, including S. y. younghusbandi (n=21), O. stewartii (n=35), Triplophysa stenura (n=2), Triplophysa orientalis (n=4), and Triplophysa tibetana (n=1); together they accounted for only 2.15% of the sampled fishes, which is significantly below the estimates recorded for exotic fish. In 2013, 2116 fish belonging to 5 species were captured, and there was no native fish (Table 1). The results showed that the percentage of P. parva in the fish community had significantly increased from 33.64% in 2009 to 64.08% in 2013. Furthermore, the occurrence of both juveniles and adults of P. parva indicated the establishment of breeding population.
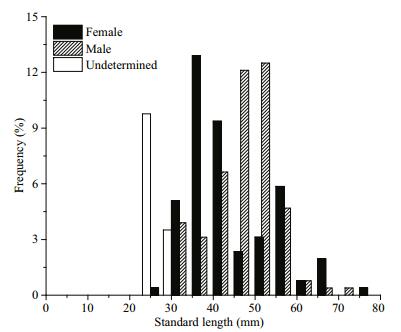
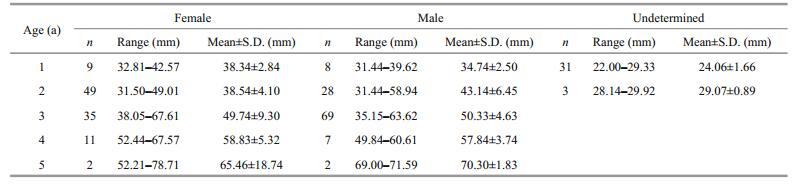
In total, 256 P. parva were subsampled randomly for age determination; 108 were females (SL 32.81– 78.71 mm), 114 were males (SL 31.44–71.59 mm), and 34 were unsexed (SL 22.00–29.92 mm). The length-frequency distributions differed significantly between sexes (Kolmogorov-Smirnov; Z=2.804, P < 0.001) (Fig. 2). The sexual ratio (F:M) was 1:1.08, which was not significantly different from 1:1 (χc2=0.082, P=0.775).
|
| Fig.2 Frequency distributions of standard length of P. parva |
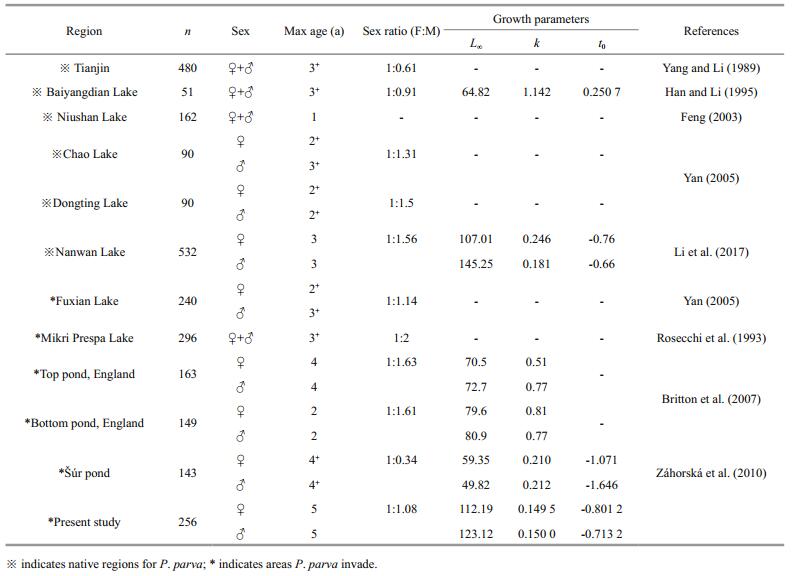
Of the 256 P. parva, only 2 (approximately 0.78%) were discarded due to regeneration or unidentifiable annulus, the estimated max age was 5 years for both females and males. Age structure differed significantly between sexes (Kolmogorov-Smirnov; Z=1.715, P=0.006). Most females were 3 a (60.53%), while most males were 2 a (46.23%) (Table 2). The SL-BW relationship was BW=2.069×10-5SL3.006 (R2=0.978, n=108), BW=8.731×10-6SL3.208 (R2=0.969, n=114), and BW=1.516×10-5SL3.061 (R2=0.868, n=34) for females, males, and unsexed, respectively. Significant differences were found in the SL-BW relationship between sexes (ANCOVA, F=44.046, P < 0.01). The allometric index b differed significantly from 3 for males (t-test, t=3.836, P < 0.01), whereas there was no statistical difference from 3 for females (t-test, t=0.137, P > 0.05). This suggests that the females were isokinetic, while the males were allometric. The von Bertalanffy functions fitted to the observed body length-at-age were given as Lt= 112.19(1–e-0.1495(t+0.8012)) and Lt= 123.12(1–e-0.1500(t+0.7132)) for females and males, respectively.
