Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SU Fang, LIU Jianguo
- The carotenoid characteristics of the important wild shrimp Trachysalambria curvirostris (Stimpson, 1860) in China
- Journal of Oceanology and Limnology, 37(2): 706-712
- http://dx.doi.org/10.1007/s00343-019-8018-z
Article History
- Received Jan. 25, 2018
- accepted in principle Apr. 4, 2018
- accepted for publication Apr. 27, 2018
2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China
The cocktail shrimp, Trachysalambria curvirostris (Stimpson, 1860) is a commercially important shrimp species that is widely distributed in the coastal waters of the Western Indo-Pacific (a range from Korea, Japan, China, Australia, Sri Lanka, India and the East Africa) (Cha et al., 2004). This shrimp is one of the most important target species of the shrimp fishery, which is responsible for almost one fifth of the total shrimp catches in China from 2010 to 2016, with annual harvests of more than 310 000 tonnes (Bureau of Fisheries of the Ministry of Agriculture of the People's Republic of China, 2017) and contributing over 50% of the total shrimp catch in Korea (Cha et al., 2004).
As a small shrimp, T. curvirostris is mainly used for producing shelled fresh shrimp and further processing to dried shrimp. The products made from T. curvirostris are popular among consumers because of their color, texture and superior flavor compared to similar products from other shrimps. Color, being the most important sensory attribute for consumers to evaluate product quality, has a direct effect on the price and marketability of shrimp products (Latscha, 1989). For example, in the Chinese market, the price of well-colored T. curvirostris dried shrimp is $10–24/kg USD higher than poorly colored products. However, there is still no information available on the pigment composition or distribution in this shrimp.
In addition, as most dried shrimp are traditionally prepared by sun drying or hot air drying, loss of color and other physical and sensory properties often occur during the drying process (Namsanguan et al., 2004; Niamnuy et al., 2008), but information about the pigment profile in dried shrimp is still limited.
This study is focused on the carotenoid profiles, including concentration, composition, molecular forms (free or esterified) and configuration (geometrical and optical isomers) in the raw and dried shrimp T. curvirostris, so as to better understand and utilize this bioresource.
2 MATERIAL AND METHOD 2.1 Animals and sample preparationThree lots (60 shrimps per lot) of wild adult T. curvirostris of both sexes (female: male=1:1) were caught off the west coast of China in February, 2017. The shrimp samples were placed in a styrofoam box with ice and transported to the Key Laboratory of Experimental Marine Biology of the Institute of Oceanology of the Chinese Academy of Sciences. All the shrimps were healthy and in their intermolt period. The gonads of the females were immature. The mean body length and weight of T. curvirostris were 12.26±0.17 cm, 13.32±0.63 g, respectively. Each lot shrimps were equally divided into two portions. One portion was fresh-processed to obtain the raw meat and waste (including head, shell and tail) samples and the remaining portion was used for dried meat preparation.
The raw meat and waste were weighed immediately to determine the yield of the different body parts (% of total body weight) and then were stored separately at -80℃ for later analysis.
Dried meat was prepared according to the method reported by Namsanguan et al. (2004) by boiling raw shrimp in NaCl solution and then drying in a hot air dryer at 50℃ until the average moisture reached 28%–30% before shucking the shell. The determination of moisture content was conducted according to AOAC procedures (AOAC, 1995).
The raw meat was well chopped and then homogenized before subsampling. Both the waste of raw shrimp and the dried meat samples were vacuum freeze dried and macerated before subsampling.
2.2 Measurement of total carotenoidsThe carotenoids in shrimp samples were extracted with acetone (AR grade) according to the modified method Johnston et al. (2000). The total carotenoid content was determined by using an ultraviolet-visible spectrophotometer (U-2900, HITACHI Co. Ltd., Japan) at 478 nm. The carotenoid content, expressed as mg carotenoid/kg dry weight, was calculated according to the following formula:
Carotenoid concentration (mg/kg)=(A478 nm×D)/ (0.22×W),
where A is the absorbance, D is the dilution multiple of the extract and 0.22 is absorbance at 478 nm of a 1-mg/kg astaxanthin standard and W is the dry weight of the sample, in grams.
