Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CAO Min, ZHANG Fangfang, MAO Yunxiang, KONG Fanna, WANG Dongmei
- Characterization of the squalene-rich Botryococcus braunii Abt02 strain
- Journal of Oceanology and Limnology, 37(2): 675-684
- http://dx.doi.org/10.1007/s00343-019-8053-9
Article History
- Received Mar. 13, 2018
- accepted in principle May. 15, 2018
- accepted for publication Jun. 12, 2018
2 Key Laboratory of Marine Genetics and Breeding(OUC), Ministry of Education, Qingdao 266003, China;
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266000, China
Botryococcus braunii, belonging to Chlorophyta, Trebouxiophyceae, Trebouxiales, Botryococcaeae, Botryococcus, is a colonial, slow-growing fresh water microalga, which is widely distributed in tropical, subtropical and temperate zones (Wake and Hillen, 1981; Banerjee et al., 2002; Senousy et al., 2004). Studies have shown that, under appropriate culture conditions, as much as 86% of its dry weight may be composed of hydrocarbons (Brown et al., 1969). Therefore, B. braunii's capacity for hydrocarbon synthesis ability make it useful for industrial applications.
Under normal growth conditions, B. braunii colonies are composed of tens to hundreds of cells, with intercellular bonds formed through cross linking of extracellular hydrocarbon lipids and polysaccharides (Chiang et al., 2004; Metzger and Largeau, 2005; Tanoi et al., 2011). Under an optical microscope, cells appear pear-shaped and form colonies of various sizes, in which large algal colonies are formed through the aggregation of small algal colonies and have the appearance of a cluster of grapes (Metzger et al., 1990). On the basis of the chemical structures of their metabolic hydrocarbon products, the strains of B. braunii can be classified into different races A, B, S and L (Metzger et al., 1990). Furthermore, studies have shown that these races have different morphological and physiological characteristics. For example, the average individual length of cells from races A and B is approximately 13 μm, with a width of approximately 8 μm, whereas the cells from race L measure about 9 μm×5 μm (Metzger et al., 1988). Another difference between the algal races is their color after entering the stationary growth phase. Races B and L change from green to orange and orange-brown, respectively, whereas race A changes from green to yellow due to the accumulation of ketones and carotenoids (Metzger and Casadevall, 1989). However, because the appearance of the algae is also influenced by culture conditions, it is difficult to accurately distinguish races based solely on visual characteristics. In 2012, Kawachi et al. used 31 B. braunii strains from known races to construct a phylogenetic tree based on 18S rDNA sequences. Their results showed that the tested strains of B. braunii could be divided into three main clusters which matched their races well, indicating that there is a relationship between the genetic lineage and hydrocarbon production in B. braunii (Kawachi et al., 2012). Thus, race can be determined by analysis of B. braunii 18S rDNA sequences.
According to metabolite analyses, race A mainly produces unbranched straight-chain n-dienes and trienes with an odd number of carbons, ranging from C22-C33 and shorter n-alkenes (Metzger et al., 1990). These hydrocarbons are synthesized by an extension process followed by a decarboxylation step in the fatty acid synthesis pathway. Race B produces mainly triterpenoids, including branched isoprenoids with carbon numbers of C30-C37 (referred to as Botryococcene) and squalenes with C31-C34 (Hillen et al., 1982). Race L produces mainly C40-C78 lycopadiene with a benzene ring or a heterocyclic chemical group on the side chain. The synthesis of lycopadiene is hypothesized to occur via the tail-to- tail combination of two phytyl groups (Wake and Hillen, 1980). Among these races, A and B have higher hydrocarbon content, and thus higher potential for industrial application, than race L.
Both botryococcenes and methylsqualenes are synthesized by the mevalonate-independent pathway from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are produced by the mevalonate and methylerythritol 4-phosphate (MEP) pathways, respectively (Liao et al., 2006). Synthesis of IPP and DMAPP is followed by triterpene synthesis. Previous studies have demonstrated that the MEP pathway is the major route for oil biosynthesis in race B (Sato et al., 2003; Ioki et al., 2012). The enzyme 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase (MCS) participates in the synthesis of IPP and DMAPP precursor. The first step of the MEP pathway is catalyzed by 1-deoxy-D-xylulose 5-phosphate synthase (DXS), and B. braunii is known to possess three isoforms of this enzyme (Matsushima et al., 2012). Dimethylallyltranstransferase (DLS) and squalene synthase (SQS) catalyze the subsequent triterpene synthesis. Ioki et al believed that the reactions catalyzed by these enzymes were rate- limiting steps in oil biosynthesis by race B (Ioki et al., 2012). Many studies have confirmed that both the lipid and hydrocarbon contents of B.braunii increase under nitrogen deficient growth conditions (Singh and Kumar, 1992; Choi et al., 2011; Fang et al., 2015). Therefore, real-time PCR quantification was performed to determine the expression levels of the four hydrocarbons biosynthesis-related genes at different culture times under nitrogen replete and nitrogen depleted conditions. Determining the expression of these enzyme genes is useful for understanding the hydrocarbon synthesis pathway in B. braunii Abt02.