|
Of the 156 intestines collected randomly for diet analysis, 84 were empty (53.8%), and the remaining 72 contained at least one prey item (46.2%). Thirtyeight specimens with good fullness (SL 36–76 mm) were chosen for food items identification. The diets consisted of a wide variety of algae (57 genera, belonging to 7 phyla) and animal organisms (23 genera and 5 other taxa, belonging to 5 phyla). A small amount of sand, rotted plant debris, and fish scales were also observed among the intestinal contents (Appendix 1).
According to the frequency of occurrence (O%) data, the prey items most frequently ingested by P. parva were algae, Rotifera, Cladocera, Copepod, and aquatic insects, each accounting for 100%. Algae were the most abundant prey item (99.99%) in terms of the index of abundance (N%), which primarily included Chlorophyta (40.60%), Bacillariophyta (30.17%), and Cyanophyta (28.61%). Bacillariophyta were the most important food in terms of weight (45.53%), IRI (41.18%), and IP (47.88%). Chironomidae larvae followed this group in terms of weight (35.01%) and IP (35.63%).
The dietary pattern of P. parva based on the Amundsen method is graphically represented in Fig. 3a. Most prey points located toward the lower part indicate a generalized feeding strategy, in which a large number of prey items were usually consumed at a low percent. A preference for the Chironomidae larvae was observed. The Chironomidae larvae were the most important prey item of P. parva, with the highest frequency of occurrence (O%=100%) and prey-specific abundance (Pi%=28.45%), followed by Nitzschia (O%=100%, Pi%=20.66%), and Cyclotella (O%=97.34%, Pi%=12.98%). To the niche width, the points toward the lower right part of the graph indicate a relatively high within-phenotype component (WPC), i.e., high diet overlap within individuals. Therefore, P. parva is a generalized predator, which relied on a wide trophic spectrum, and Bacillariophyta and the Chironomidae larvae were the major prey items.
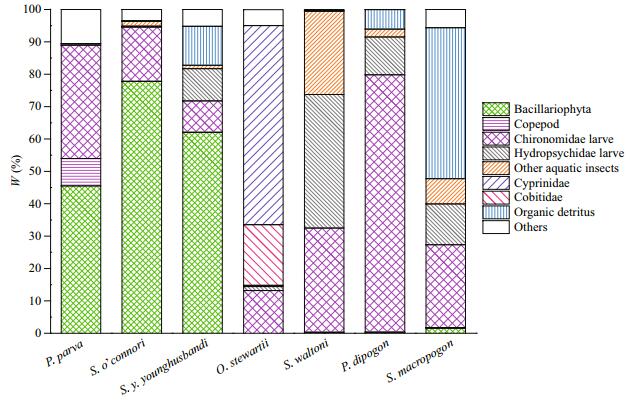
3.4 Dietary overlaps between P.parva and native fishesThe food compositions (in weight percent, W%) of P. parva and 6 native Schizothoracinae fishes are listed in Table 3. The Schizothoracinae fishes can be divided into four feeding types. S. o'connori and S. y. younghusbandi are phytophagous feeding mainly on Bacillariophyta, that accounted for 77.77% and 66.16% of their diet, respectively. S. waltoni and P. dipogon are zoobenthivores. Hydropsychidae larvae (41.25%) and Chironomidae larvae (32.20%) were the main prey items of S. waltoni, while the Chironomidae larvae (79.50%) were the main prey item of P. dipogon. O. stewartii was the only piscivore among the 6 native fishes. Fish accounted for 84.95% in the food composition, followed by the Chironomidae larvae (13.19%). Further, S. macropogon was omnivores, which mainly ingested detritus (46.64%) and aquatic insects (45.95%). The Chironomidae larvae accounted for 25.54% of their diet.