2.3 Identification of major carotenoids by HPLCThe separation of carotenoids was performed on an Agilent 1200 HPLC system (Agilent Technologies Inc., CA, USA) with a Luna 3 μ Silica column (150 mm×4.6 nm, Phenomenex, USA) following a modified method described by Capelli and Cysewski (2007). An isocratic elution program with two solvents, 83:17 (v/v) hexane (HPLC grade, Merck & Co. Inc., Kenilworth, NJ, USA)/acetone (HPLC grade, Merck & Co. Inc., Kenilworth, NJ, USA), using a flow rate of 1.0 mL/min and an injection volume of 20 μL at 25℃. Carotenoids were identified at 478 nm based on their retention times in comparison with commercially available standards. Other carotenoids without standards were identified by comparing their retention times with published data (Schiedt K and Liaaen-Jensen, 1995; Capelli and Cysewski, 2007).
To determine the composition of free and esterified astaxanthin, the amount of free astaxanthin was measured both before and after hydrolysis of the carotenoid extract. Hydrolysis of astaxanthin esters was achieved by using the method of Wade et al. (2005).
Further separation of optical isomers of astaxanthin was performed according a modified method of Wang et al. (2008). Baseline separation of three stereomers of astaxanthin was achieved using a Chiralpack IC column (25 cm×4.6 cm, Daicel Chiral Technologies [China] Co. Ltd., China) with methyl tert-butyl ether and acetonitrile (35:65, v/v) at a flow rate of 1.0 mL/ min and measured at 470 nm at room temperature. Peaks were identified by comparing the retention times of sample peaks using (3S, 3'S) astaxanthin, racemic astaxanthin (1:2:1 mixture of the [3S, 3'S], [3R, 3'S] and [3R, 3'R] isomers) standards, purchased from CaroteNature (Lupsingen, Switzerland).
2.4 Statistical analysisTo verify differences in carotenoid values among different body parts, one factor analysis of variance (ANOVA) was employed using SPSS 19.0 statistical software (SPSS Inc, Chicago, IL, USA). For all tests, P < 0.05 was accepted as the level of statistical significance.
3 RESULT 3.1 Carotenoid concentration and composition in raw and dried shrimpThe raw shrimp biomass yielded 42.3% raw meat and 57.7% raw waste. The moisture content of these was 74.4% and 60.5%, respectively. Raw shrimp biomass yielded 15.3% dry meat, with a moisture content of 29.3% (Table 1). The total carotenoids in raw meat, waste, and dried meat were 49.69±2.85, 80.28±1.54 and 26.17±1.25 mg/kg dry weight tissue, respectively (Table 1), which are statistically significant differences (P < 0.001).

|
The composition of carotenoids in raw meat, waste and dried meat are given in Table 2. In the raw shrimp, astaxanthin and β-carotene were the two major carotenoids constituting 88.86% (meat)–75.18% (waste) and 2.86% (meat)–16.83% (waste) of the total carotenoids, respectively. Echinenone, canthaxanthin (waste only) and astacene (waste only) along with other unidentified carotenoids were also detected, although their levels were low. Compared to the raw materials, astaxanthin and β-carotene in the dried meat decreased significantly (P < 0.05). Astacene, undetected in the raw meat, appeared in the dried meat.

|
Astaxanthin in T. curvirostris exists in free and esterified forms. In all samples, the levels of esterified astaxanthin were always higher than the free form (Fig. 1). The highest degree of esterification was found in the raw meat (90.59%), whereas the lowest was in the dried meat (73.49%).

|
| Fig.1 The proportion (% of total astaxanthin) of free and esterified astaxanthin in the raw meat, waste and dried meat of T. curvirostris (by HPLC) Data are presented as mean±SD (n=3). |
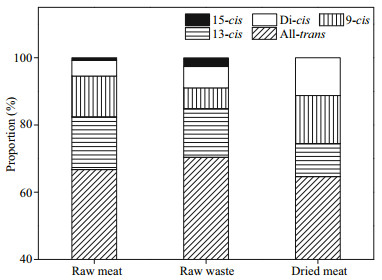
With respect to astaxanthin geometrical isomers (Fig. 2), all-trans was the predominant form followed by 13-cis, 9-cis, di-cis and 15-cis. All five geometrical isomers were detected in raw shrimp but 15-cis was not detected in dried meat. The highest ratio (70.38%) and the lowest ratio (64.64%) of all-trans astaxanthin were obtained in the waste and the dried meat, respectively. In raw shrimp, the 13-cis isomer was the predominant cis-astaxanthin constituting 14.45%-15.65% of the total astaxanthin. The ratio of 9-cis, di-cis and 15-cis astaxanthin varied around 6.21%-12.18%, 4.40%–6.71% and 0.82%–2.25%, respectively. In the dried meat, 9-cis astaxanthin was the highest cis isomer (14.30%) followed by the di-cis isomer (11.24%) and 13-cis isomer (9.83%).