2 MATERIAL AND METHOD 2.1 Strain and culture manipulationThe experimental strain B. braunii Abt02 was isolated from Fuxian Lake, Yunnan Province, China. The strain was cultured in BG-11 medium at 24±1℃ and with a light-dark cycle of 12 h:12 h, with a light intensity of 50 μmol/(m2·s). The nitrogen-depleted treatment was carried out by culturing algae in BG-11 medium without nitrogen. The OD680 of cultured B. braunii Abt02 (3 mL) was measured every five days and the values used to generate growth curves. Simultaneously, B. braunii Abt02 biomass generated under both growth conditions was determined by centrifuging and drying 200 mL of culture every five days.
2.2 Microscopic analysisThe cell morphologies, cell sizes (length, width, and aspect ratio), and colony status of algae in the various solutions were determined at different growth stages using an Olympus microscope (BX53, Olympus Co. Tokyo, Japan). Nile red (AAT-22190, AAT Bioquest Inc. America) solution was added to the B. braunii Abt02 suspension at a final concentration of 50 μg/mL for 5 min, after which the cells were washed with the PES three times. Following this, a fluorescent microscope (Eclipse 80i, Niko Co. Tokyo, Japan) was used to observe cells status and oil drops in cultures of different ages.
2.3 PCR amplificationThe genomic DNA of B. braunii Abt02 was extracted using a DNA extraction kit (Tiangen, Tiangen Biotech Co. Ltd., Beijing, China), and the 18S rDNA sequence was amplified using the following primers (F: 5'-ACGCTTGTCTCAAAGATTA-3'; R: 5'-ACGGAAACCTTGTTACGA-3') (Metzger et al., 1990). PCR amplification conditions were as follows: a 94℃ denaturation cycle for 10 min; 94℃ denaturation for 1 min, 55℃ annealing for 45 s, 72℃ extension for 30 s repeated for 30 cycles; and a final extension cycle at 72℃ for 5 min. The PCR product was sequenced by the Sanger method and the 18S gene of B. braunii Abt02 was deposited in GenBank (MH378414).
2.4 DNA sequence-based phylogenetic analysisThe resulting sequences were spliced and analyzed using SeqMan software, and a phylogenetic tree constucted using reference sequences from known races of B. braunii Abt02 and two outgroup strains belonging to Choricystis sp. (accession numbers AY195970 and AY197629). Multiple sequence alignment was performed using ClustalX 1.83 (Larkin et al., 2007). The maximum likelihood (ML)-based phylogenetic tree was constructed using a General Time Reversible model in Mega 6 (Tamura et al., 2013). For ML method, bootstrap support for each node was calculated using 1 000 replicates
2.5 Hydrocarbon content and composition analysis 2.5.1 Gravimetric analysis of cell dry weightAlgae at different growth stages were collected for cellular dry weight measurements. Firstly, 200 mL of B. braunii Abt02 of each sample was harvested into 50 mL centrifugal tubes. Tubes were centrifuged at room temperature at 12 000 r/min for 5 min and the supernatant was discare. The pellets were washed thrice with PES, after which the pellets were freeze-dried.
2.5.2 GC-MS analysis of hydrocarbonsFor hydrocarbon contents analysis, freeze-dried B. braunii Abt02 was homogenized with a mortar and pestle and then incubated in n-hexane (H1013C, Spectrum, America) for 15 min. Following this, the extractions were centrifuged at 5 000 r/min for 10 min. The extraction process was repeated twice. Then, the supernatants were pooled together and evaporated under a stream of nitrogen until dry. The resultant crude algal hydrocarbons were dissolved in an appropriate amount of n-hexane, and 1 μL of this solution was subjected to gas chromatography mass spectrometry (GC-MS) analysis (Agilent 7980A-5975C, Agilent Co, America). The column used for the analysis was the HP-5MS (30 m×250 μm×0.25 mm), the chromatographic carrier gas was helium, the inlet temperature was 300℃, the split ratio was 20:1, the initial temperature was 150℃ for 1 min, and rose to 300℃ at a rate of 15℃ per min. The scan range was 50.0 to 500.0 m/z, the ion source temperature was 230℃, and the MS quadrupole temperature was 150℃.