These results revealed that native fishes exhibit some feeding differentiation, which was probably an adaptive strategy of these fishes to deal with food shortage of the Qinghai-Tibetan Plateau. However, the freeloader P. parva might disturb this balance, leading to competition for food between the nonnative and native fishes (Tables 3 and 4, Fig. 4). According to the Morisita index (C value), food composition of P. parva was the most similar to that of S. o'connori and S. y. younghusbandi. Furthermore, significant diet overlaps were observed between P. parva and S. o'connori (C=0.90), and between P. parva and S. y. younghusbandi (C=0.88). This will lead to competition for food between P. parva and these two fishes for Bacillariophyta and Chironomidae larvae. However, considering the fewer number of abundant prey taxa and narrow dietary niche breadth, the diet spectrum of these two-native fish was actually narrow, placing them in a competitive disadvantage position. Similarly, P. parva might also have feeding stress on native P. dipogon (C=0.52) on the utilization of the Chironomidae larvae. To some extent, P. dipogon nearly fed only on the Chironomidae larvae (W%=79.50%), its food composition was unitary, and the feeding scope was narrow. The overlap index between P. parva and other native fish was 0.11–0.30; the Chironomidae larvae were also the common prey item of these fishes. Furthermore, the trophic level of the non-native and native fishes varied by 2.2–3.5.S.o'connoriandS.y.younghusbandi were at the lowest trophic level (T=2.2), while O. stewartii was at the highest level (T=3.5). Although it is piscivorous and the top consumer of the food web, O. stewartii hardly ingested P. parva (W%=0.09%) (Huo et al., 2014). Therefore, P. parva can be regarded as an exotic competitor.

|
|
| Fig.4 Diet compositions of both P. parva and native Schizothoracinae fishes in the middle reaches of Yarlung Zangbo River (in weight percentage, W%) |
During the last 50 years, P. parva has successfully invaded many countries and regions worldwide and is considered the most invasive fish species (Gozlan et al., 2010). The present study showed that P. parva has become established in the Chabalang Wetland and that its population size is constantly increasing. It has invaded many waters of the Yarlung Zangbo River and its tributaries (Shen and Guo, 2008; Chen and Chen, 2010; Fan et al., 2011; Yang et al., 2011). The water temperature and primary productivity of the Yarlung Zangbo River is relatively lower than that of other aquatic environments due to the unique and complex plateau climate, and the special hydrogeomorphology (Wei et al., 2015). The invasive success of P. parva partially relates to its strong invasiveness, with a wide ecological amplitude (Sunardi et al., 2005, 2007), high plastic life history traits (Rosecchi et al., 2001; Pinder et al., 2005; Britton et al., 2007; Onikura and Nakajima, 2013), and generalized feeding habits (Rosecchi et al., 1993; Zhang et al., 1998; Xie et al., 2000). On the other hand, based on the food web and co-evolution theories (Elton, 1958), the relatively isolated aquatic ecosystem of the Qinghai-Tibet Plateau is fragile and vulnerable to outer disturbance due to simple fish fauna, low species diversity, and simple interspecific interaction patterns; it is too weak to resist alien fish invasion (Schoenherr, 1981; Minckley and Douglas, 1991; Wu and Tan, 1991; Chen and Chen, 2010). In addition, the adult native Schizothoracinae and Sisoridae species mainly inhabit the main stream of the Yarlung Zangbo River and its tributaries, leaving plenty of favorable habitats for P. parva, such as river branches and wetlands, where the food resources are relatively abundant (Wu and Wu, 1992; Fan et al., 2011). Furthermore, anthropogenic activities, including repeated fish introduction, overexploitation of native fish, hydraulic engineering construction, and religious release (Gozlan et al., 2010; Huo et al., 2014; Zhu et al., 2017), facilitate the invasion success of P. parva in Tibet.