|
| Fig.2 Composition (% of total astaxanthin) of astaxanthin geometrical isomers in raw meat, waste and dried meat of T. curvirostris All values are averages of three independent repetitions. |
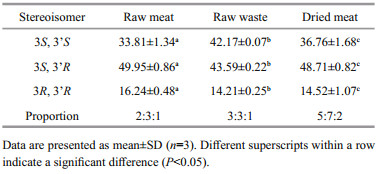
Results presented in Table 3 show that three stereoisomers, i.e. two enantiomers (3S, 3'S) and (3R, 3'R) and a meso form (3S, 3'R), were present in T. curvirostris. However, the levels of specific configurational isomers in this shrimp were significantly different (P < 0.001) in different parts. The (3R, 3'R) isomer, ranging from 4.21% to 16.24% of the total astaxanthin was always the lowest stereoisomer, and the meso (3S, 3'R) isomer was always the highest stereoisomer, no matter which part of the shrimp. However, proportions of the (3S, 3'S), (3S, 3'R) and (3R, 3'R) isomers varied greatly in the raw meat (2:3:1), waste (3:3:1) and dried meat (5:7:2).
|
The carotenoid content of shrimps is speciesspecific and varies in different body parts. For example, the total carotenoid content in the meat and waste were 17.4 and 66.65 mg/kg in P. monodon, 10.4 and 43.02 mg/kg in Penaeus indicus, 11.1 and 61.23 mg/kg in Metapenaeus dobsonii, 16.0 and 143.57 mg/kg in Parapenaeopsis stylifera (Sachindra et al., 2005), 15.9 and 76.66 mg/kg in Solonocera indica and 21.4 and 168.91 mg/kg in Aristeus alcocki (Manjabhat et al., 2006), respectively. This study indicated that the total carotenoid content in the meat of T. curvirostris is much higher than in the shrimps mentioned above and the level in the waste was comparable. In addition to the species-specific difference, the carotenoid content in wild shrimps may also influenced by sex, life stage, pathogens, different habitat, seasonal changes in food composition, etc. (Yanar and Yanar, 2004). Thus, a more detailed investigation of the biology of this wild shrimp is needed.
Like other shrimps (Mandeville et al., 1991; Sánchez-Camargo et al., 2011), the highest carotenoid content of this shrimp was observed in the waste, which comprised half of the total body weight. Combined with the statistical data (Bureau of Fisheries of the Ministry of Agriculture of the People's Republic of China, 2017), the production of T. curvirostris waste is around 180 000 tonnes per year in China and most of the waste is discarded as worthless trash. Further, calculation of the carotenoid content shows that nearly 9 tonnes high-value carotenoids (mostly astaxanthin) are discarded every year. Utilization of this waste would provide an excellent source of pigment and nutrients for animal feeds, increasing the profitability of the industry, in addition to contributing to the reduction of clandestine disposal in the environment.
Esterified astaxanthin is the dominant form of astaxanthin found in T. curvirostris. Similar distributions of astaxanthin and its esters have been reported by Sachindra et al. (2005) and Manjabhat et al. (2006) in various Indian shrimps. Previous studies reported that the percentage of esterified astaxanthin was 53.9%–68.1% in P. indicus, 63.8%–72.4.2% in P. monodon, 67.1%–76.8% in M. dobsonii, 46.2%-73.6% in P. stylifera (Sachindra et al., 2005), 65.4%-79.7% in S. indica, and 69.95%–82.4% in A. alcocki (Manjabhat et al., 2006). Compared to T. curvirostris, the amounts of astaxanthin esters reported in these shrimps was significantly lower. This may explain why the quality of products made from T. curvirostris is superior to those for other shrimps. For human consumption, foods or nutraceuticals with a higher percentage of astaxanthin esters can provide a significantly longer shelf life (Lorenz and Cysewski, 2000; Etoh et al., 2012).