2.6 qRT-PCR analysisReal-time PCR was carried out to examine transcript accumulation levels for hydrocarbon biosynthesis-related enzymes (DXS, MCS, DLS, SQS). Three biological replicates were performed for each treatment at different culture times (lag phase, log phase, early stationary growth phase, late stationary growth phase) under nitrogen-replete and nitrogen-depleted growth conditions, respectively. The expression levels of tubulin and actin were used as the standard for normalization. For each gene, the primers were optimized based on the melting curve of the PCR product as well as the PCR amplification efficiencies calculated using a cDNA dilution series. The cDNA was synthesized from total RNA (1 000 ng/ reaction) with an oligo (dT) primer using a First Strand cDNA Synthesis Kit. The real-time PCR reactions were performed using LightCycler480® SYBR Green I Master Mix (4707516001, Roche Applied Science, America). The 2-ΔΔCt method was used to calculate relative gene expression values.
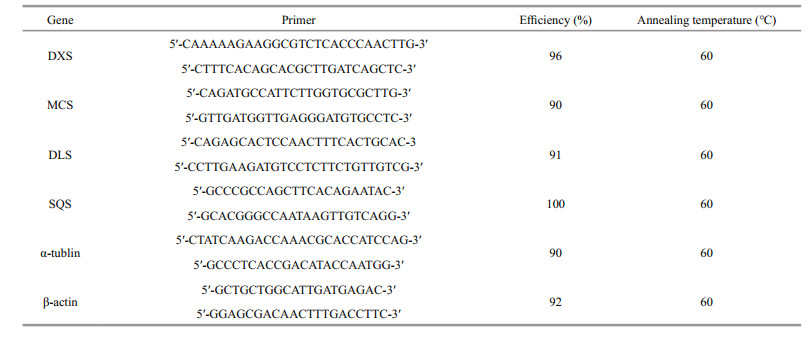
3 RESULT 3.1 Morphological characteristicsThe algal colony sizes of B. braunii Abt02 ranged from 10-100 microns. Microscopic observation revealed that small algal colonies were petal-like or fan-shaped, whereas the large algal colonies were composed of several, mainly petal-like, smaller colonies, surrounded by substantial quantities of gelatinous substances. Individual cells of B. braunii Abt02 were pear-shaped, and their size was on average 9.61±3.03 μm in width, 14.27±1.09 μm in length, and with an aspect ratio of 1.56±0.39. During the exponential growth phase, B. braunii Abt02 colonies appeared green in color (Fig. 1a), while at the stationary phase their color turned brown due to the carotenoid accumulation. When the osmotic pressure was changed, oil droplets and other contents from within the algal cells were released, and the cells turned a lighter shade of green (Fig. 1b-d). After Nile red staining, peripheral orange fluorescence from aliphatic hydrocarbon components was observed, while the intermediate crosslinked substances showed golden yellow fluorescence due to the presence of pigments and polysaccharides (Fig. 1e-q).
|
| Fig.1 Morphological characters of B. braunii Abt02 under the microscope |
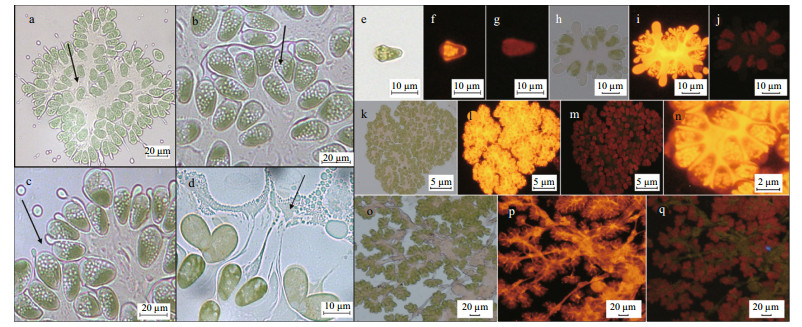
The biomass and growth curves of B. braunii Abt02 were determined under nitrogen-depleted and nitrogen-replete growth conditions. Our results showed that the growth rate and biomass of Abt02 under nitrogen-depleted conditions were lower than that under nitrogen-replete conditions. The lag phase under nitrogen depletion lasted for approximately 5 days, but only 3 days under normal culture conditions. After 5 days of cultivation, the growth rate and biomass of algae grown under nitrogen-depleted conditions were significantly lower than in cultures grown under nitrogen-replete conditions. For example, after 15 days of growth the OD680 value of cultures grown under nitrogen-depleted and nitrogen-replete conditions were 0.53 and 0.17, respectively, and the biomass of nitrogen-replete algae was 2.3 times that of algae grown without nitrogen. Furthermore, under normal culture conditions the average growth rate was 0.120 μ, but only 0.052 μ under conditions of nitrogen depletion (Fig. 2).