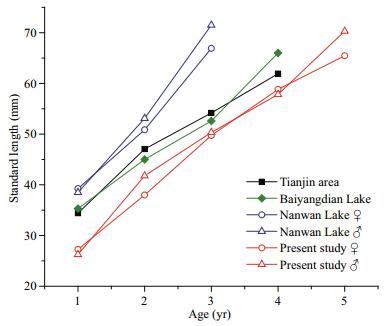
Plasticity in life-history strategies is considered as one of the most important reasons for P. parva to easily adapt to different habitats, including fast growth, early maturity, short life span, and special reproductive strategy (Rosecchi et al., 2001; Britton et al., 2007). The present study indicated that the growth rate of P. parva in the Chabalang Wetland is lower than that in the other regions (Table 5, Fig. 5). This might be attributed to the high altitude and microthermal climate of the Qinghai-Tibet Plateau resulting in low mean water temperatures and short growth seasons, which are important determinants of the life history of fish (Cowx, 2001). According to Gaspar et al. (1999), with decrease in water temperature, the lifespan and body size of fish increases, while the growth rate of fish decreases. Studies have shown that the growth rate of P. parva varies widely in different places, which is attributed to population density, niche overlap, and food abundance (Yan, 2005; Britton et al., 2007, 2008). These intraspecific differences in the life-history traits between populations inhabiting different habitats indicate that this species can modify its life-history strategy according to environment conditions. This can be regarded as an adaptive evolutionary response of P. parva to environmental change.
|
| Fig.5 Comparison of observed SL of P. parva from different regions |
The results of the present study indicated that P. parva in the Chabalang Wetland relies on a wide trophic spectrum and ingested mainly Bacillariophyta and the Chironomid larvae. This is consistent with the findings of previous studies, which described P. parva as an omnivore or planktivorous with a broad diet breadth (Rosecchi et al., 1993; Zhang et al., 1998; Wolfram-Wais et al., 1999; Xie et al., 2000). A preference of P. parva for the Chironomidae larvae has also been reported by other studies (Wolfram-Wais et al., 1999; Declerck et al., 2002). Therefore, as a generalized and opportunistic predator, P. parva can readjust its diet composition based on the abundance and availability of food. The broad diet spectrum enhances their adaptability to different environmental conditions. The results of the present study indicated certain overlaps between P. parva and six endemic Schizothoracinae species in the trophic niche. Bacillariophyta and Chironomid larvae were their common main prey items. The native fishes are probably well adapted to resist harsh environmental conditions by feeding differentiation; however, they are unable to contend with intruders, such as P. parva—a more effective hunter (Schoenherr, 1981; Minckley and Douglas, 1991; Rosecchi et al., 1993). As an exotic intruder, high grazing pressure exerted by dense P. parva population can change the prevalent environmental conditions through top-down effects characterized by increased development of phytoplankton and accelerated eutrophication (Adámek and Sukop, 2000; Gozlan et al., 2010). The decline of primary and/or second producers will have bottom-up effects on the upper trophic levels through the aquatic food webs (Power, 1990).
The adult Schizothoracinae fishes mainly inhabit swift current, while P. parva inhabit slow-flow waters. Therefore, the direct competition with adult Schizothoracinae for food might not occur very frequently. The competition for food will mainly occur between P. parva and the Schizothoracinae fish larvae and/or fry. However, because of the slow early development and relatively hysteretic first-feeding on live bait (Shao et al., 2012), the Schizothoracinae fish larvae and/or fry were at a competitive disadvantage than P. parva. The mortality of these small-sized native fish might increase, which will lead to further decline of recruitment of these native fishes (Huo et al., 2014). Although the trophic competition might not occur frequently between P.parva and adult Schizothoracinae, the studies on feeding habits of native Schizothoracinae did not include individuals with SL < 100 mm (Yang et al., 2011; Huo et al., 2014; Ma et al., 2014; Zhou, 2014; Yang, 2015; Liu, 2016). Therefore, further studies are necessary to demonstrate food relationships between P. parva and these small-sized native fishes.