In nature, the predominant geometric isomer of astaxanthin is the all-trans astaxanthin, the kinetically and thermodynamically most stable form (Bjerkeng et al., 1997). Cis isomers at the 9, 13 and 15 position are also observed (Rodriguez-Saiz et al., 2010). In Haematococcus pluvialis, the ratio of all-trans to cis isomers is nearly 3:1 and the main cis isomers were 9-cis and 13-cis astaxanthin (Yuan and Chen, 2000). The level of 13-cis astaxanthin detected in the raw P. vannamei was 2.25%(Yang et al., 2015).Cis isomers deposited in coho salmon (Oncorhynchus kisutch) comprise 3%–8% of total astaxanthins (Arai et al., 1987). In this study, all-trans, 9-cis, 13-cis, 15-cis and di-cis astaxanthin were detected, and constituted 30%-35% of the astaxanthin, which is a higher level than that reported for other species. Several studies have reported that cis astaxanthin isomers are selectively absorbed into human plasma (Østerlie et al., 2000) and have higher antioxidant potential in vitro and in vivo (Yang et al., 2017). Combining these studies with the results obtained in the current work suggests that T. curvirostris could be used as a natural source of cisastaxanthin-enriched functional food.
Astaxanthin contains two chiral carbons and therefore may be present in varies stereoisomeric forms (Britton, 1995). In general, astaxanthin from natural sources trends to occur predominantly as either the (3S, 3'S) or (3R, 3'R) form, while synthetic astaxanthin contains a mixture of the three isomers in approximately a 1:2:1 ratio (Turujman et al., 1997). Three stereoisomers were identified in T. curvirostris and the ratio of these isomers was similar to those found in previous studies on other shrimps in the Penaeidae, where the percentage of (3S, 3'S) astaxanthin ranged from 32% to 45%, meso astaxanthin 40%–50% and (3R, 3'R) astaxanthin 15%–30% (Matsuno et al., 1984; Latscha, 1989). However, this differs from the distribution in E. superba in the Euphausiidae, which contains over 99% of (3R, 3'R) astaxanthin and in Macrobrachium nipponense, Palaemon paucidens and Paratya compressa compressa in the Palaemonidae, which have over 99% of (3S, 3'S) astaxanthin (Matsuno et al., 1984). Little is yet known as to why shrimps in the Penaeidae have such high percentages of meso astaxanthin, or where this meso isomer originates.
4.2 Comparison the carotenoid profiles of raw and dried shrimp meatThe dried meat of T. curvirostris obtained after hot air drying, had a substantial loss of total carotenoids, including β-carotene and astaxanthin. Astacene, an oxidation product of astaxanthin and not found in raw meat, was detected in the dried meat, which indicates that the shrimp drying process not only caused some degradation of the original carotenoids but also produced by-products. The percentage of free astaxanthin was increased nearly 3-fold compared to that in raw shrimp. The dehydration process also led to isomerization of astaxanthin. The all-trans astaxanthin showed a slight decline after drying. The 9-cis astaxanthin increased and the 13-cis and 15-cis isomers decreased. The most remarkable change was the 2-fold increase of di-cis astaxanthin in the dried shrimp as compared to the raw shrimp. The optical patterns of astaxanthin in dried shrimp were also changed compared to the raw meat. The proportion of (3S, 3'S) isomer increased while those of meso and (3R, 3'R) isomers decreased. The severe degradation, hydrolysis and isomerization of carotenoids during the drying of shrimp may be due to the traditional hot air drying conditions. From the carotenoid protection perspective, new drying techniques should be able to minimize the hydrolysis of astaxanthin esters and isomerization of astaxanthin, thereby preserving the maximum amount of carotenoids in the dried shrimps.
5 CONCLUSIONTrachysalambria curvirostris contains a high concentration of carotenoids, with the predominant carotenoid being esterified astaxanthin. Five geometrical isomers, i.e. all-trans, 9-cis, 13-cis, 15-cis and di-cis astaxanthin, and three optical isomers, i.e. (3S, 3'S), (3S, 3'R) and (3R, 3'R) astaxanthin were observed. The study also revealed that the levels of carotenoids, degree of esterification of astaxanthin and ratio of different geometrical and optical isomers in the raw meat, waste and dried meat of T. curvirostris were significantly different. The high content of carotenoids in shrimp waste coupled with of the huge amount of unused shrimp biomass available, suggests this waste could be exploited for recovery of carotenoids (mainly astaxanthin esters) for use as pigment additives in feeds. Severe degradation and isomerization of carotenoids occurred during the drying process suggesting that the method of dehydrating shrimp should be carefully controlled, and improved, in the manufacturing of shrimp products.