|
| Fig.2 Growth curve and biomass of B. braunii Abt02 under nitrogen deplete and nitrogen replete growth conditions |
Analysis of sequencing results showed that the 1 747 bp 18S rDNA sequence of B. braunii Abt02 was highly similar to the reference sequences in the database. In particular, it had 99% similarity to Bot30- 1, a race B strain. The Abt02 18S rDNA sequence, along with the database sequences of known braunii strains andthe two out-group strains of Choricystis sp. were used to construct a phylogenetic tree (Fig. 3). The results showed that: (1) the 32 B. braunii samples used in this study could be divided into three clades. Race A made up clade Ⅰ, race S and L were clustered into clade Ⅱ and race B belongs to clade Ⅲ; (2) in clade Ⅲ, 15 algal strains (including Abt02) clustered into subclade Ⅰ, while another 5 strains were clustered into subclade Ⅱ.

|
| Fig.3 Phylogenetic tree based on 18S rDNA sequences |
The hydrocarbon content of B. braunii Abt02 could reach as high as 43.75%±5.79% of the culture's dry mass. GC-MS analysis of B. braunii Abt02 showed that, despite this, only a limited variety of hydrocarbon compounds were present in this strain. Upon comparison with the mass spectrum library, these found to be four kinds of terpenes, with their retention times related to the complexity of their chemical structures and carbon atom number (Table 1). Following the order of appearance of their respective peaks, the compound with a retention time of 16.851 was a straight-chain two terpenoid geranylgeraniol (C20H34O). The compound with a retention time of 16.950 was squalene(C30H50), which is the characteristic component of the race B strain. The compounds with retention times of 17.158 and 17.317 were both hydroxylated squalene (C30H50O), with identical chemical formulas, but different conformations of structure. The first hydroxylated squalene was a squalene derivative, which comprised 77.49% of the total hydrocarbon content of the Abt02 strain, while the second hydroxylated squalene was a double bond cyclohexanol, proposedly formed through the cyclization of a straight-chain C30H50O.
 |
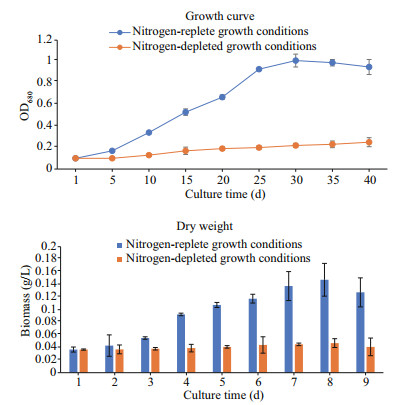
As one of the basic elements of nucleic acids and proteins, nitrogen plays a key role in controlling algal growth and composition. Studies have found that nitrogen deficiency can led to the decrease of the biomass, while can accelerate the hydrocarbon synthesis in B. braunii. By comparing the hydrocarbon composition of algal cultures, we found that crude hydrocarbon contents increased significantly with culture time (P < 0.05) under both nitrogen-replete and nitrogen-depleted conditions. When algae in lag to stationary growth phases were compared the crude hydrocarbon content increased, as a proportion of dry culture mass, from 16.71% to 43.75% under nitrogen- replete conditions and from 18.05% to 56.89% under conditions of nitrogen starvation. Statistical analysis showed no significant difference between the crude hydrocarbon contents of cultures grown under the different treatment regimens during log phase (P > 0.05), but a significant difference during lag phase (P < 0.05), and extreme differences during the stationary growth phase (P < 0.01) (Fig. 4). Analysis of peak areas showed a significant increase in squalene and its derivatives as culture time increased (P < 0.05). Furthermore, the amount of squalene and its derivatives increased from 78.26% to 83.32% under normal culture conditions and from 79.80% to 85.95% under conditions of nitrogen deficiency when algae in the lag to late stationary growth phases were compared. After equal culture periods, the amount of squalene and its derivatives produced during nitrogen- deficient culture was significantly higher than that produced under normal culture conditions (P < 0.05) (Fig. 5).