Although the biological information collected in Chabalang Wetland is incomplete, some more ecological effects of P. parva can be speculated. Firstly, P. parva has been reported to feed on the eggs and larvae of native fish species (Jin et al., 1996; Xie et al., 2000). In the present study, some fish eggs and scales were found in the intestine of P. parva. Further studies are necessary to elucidate if these belonged to native fish. Secondly, the high abundance of P. parva will result in intense competition with native fish for habitat, leading to habitat loss and niche displacement among native fish. This is because the waters that the invader inhabits are exactly the favorable feeding and fattening grounds of native Triplophysa and Schizothoracinae fish larvae and/or fry (Wu and Wu, 1992; Fan et al., 2011). Furthermore, P. parva has been found to be a vector of many parasites and pathogens, some of which have been documented to cause emergent infectious disease and high rates of mortalities of fish (Urabe et al., 2007; You et al., 2008; Gozlan et al., 2010). Therefore, their invasion might pose new potential threats to native fish and aquatic ecosystem. Further, P. parva is a non-target species that was unknowingly released along with intentionally-introduced species (Gozlan et al., 2010). Regarding their increasing distribution and their presence in waters that connect to major river catchments, fluvial dispersal is going on (Pinder et al., 2005). There might be few waters in Tibet immune from their invasion, numerical dominance, and subsequent impacts. To protect the endemic fish and the aquatic ecosystem in Tibet from the invasion, thorough investigation of P. parva in Tibet is needed. Further studies on the reproductive strategy, population dynamics, spreading tendency of P. parva, and the position it occupies in the plateau aquatic food web are also needed to establish effective risk assessment and management policies.
5 CONCLUSIONThe invasive P. parva in Chabalang Wetland shows plasticity of some life history traits with slow growth and high growth potential. This appeared to be related to the low water temperature and short growth season in the plateau. It can be considered a generalized and opportunistic predator, competing with native fish, especially S. o'connori, S. younghusbandi younghusbandi, and P. dipogon, for Bacillariophyta and Chironomid larvae. However, because the habitats are different, direct competition with adult Schizothoracinae for food might not occur very frequently, the trophic effects of P. parva on native adult Schizothoracinae are limited; however, for small-sized native fish living in the same environment, such as Schizothoracinae fish larvae and/or fry, the effects may be significant. Further studies are needed to clarify the food relationships between P. parva and small-sized native fish.
6 ACKNOWLEDGMENTThe authors would like to thank DUAN Youjian, YANG Xin, and ZHOU Xianjun for help in sample collection.
Electronic supplementary materialSupplementary material (Appendix 1) is available in the online version of this article at https://doi.org/10.1007/s00343-019-8004-5.
Adámek Z, Sukop I. 2000. The impact of topmouth gudgeon (Pseudorasbora parva) populations upon pond environmental determinants. In: Lusk S, Halačka K eds. Biodivesity of Fishes in the Czech Republic (Ⅲ.). Institute of vertebrate Biology Academy of Sciences, Brno, Czech Republic. p.37-43.
|
Amundsen P A, Gabler H M, Staldvik F J. 1996. A new approach to graphical analysis of feeding strategy from stomach contents data-modification of the Costello (1990) method. Journal of Fish Biology, 48(4): 607-614.
|
Bai W Q, Shang E P, Zhang Y L. 2012. Application of a new method of wetland vulnerability assessment to the Lhasa River basin. Resources Science, 34(9): 1761-1768.
(in Chinese with English abstract) |
Bianco P G. 1988. Occurrence of the Asiatic gobionid Pseudorasbora parva (Temminck and Schlegel) in southeastern Europe. Journal of Fish Biology, 32(6): 973-974.
DOI:10.1111/jfb.1988.32.issue-6 |
Britton J R, Davies G D, Brazier M, Pinder A C. 2007. A case study on the population ecology of a topmouth gudgeon (Pseudorasbora parva) population in the UK and the implications for native fish communities. Aquatic Conservation: Marine and Freshwater Ecosystems, 17(7): 749-759.
DOI:10.1002/(ISSN)1099-0755 |
Britton J R, Davies G D, Brazier M. 2008. Contrasting life history traits of invasive topmouth gudgeon (Pseudorasbora parva) in adjacent ponds in England. Journal of Applied Ichthyology, 24(6): 694-698.
DOI:10.1111/j.1439-0426.2008.01163.x |
Chen F, Chen Y F. 2010. Investigation and protection strategies of fishes of Lhasa River. Acta Hydrobiologica Sinica, 34(2): 278-285.
(in Chinese with English abstract) |
Chu X L, Chen Y R. 1989. Fishes of Yunnan. Science Press, Beijing.
(in Chinese)
|
Cortés E. 1997. A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences, 54(3): 726-738.
DOI:10.1139/f96-316 |
Cowx I G. 2001. Factors Influencing Coarse Fish Populations in Rivers: a Literature Review. Environment Agency Research and Development Publication 18, the Stationery Office, London.
|
Declerck S, Louette G, De Bie T, De Meester L. 2002. Patterns of diet overlap between populations of non-indigenous and native fishes in shallow ponds. Journal of Fish Biology, 61(5): 1182-1197.