6 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article
7 ACKNOWLEDGEMENTWe are also particularly indebted to Dr. John van der Meer, Pan-American Marine Biotechnology Association, for his assistance in proofreading.
Arai S, Mori T, Miki W, Yamaguchi K, Konosu S, Satake M, Fujita T. 1987. Pigmentation of juvenile coho salmon with carotenoid oil extracted from antarctic krill. Aquaculture, 66(3-4): 255-264.
DOI:10.1016/0044-8486(87)90111-6 |
Association of Official Analytical Chemists (AOAC). 1995. Official Methods of Analysis. 16th edn. Association of Official Analytical Chemists, Arlington, Virginia. p.13.
|
Bjerkeng B, Følling M, Lagocki S, Storebakken T, Olli J J, Alsted N. 1997. Bioavailability of all-E-astaxanthin and Z-isomers of astaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture, 157(1-2): 63-82.
DOI:10.1016/S0044-8486(97)00146-4 |
Britton G, Liaaen-Jensen S, Pfander H. 1995.Carotenoids.Volume 1A: Isolation and Analysis. Birkhäuser Verlag, Basel. p.94-95.
|
Britton G. 1995. Structure and properties of carotenoids in relation to function. FASEB Journal, 9(15): 1 551-1 558.
DOI:10.1096/fasebj.9.15.8529834 |
Bureau of Fisheries of the Ministry of Agriculture of the People's Republic of China (BFMOA). 2017. China Fishery Statistical Yearbook. China Agriculture Press, Beijing, China. p.15-46. (in Chinese)
|
Capelli B, Cysewski G. 2007. Astaxanthin Natural Astaxanthin: King of the Carotenoids. Cyanotech Corporation, KailuaKona, HI, USA. p.107-109.
|
Cha H K, Oh C W, Choi J H. 2004. Biology of the cocktail shrimp, Trachysalambria curvirostris (Decapoda:Penaeidae) in the Yellow Sea of Korea. Journal of the Marine Biological Association of the United Kingdom, 84(2): 351-357.
DOI:10.1017/S0025315404009270h |
Etoh H, Suhara M, Tokuyama S, Kato H, Nakahigashi R, Maejima Y, Ishikura M, Terada Y, Maoka T. 2012. Autooxidation products of astaxanthin. Journal of Oleo Science, 61(1): 17-21.
DOI:10.5650/jos.61.17 |
Johnston I A, Alderson R, Sandham C, Dingwall A, Mitchell D, Selkirk C, Nickell D, Baker R, Robertson B, Whyte D, Springate J. 2000. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture, 189(3-4): 335-349.
DOI:10.1016/S0044-8486(00)00373-2 |
Latscha T. 1989. The role of astaxanthin in shrimp pigmentation. In:Advances in Tropical Aquaculture. IFREMER, Tahiti, French Polynesia, 9: 319-325.
|
Lorenz R T, Cysewski G R. 2000. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends in Biotechnology, 18(4): 160-167.
DOI:10.1016/S0167-7799(00)01433-5 |
Mandeville S, Yaylayan V, Simpson B, Ramaswamy H. 1991. Isolation and identification of carotenoid pigments, lipids and flavor active components from raw commercial shrimp waste. Food Biotechnology, 5(2): 185-195.
DOI:10.1080/08905439109549801 |
Manjabhat S N, Narayan B, Subbanna M N. 2006. Carotenoids in Solonocera indica and Aristeus alcocki, deep-sea shrimp from Indian waters. Journal of Aquatic Food Product Technology, 15(2): 5-16.
DOI:10.1300/J030v15n02_02 |
Matsuno T, Maoka T, Katsuyama M, Ookubo M, Katagiri K, Jimura H. 1984. The occurrence of enantiomeric and meso-astaxanthin in aquatic animals. Nippon Suisan Gakkaishi, 50(9): 1 589-1 592.