|
| Fig.4 Changer of crude hydrocarbon content of B. braunii Abt02 under different culture condition |

|
| Fig.5 Changer of Squalene and its derivatives content of B. braunii Abt02 under different culture condition |
The above result showed that the level of hydrocarbon accumulation under nitrogen-depleted condition was higher than that under nitrogen-replete condition. Therefore, the expression levels of the four hydrocarbons biosynthesis-related genes were determined during different growth phases under normal and nitrogen depleted conditions. The expression profiles of hydrocarbon biosynthesis- related genes were presented in Table 2. And the results of the real-time PCR experiment (Fig. 6) showed that there was no apparent difference in the expression levels of DXS and MCS between the control and treated groups during the lag phase. However, the expression levels of DLS and SQS in the treated groups were slightly lower than those in the control. It seems that there is an adaptive phase for the biosynthesis of hydrocarbons. During the log phase and early stationary phase, all four genes were expressed at higher levels, especially SQS, which expressed at 2.2-folds higher than that in control. Then on the late stationary phase, the expression levels of DXS and MCS increased while those of DLS and QSQ decreased but their absolute level remained to be higher than the control. The elevated expression levels of these four limiting-rate genes under conditions of nitrogen deficiency is beneficial to the accumulation of hydrocarbons.
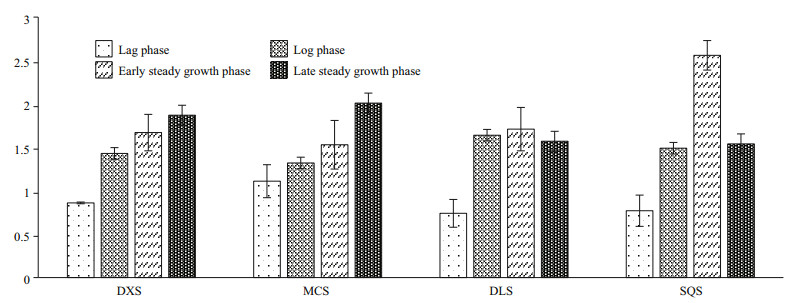
|
| Fig.6 The expression levels of the four hydrocarbons biosynthesis related genes in different culture time under nitrogen deficiency conditions |
Identification and classification of microalgae are a sound basis for biological research, and hydrocarbon content and composition are the key points of interest in the study of B. braunii. The correlation between algal strain phylogeny and its chemical composition is also a current research focus. Currently, morphological identification, chemical composition testing, and gene sequence analysis have been applied in the classification and identification of B. braunii (Wang et al., 2011). Liu et al studied the morphological characteristics of three strains of B. braunii, ZJU3001, ZJU3008, and ZJU3013, comparing the size and color differences in cells and colonies, classifying them as three strains (Liu, 2013). In the present study, the B. braunii Abt02 strain was found to be a size of (9.61±3.03) μm ×(14.27±1.09)μm and was brown during the stationary growth stage due to the accumulation of carotenoids, which indicates that this strain belongs to race B. However, due to the plasticity of microalgal cells, cellular morphology can change according to the environmental factors and the growth period. Therefore, strain classification based on morphology alone is unreliable.
Numerous studies have shown that 18S rDNA sequences are highly conserved and can be used for evolutionary and classification studies of strains from the same genus or at higher levels of taxonomy (Huss and Sogin, 1990). A phylogenetic analysis was performed using 18S rRNA gene sequences from nine B. braunii strains (five from race A, three from race B, and one from race L). They found that strains in each race were clustered into single clades, with the grouping revealing a close genetic relationship between the B and L races, which indicated a correlation between the evolutionary relationship of the 18S rDNA in B. braunii and their hydrocarbon production. This suggested the possibility of classifying B. braunii strains based on their 18S rDNA sequences (Schwender et al., 1996). Kawachi et al. analyzed the relationships between the 18S rDNA sequences from 31 strains of B. braunii, and suggested clustering currently known strains into at least two "species", the first correspondsing to race A and the second to races B + L. In addition, they recognized that the classification could be based on biochemical characteristics, such as the cellular hydrocarbon profile (Kawachi et al., 2012). Our results showed that strain Abt02 belongs to race B.