DOI:10.1111/jfb.2002.61.issue-5 |
Elton C S. 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
|
Fan L Q, Tu Y L, Li J C, Fang J P. 2011. Fish assemblage at the Lhalu Wetland: does the native fish still exist. Resources Science, 33(9): 1742-1749.
|
Feng G P. 2003. Studies on Age Structure and Diversity of Fish Community in the Niushan Lake. Huazhong Agricultural University, Wuhan. (in Chinese with English abstract)
|
Fishery Bureau in Tibet Municipality. 1995. The Fishes and Fishes' Resource of Tibet. Agriculture Press, Beijing.
(in Chinese)
|
Gaspar M B, Ferreira R, Monteiro C C. 1999. Growth and reproductive cycle of Donax trunculus L., (Mollusca: Bivalvia) off Faro, southern Portugal. Fisheries Research, 41(3): 309-316.
DOI:10.1016/S0165-7836(99)00017-X |
Gozlan R E, Andreou D, Asaeda T, Beyer K, Bouhadad R, Burnard D, Caiola N, Cakic P, Djikanovic V, Esmaeili H R, Falka I, Golicher D, Harka A, Jeney G, Kováč V, Musil J, Nocita A, Povz M, Poulet N, Virbickas T, Wolter C, Tarkan A S, Tricarico E, Trichkova T, Verreycken H, Witkowski A, Zhang C G, Zweimueller I, Britton J R. 2010. Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions. Fish and Fisheries, 11(4): 315-340.
DOI:10.1111/faf.2010.11.issue-4 |
Hall S J, Raffaelli D, Basford D J, Robertson M R, Fryer R. 1990. The feeding relationships of the larger fish species in a Scottish sea loch. Journal of Fish Biology, 37(5): 775-791.
DOI:10.1111/jfb.1990.37.issue-5 |
Han X F, Li S H. 1995. The biology of Pseudorasbora parva in Baiyangdian Lake. Hebei Fisheries, (2): 3-6.
(in Chinese with English abstract) |
Hill M O. 1973. Diversity and evenness: a unifying notation and its consequences. Ecology, 54(2): 427-432.
DOI:10.2307/1934352 |
Huo B, Xie C X, Madenjian C P, Ma B S, Yang X F, Huang H P. 2014. Feeding habits of an endemic fish, Oxygymnocypris stewartii, in the Yarlung Zangbo River in Tibet, China. Environmental Biology of Fishes, 97(11): 1279-1293.
DOI:10.1007/s10641-013-0213-8 |
Jin K W, Shi W L, Yu X Y, Hu H Y, Li W. 1996. The preliminary observation on eight small freshwater fishes eating adhesive fish eggs. Journal of Dalian Fisheries College, 11(3): 24-30.
(in Chinese with English abstract) |
Krebs C J. 1989. Ecological Methodology. Harper & Row, New York.
|
Le Cren E D. 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). Journal of Animal Ecology, 20(2): 201-219.
DOI:10.2307/1540 |
Li H J, Wang Y P, Leng Q L, Li X J, Li X F, Yu T L, Huang B. 2017. Study on the age and growth of Pseudorasbora parva from Nanwan Lake upstream the Huaihe River. Acta Hydrobiologica Sinica, 41(4): 835-842.
(in Chinese with English abstract) |
Li S Z. 1982. Studies on Zoogeographical Divisions for Fresh Water Fishes of China. Science Press, Beijing.
(in Chinese)
|
Liu J K. 1999. Hydrobiology. Science Press, Beijing.
(in Chinese)
|
Liu J Y. 2016. Study on Biology and Population Dynamics of Schizothorax Macropogon in the Yarlung Tsangpo River. Tarim University, Alaer. (in Chinese with English abstract)
|
Liu X Q, Wang H Z, Liang X M. 2006. Food web of macroinvertebrate community in a Yangtze shallow lake: trophic basis and pathways. Hydrobiologia, 571(1): 283-295.
DOI:10.1007/s10750-006-0248-3 |
Ma B S, Xie C X, Huo B, Yang X F. 2014. Feeding habits of Schizothorax oconnori lloyd, 1908 in the Yarlung Zangbo River, Tibet. Journal of Applied Ichthyology, 30(2): 286-293.