DOI:10.2331/suisan.50.1589 |
Namsanguan Y, Tia W, Devahastin S, Soponronnarit S. 2004. Drying kinetics and quality of shrimp undergoing different two-stage drying processes. Drying Technology, 22(4): 759-778.
DOI:10.1081/DRT-120034261 |
Niamnuy C, Devahastin S, Soponronnarit S, Raghavan G S V. 2008. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. Journal of Food Engineering, 87(4): 591-600.
DOI:10.1016/j.jfoodeng.2008.01.013 |
Østerlie M, Bjerkeng B, Liaaen-Jensen S. 2000. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. The Journal of Nutritional Biochemistry, 11(10): 482-490.
DOI:10.1016/S0955-2863(00)00104-2 |
Rodriguez-Saiz M, de la Fuente J L, Barredo J L. 2010. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Applied Microbiology and Biotechnology, 88(3): 645-658.
DOI:10.1007/s00253-010-2814-x |
Sachindra N M, Bhaskar N, Mahendrakar N S. 2005. Carotenoids in different body components of Indian shrimps. Journal of the Science of Food and Agriculture, 85(1): 167-172.
DOI:10.1002/(ISSN)1097-0010 |
Sánchez-Camargo A P, Meireles M Â A, Lopes B L F, Cabral F A. 2011. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). Journal of Food Engineering, 102(1): 87-93.
DOI:10.1016/j.jfoodeng.2010.08.008 |
Schiedt K and Liaaen-Jensen S. 1995. Isolation and Analysis.In: Britton G, Liaaen-Jensen S, Pfander H eds.Carotenoids. Volume 1A: Isolation and Analysis., Birkhäuser Verlag, : Basel. p.81-107.
|
Stimpson W. 1860. Prodromus descriptionis animalium evertebratorum, quae in Expeditione ad Oceanum Pacificum Septentrionalem, a Republic Federata missa, Cadwaladore Ringgold et Johanne Rodgers Ducibus, observavit et descripsit. Pars Ⅷ, Crustacea Macrura. Crustacea Macrura.Proceedings of the Academy of Natural Sciences of Philadelphia, 1860: 22-47.
|
Turujman S A, Wamer W G, Wei R R, Albert R H. 1997. Rapid liquid chromatographic method to distinguish wild salmon from aquacultured salmon fed synthetic astaxanthin. Journal of AOAC International, 80(3): 622-632.
|
Wade N, Goulter K C, Wilson K J, Hall M R, Degnan B M. 2005. Esterified astaxanthin levels in lobster epithelia correlate with shell colour intensity:potential role in crustacean shell colour formation. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 141(3): 307-313.
DOI:10.1016/j.cbpc.2005.04.004 |
Wang C L, Armstrong D W, Chang C D. 2008. Rapid baseline separation of enantiomers and a mesoform of all-transastaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. Journal of Chromatography A, 1194(2): 172-177.
DOI:10.1016/j.chroma.2008.04.063 |
Yanar Y, Çelik M, Yanar M. 2004. Seasonal changes in total carotenoid contents of wild marine shrimps (Penaeus semisulcatus and Metapenaeus monoceros) inhabiting the eastern Mediterranean. Food Chemistry, 88(2): 267-269.
DOI:10.1016/j.foodchem.2004.01.037 |
Yang C, Zhang L F, Zhang H, Sun Q R, Liu R H, Li J, Wu L Y, Tsao R. 2017. Rapid and efficient conversion of all-Eastaxanthin to 9Z-and 13Z-isomers and assessment of their stability and antioxidant activities. Journal of Agricultural and Food Chemistry, 65(4): 818-826.
DOI:10.1021/acs.jafc.6b04962 |
Yang S, Zhou Q X, Yang L, Xue Y, Xu J, Xue C H. 2015. Effect of thermal processing on astaxanthin and astaxanthin esters in pacific white shrimp Litopenaeus vannamei. Journal of Oleo Science, 64(3): 243-253.
DOI:10.5650/jos.ess14219 |
Yuan J P, Chen F. 2000. Purification of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga Haematococcus pluvialis. Food Chemistry, 68(4): 443-448.
DOI:10.1016/S0308-8146(99)00219-8 |
 2019, Vol. 37
2019, Vol. 37