The hydrocarbon content of B. braunii Abt02 can reach up to 43.75% of cell dry weight during the log phase, which is higher than the total hydrocarbon content found in Maddingley (26.1%) and N-836 (13%) strains (Metzger et al., 1985), but lower than that found in Darwin and Berkeley strains (in which hydrocarbon content reached more than 50%) (Talukdar et al., 2013). Analysis of the hydrocarbon components of Abt02 by GC-MS showed that the algae was enriched in squalene and its hydroxylated derivatives, but not in botryococcenes. Squalene is an isoprene terpene whose synthetic precursor is IPP or DMAPP. Synthesis of IPP or DMAPP in plants takes place through two pathways, namely the mevalonate pathway (MVA) and 2-methyl-D-erythritol-4-phosphate pathway (MEP). The MEP pathway is the main pathway for the synthesis of botryococcene and squalene, which was established using 13C-labeled glucose (Schwender et al., 1996). The synthesized IPP or DMAP enters the terpenoid synthesis process and is then synthesized into farnesoid pyrophosphate (FPP) through a series of reactions. Subsequently, two molecules of FPP are condensed into precursor squalene pyrophosphate (PSPP) through the isopentenyl transfer reaction (Poulter, 1990). PSPP is the common precursor of both squalene and botryococcene (White et al., 1986, 1992). With NADPH as a hydrogen donor, rearrangement of the carboniumion and bond breaking at distinct locations in the PSPP molecule result in the formation of the squalene or botryococcene. Taking these common characteristics into account, it was proposed that the synthesis of both botryococcene and squalene are catalyzed by squalene synthase, whose structure can be adapted for the necessary reactions to synthesize the two products (Zhang and Poulter, 1995; Jarstfer et al., 1996). However, other studies suggested that the synthesis of these two compounds may involve two different enzymes (Niehaus et al., 2011; Ioki et al., 2012). Moreover, we found that Abt02 contained high levels of diterpenoid substances, such as geranylgeraniol, which has a wide range of physiological activities. Previous studies have reported that geranylgeraniol can arrest human prostate cancer cells DU145 in the G1 phase of the cell cycle and induce apoptosis, indicating that it has potential as an anti-tumor agent (Fernandes et al., 2013). Moreover, geranylgeraniol can be used to treat a variety of conditions, such as ulcers, neurasthenia, skin aging, thrombosis, atherosclerosis, and immune deficiency. Geranylgeraniol is also a precursor for the synthesis of other important compounds, such as terpenes, carotenoids, steroids, cholesterol, and taxol. Therefore, the Abt02 algal strain we have isolated in this study may be used for the preparation of terpenes for industrial purposes. According to the hydrocarbon component analysis, the Abt02 strain contained squalene and squalene derivatives, one of the characteristics of race B strains. It is consistent with the results of the molecular identification.
What's more, the researchers from all over the world have conducted extensive research on the conditions of growth and hydrocarbon production of B. braunii. Studies about nitrogen limitation and deficiency mainly include the change of physical characteristics and lipid composition (White et al., 1992; Zhang and Poulter, 1995; Schwender et al., 1996). We studied the expression levels of four hydrocarbons biosynthesis-related enzymes. They were all expressed at elevated levels during log growth under nitrogen deficient conditions. In particular, the increase in squalene synthase induced under nitrogen-deficient conditions can lead to an increased production of squalene and its derivative. By comparing the gene expressions levels of hydrocarbon synthesis gene in races A and B, Ioki et al speculated DXS and DLS may participate in hydrocarbon limiting step in the synthesis pathway (Ioki et al., 2012). We studied the DXS, MCS and DLS expression levels under the condition of nitrogen deficiency. During the lag phase, DXS, MCS and DLS gene expression did not differ significantly from that of the control. However, as the culture moved into the stationary phase, these genes were expressed at significantly higher levels than were seen in the normal control. Thus, it can be seen that nitrogen deficiency affects the hydrocarbon synthesis during the second growth phase, affecting the MEP pathway, which supported the DXS, MCS and DLS limiting step in hydrocarbon synthesis. The synthesis of hydrocarbon in B. braunii is a complex process, in which a large number of enzymes take part. Therefore, further research employing a variety of technologically advanced methodologies is required to fully understand the mechanisms underlying hydrocarbon synthesis.
5 CONCLUSIONIn this study, a B. braunii strain isolated from Fuxian Lake, China, was systematically analyzed. Microscopic observation revealed the individual cell sizes of B. braunii Abt02 to be, on average, 9.61± 3.03 μm in width and 14.27±1.09 μm in length. Fluorescent detection revealed a high quantity of aliphatic hydrocarbon components within the cells. Phylogenetic analysis based on 18S rDNA sequences assigned B. braunii Abt02 to race B. GC-MS analysis of B. braunii Abt02 showed that its hydrocarbon content can reach up to 43.75%±5.79% of total culture dry mass. Hydrocarbon composition analysis showed that the crude hydrocarbon content of cultures increased significantly under nitrogen-depleted conditions, especially during the lag and stationary growth phases. The elevated expression levels of four rate-limiting genes (DXS, MCS, DLS and SQS) under conditions of nitrogen limitation were conducive for accumulation of hydrocarbons. The results of study will inevitably prove useful information for future research on the industrial applicability of this strain.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during this the current are available from the corresponding author on reasonable request.
Banerjee A, Sharma R, Chisti Y, Banerjee U C. 2002. Botryococcus braunii:a renewable source of hydrocarbons and other chemicals. Critical Reviews in Biotechnology, 22(3): 245-279.
DOI:10.1080/07388550290789513 |
Brown A C, Knights B A, Conway E. 1969. Hydrocarbon content and its relationship to physiological state in the green alga Botryococcus braunii. Phytochemistry, 8(3): 543-547.