DOI:10.1111/jai.2014.30.issue-2 |
Minckley W L, Douglas M E. 1991. Discovery and extinction of western fishes: a blink of the eye in geologic time. In: Minckley W L, Deacon J E eds. Battle against Extinction: Native Fish Management in the American West. The University of Arizona Press, Tucson. p.7-17.
|
Mohan M V, Sankaran T M. 1988. Two new indices for stomach content analysis of fishes. Journal of Fish Biology, 33(2): 289-292.
DOI:10.1111/jfb.1988.33.issue-2 |
Odum W E, Heald E J. 1975. The detritus-based food web of an estuarine mangrove community. In: Wiley M ed. Estuarine Research. Academic Press, New York. 1: 265-286.
|
Onikura N, Nakajima J. 2013. Age, growth and habitat use of the topmouth gudgeon, Pseudorasbora parva in irrigation ditches on northwestern Kyushu Island, Japan. Journal of Applied Ichthyology, 29(1): 186-192.
DOI:10.1111/jai.2012.29.issue-1 |
Pinder A C, Gozlan R E, Britton J R. 2005. Dispersal of the invasive topmouth gudgeon, Pseudorasbora parva in the UK: a vector for an emergent infectious disease. Fisheries Management and Ecology, 12(6): 411-414.
DOI:10.1111/fme.2005.12.issue-6 |
Pinkas L, Oliphant M S, Iverson I L K. 1971. Food habits of albacore, bluefin tuna, and bonito in California waters. Fish Bull etin, 152: 1-105.
|
Pollux B J A, Korosi A. 2006. On the occurrence of the Asiatic cyprinid Pseudorasbora parva in the Netherlands. Journal of Fish Biology, 69(5): 1575-1580.
DOI:10.1111/jfb.2006.69.issue-5 |
Power M E. 1990. Effects of fish in river food webs. Science, 250(4982): 811-814.
DOI:10.1126/science.250.4982.811 |
Rosecchi E, Crivelli A J, Catsadorakis G. 1993. The establishment and impact of Pseudorasbora parva, an exotic fish species introduced into Lake Mikri Prespa (north-western Greece). Aquatic Conservation: Marine and Freshwater Ecosystems, 3(3): 223-231.
DOI:10.1002/(ISSN)1099-0755 |
Rosecchi E, Thomas F, Crivelli A J. 2001. Can life-history traits predict the fate of introduced species? A case study on two cyprinid fish in southern France. Freshwater Biology, 46(6): 845-853.
DOI:10.1046/j.1365-2427.2001.00715.x |
Schoenherr A A. 1981. The role of competition in the replacement of native fishes by introduced species. In: Naiman R J, Soltz D L eds. Fishes in North American Deserts. John Wiley, New York. p.173-203.
|
Shannon C E. 1948. A mathematical theory of communication. The Bell System Technical Journal, 27(3): 379-423.
DOI:10.1002/bltj.1948.27.issue-3 |
Shao J, Xie C X, Xu J, Yang X F, Gesang D, Lin S Q. 2012. Effects of different diets on growth and survival of three species of Tibetan fish larvae. Freshwater Fisheries, 42(6): 49-53.
(in Chinese with English abstract) |
Shen H B, Guo L. 2008. Survey and analysis on fish composition of Niyang River in Tibet. Hebei Fisheries, (5): 51-54, 60.
(in Chinese with English abstract) |
Sunardi, Asaeda T, Manatunge J. 2005. Foraging of a small planktivore (Pseudorasbora parva: Cyprinidae) and its behavioral flexibility in an artificial stream. Hydrobiologia, 549(1): 155-166.
DOI:10.1007/s10750-005-5442-1 |
Sunardi, Asaeda T, Manatunge J. 2007. Physiological responses of topmouth gudgeon, Pseudorasbora parva, to predator cues and variation of current velocity. Aquatic Ecology, 41(1): 111-118.