DOI:10.1016/S0031-9422(00)85397-2 |
Chiang I Z, Huang W Y, Wu J T. 2004. Allelochemicals of Botryococcus braunii (Chlorophyceae). Journal of Phycology, 40(3): 474-480.
DOI:10.1111/jpy.2004.40.issue-3 |
Choi G G, Kim B H, Ahn C Y, Oh H M. 2011. Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii. Journal of Applied Phycology, 23(6): 1031-1037.
DOI:10.1007/s10811-010-9636-1 |
Fang L, Sun D Y, Xu Z Y, He J, Qi S Y, Chen X, Chew W, Liu J H. 2015. Transcriptomic analysis of a moderately growing subisolate Botryococcus braunii 779(Chlorophyta) in response to nitrogen deprivation. Biotechnology for Biofuels, 8: 130.
DOI:10.1186/s13068-015-0307-y |
Fernandes N V, Yeganehjoo H, Katuru R, DeBose-Boyd R A, Morris L L, Michon R, Yu Z L, Mo H B. 2013. Geranylgeraniol suppresses the viability of human DU145 prostate carcinoma cells and the level of HMG CoA reductase. Experimental Biology and Medicine, 238(11): 1265-1274.
DOI:10.1177/1535370213492693 |
Hillen L W, Pollard G, Wake L V, White N. 1982. Hydrocracking of the oils of Botryococcus braunii to transport fuels. Biotechnology and Bioengineering, 24(1): 193-205.
DOI:10.1002/(ISSN)1097-0290 |
Huss V A R, Sogin M L. 1990. Phylogenetic position of some Chlorella species within the chlorococcales based upon complete small-subunit ribosomal RNA sequences. Journal of Molecular Evolution, 31(5): 432-442.
DOI:10.1007/BF02106057 |
Ioki M, Baba M, Bidadi H, Suzuki I, Shiraiwa Y, Watanabe M M, Nakajima N. 2012. Modes of hydrocarbon oil biosynthesis revealed by comparative gene expression analysis for race A and race B strains of Botryococcus braunii. Bioresource Technology, 109: 271-276.
DOI:10.1016/j.biortech.2011.11.078 |
Jarstfer M B, Blagg B S J, Rogers D H, Poulter C D. 1996. Biosynthesis of squalene. Evidence for a tertiary cyclopropylcarbinyl cationic intermediate in the rearrangement of presqualene diphosphate to squalene. Journal of the American Chemical Society, 118(51): 13089-13090.
|
Kawachi M, Tanoi T, Demura M, Kaya K, Watanabe M M. 2012. Relationship between hydrocarbons and molecular phylogeny of Botryococcus braunii. Algal Research, 1(2): 114-119.
DOI:10.1016/j.algal.2012.05.003 |
Larkin M A, Blackshields G, Brown N P, Chenna R, McGettigan P A, McWilliam H, Valentin F, Wallace I M, Wilm A, Lopez P, Thompson J D, Gibson T J, Higgins D G. 2007. Clustal W and Clustal X version 2.0. Bioinformatics, 23(21): 2947-2948.
DOI:10.1093/bioinformatics/btm404 |
Liao Z H, Chen M, Gong Y F, Miao Z Q, Sun X F, Tang K X. 2006. Isoprenoid biosynthesis in plants:pathways, genes, regulation and metabolic engineering. Journal of Biological Sciences, 6(1): 209-219.
DOI:10.3923/jbs.2006.209.219 |
Liu X Y. 2013. Studies on the Separation, Identification and Mutation Breeding of Botryococcus braunii. Zhejiang University, Hangzhou. (in Chinese)
|
Matsushima D, Jenke-Kodama H, Sato Y, Fukunaga Y, Sumimoto K, Kuzuyama T, Matsunaga S, Okada S. 2012. The single cellular green microalga Botryococcus braunii, race B possesses three distinct 1-deoxy-D-xylulose 5-phosphate synthases. Plant Science, 185-186: 309-320.
DOI:10.1016/j.plantsci.2012.01.002 |
Metzger P, Allard B, Casadevall E, Berkaloff C, Couté A. 1990. Structure and chemistry of a new chemical race of Botryococcus braunii (Chlorophyceae) that produces lycopadiene, a tetraterpenoid hydrocarbon. Journal of Phycology, 26(2): 258-266.
DOI:10.1111/j.0022-3646.1990.00258.x |
Metzger P, Berkaloff C, Casadevall E, Coute A. 1985. Alkadiene-and botryococcene-producing races of wild strains of Botryococcus braunii. Phytochemistry, 24(10): 2305-2312.
DOI:10.1016/S0031-9422(00)83032-0 |
Metzger P, Casadevall E, Coute A. 1988. Botryococcene distribution in strains of the green alga Botryococcus braunii. Phytochemistry, 27(5): 1383-1388.
DOI:10.1016/0031-9422(88)80199-7 |
Metzger P, Casadevall E. 1989. Aldehydes, very long chain alkenylphenols, epoxides and other lipids from an alkadiene-producing strain of Botryococcus braunii. Phytochemistry, 28(8): 2097-2104.
DOI:10.1016/S0031-9422(00)97927-5 |
Metzger P, Largeau C. 2005. Botryococcus braunii:a rich source for hydrocarbons and related ether lipids. Applied Microbiology and Biotechnology, 66(5): 486-496.
DOI:10.1007/s00253-004-1779-z |
Niehaus T D, Okada S, Devarenne T P, Watt D S, Sviripa V, Chappell J. 2011. Identification of unique mechanisms for triterpene biosynthesis in Botryococcus braunii. Proceedings of the National Academy of Sciences of the United States of America, 108(30): 12260-12265.
DOI:10.1073/pnas.1106222108 |
Poulter C D. 1990. Biosynthesis of non-head-to-tail terpenes.Formation of 1'-1 and 1'-3 linkages. Accounts of Chemical Research, 23(3): 70-77.
DOI:10.1021/ar00171a003 |
Sato Y, Ito Y, Okada S, Murakami M, Abe H. 2003. Biosynthesis of the triterpenoids, botryococcenes and tetramethylsqualene in the B race of Botryococcus braunii via the non-mevalonate pathway. Tetrahedron Letters, 44(37): 7035-7037.
DOI:10.1016/S0040-4039(03)01784-2 |
Schwender J, Seemann M, Lichtenthaler H K, Rohmer M. 1996. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate nonmevalonate pathway in the green alga Scenedesmus obliquus. Biochemical Journal, 316(Pt 1): 73-80.
|
Senousy H H, Beakes G W, Hack E. 2004. Phylogenetic placement of Botryococcus braunii (Trebouxiophyceae) and Botryococcus sudeticus isolate UTEX 2629(Chlorophyceae). Journal of Phycology, 40(2): 412-423.
DOI:10.1046/j.1529-8817.2004.03173.x |
Singh Y, Kumar H D. 1992. Lipid and hydrocarbon production by Botryococcus spp. under nitrogen limitation and anaerobiosis. World Journal of Microbiology and Biotechnology, 8(2): 121-124.
|
Talukdar J, Kalita M C, Goswami B C. 2013. Characterization of the biofuel potential of a newly isolated strain of the microalga Botryococcus braunii Kützing from Assam, India. Bioresource Technology, 149: 268-275.
DOI:10.1016/j.biortech.2013.09.057 |
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6:molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729.
DOI:10.1093/molbev/mst197 |
Tanoi T, Kawachi M, Watanabe M M. 2011. Effects of carbon source on growth and morphology of Botryococcus braunii. Journal of Applied Phycology, 23(1): 25-33.
DOI:10.1007/s10811-010-9528-4 |
Wake L V, Hillen L W. 1980. Study of a "bloom" of the oil-rich alga Botryococcus braunii in the Darwin River Reservoir. Biotechnology and Bioengineering, 22(8): 1637-1656.
DOI:10.1002/(ISSN)1097-0290 |
Wake L V, Hillen L W. 1981. Nature and hydrocarbon content of blooms of the alga Botryococcus braunii occuring in Australian freshwater lakes. Marine and Freshwater Research, 32(3): 353-367.
DOI:10.1071/MF9810353 |
Wang P Y, Mao Y X, Kong F N, Ma M, Ma F. 2011. Morphological and genetic diversity of Botryococcus braunii. Periodical of Ocean University of China, 41(5): 63-70.
(in Chinese) |
White J D, Somers T C, Reddy G N. 1986. The absolute configuration of (-)-botryococcene. Journal of the American Chemical Society, 108(17): 5352-5353.
DOI:10.1021/ja00277a054 |
White J D, Somers T C, Reddy G N. 1992. Degradation and absolute configurational assignment to C34-botryococcene. The Journal of Organic Chemistry, 57(18): 4991-4998.
DOI:10.1021/jo00044a039 |
Zhang D L, Poulter C D. 1995. Biosynthesis of non-head-totail isoprenoids. Synthesis of 1'-1 and 1'-3 structures by recombinant yeast squalene synthase. Journal of the American Chemical Society, 117(5): 1641-1642.
DOI:10.1021/ja00110a022 |
 2019, Vol. 37
2019, Vol. 37