DOI:10.1007/s10452-006-9048-0 |
Urabe M, Ogawa K, Nakatsugawa T, Nakai K, Tanaka M, Wang G T. 2007. Morphological description of two bucephalid trematodes collected from freshwater fishes in the Uji River, Kyoto, Japan. Parasitology International, 56(4): 269-272.
|
Wei X, Deng Y, Zhang L L, Tuo Y C. 2015. Analysis of water temperature characteristics in middle reach of the Yarlung Zangbo River. Journal of Sichuan University (Engineering Science Edition), 47(S2): 17-23.
(in Chinese with English abstract) |
Wildekamp R H, Van Neer W, Küçük F, Ünlüsayin M. 1997. First record of the eastern Asiatic gobionid fish Pseudorasbora parva from the Asiatic part of Turkey. Journal of Fish Biology, 51(4): 858-861.
|
Wolfram-Wais A, Wolfram G, Auer B, Mikschi E, Hain A. 1999. Feeding habits of two introduced fish species (Lepomis gibbosus, Pseudorasbora parva) in Neusiedler See (Austria), with special reference to chironomid larvae (Diptera: Chironomidae). Hydrobiologia, 408-409: 123-129.
DOI:10.1023/A:1017014130103 |
Wu Y F, Tan Q J. 1991. Characteristics of the fish-fauna of the characteristics of Qinghai-Xizang Plateau and its geological distribution and formation. Acta Zoologica Sinica, 37(2): 135-152.
(in Chinese with English abstract) |
Wu Y F, Wu C Z. 1992. The Fishes of the Qinghai-Xizang Plateau. Sichuan Publishing House of Science & Technology, Chengdu. (in Chinese)
|
Xie C X. 2010. Ichthyology. China Agriculture Press, Beijing.
(in Chinese)
|
Xie S, Cui Y, Zhang T, Li Z. 2000. Seasonal patterns in feeding ecology of three small fishes in the Biandantang Lake, China. Journal of Fish Biology, 57(4): 867-880.
DOI:10.1111/jfb.2000.57.issue-4 |
Yan Y Z. 2005. Adaptive Evolution in Life-History Strategies of Invasive Fishes in Lake Fuxian. Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan. (in Chinese with English abstract)
|
Yang H Y, Huang D M. 2011. A preliminary investigation on fish fauna and resources of the upper and middle Yalu Tsangpo River. Journal of Central China Normal University (Natural Sciences), 45(4): 629-633.
(in Chinese with English abstract) |
Yang X F, Xie C X, Ma B S, Huo B, Huang H P, Zhang H J, Xu J. 2011. Feeding habits of Schizopygopsis younghusbandi younghusbandi. Freshwater Fisheries, 41(4): 41-44, 49.
(in Chinese with English abstract) |
Yang X. 2015. Study on Age, Growth, Feeding Habits and Population Dynamics of Ptychobarbus Dipogon in the Yarlung Tsangpo River. Huazhong Agricultural University, Wuhan. (in Chinese with English abstract)
|
Yang Z F, Li M D. 1989. The biology of Pseudorasbora parva in Tianjin. Chinese Journal of Zoology, 24(1): 11-14.
(in Chinese with English abstract) |
You P, Easy R H, Cone D K. 2008. Gyrodactylus parvae n. sp. (Monogenea) from the fins and body surface of Pseudorasbora parva (Cyprinidae) in central China. Comparative Parasitology, 75(1): 28-32.
|
Záhorská E, Kováč V, Katina S. 2010. Age and growth in a newly-established invasive population of topmouth gudgeon. Central European Journal of Biology, 5(2): 256-261.
|
Zhang T L, Cui Y B, Fang R L, Xie S G, Li Z J. 1998. Population biology of topmouth gudgeon (Pseudorasbora parva) in Baoan Lake. Ⅲ. Food habit. Acta Hydrobiologica Sinica, 22(S1): 155-164.
(in Chinese) |
Zhou X J. 2014. Study on the Biology and Population Dynamics of Schizothorax waltoni. Huazhong Agricultural University, Wuhan. (in Chinese with English abstract)
|
Zhu S Q. 1989. The Loaches of the Subfamily Nemacheilinae in China (Cypriniformes: Cobitidae). Jiangsu Science and Technology Press, Nanjing.
(in Chinese)
|
Zhu T B, Chen L, Yang D G, Ma B, Li L. 2017. Distribution and habitat character of Schizothoracinae fishes in the middle Yarlung Zangbo River. Chinese Journal of Ecology, 36(10): 2817-2823.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37