Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MA Baoshan, NIE Yuanyuan, WEI Kaijin, XU Bin, ZHU Xiangyun, XU Jin
- Estimates on age, growth, and mortality of Gymnocypris firmispinatus (Cyprinidae: Schizothoracinae) in the Anning River, China
- Journal of Oceanology and Limnology, 37(2): 736-744
- http://dx.doi.org/10.1007/s00343-019-7372-1
Article History
- Received Dec. 6, 2017
- accepted in principle Feb. 14, 2018
- accepted for publication May. 10, 2018
2 Development Center for Animal Husbandry in Mountain Area in Miao Autonomous County of Songtao, Tongren 554100, China
The Anning River Basin is located in the transitional zone of the Qinghai-Tibet Plateau, Yunnan-Kweichow Plateau, and Sichuan Basin. The transitional zone maintains high fish diversity and plays significant role in the ecological security and ecological construction of Sichuan Province, China. Eco-environmental problems, such as minerals, hydropower, agriculture and animal husbandry resources, in the Anning River Basin pose a threat to the ecological security in the region (Xian, 2011). Based on our and other investigations in the field (Ru et al., 2016; Ma et al., 2017b), fish resource in the Anning River is severely threatened by intensive human activities, including damming, sand excavation, and overfishing. Compared with the previous studies, the number of fish species reduced from 82 to 52, the fish size became smaller and smaller, and daily fish catches were less than 10% of those captured in ten years ago (Ru et al., 2016). Thus, many researchers (Ru et al., 2016; Cao, 2017) strongly suggested that conservation of fish resources in the Anning River should be carried out as soon as possible.
Gymnocypris firmispinatus Wu et Wu, 1988 is a small-size Schizothoracinae fishes, only distributes in the Jinsha River and its tributaries (Chen and Cao, 2000), and mainly resides in the Anning River and its tributaries (Wang, 2008; Ru et al., 2016; Ma et al., 2017b). G. firmispinatus preferentially inhabits in fast-flowing water and the spawner often moves to nearby upstream for reproduction (Cao et al., 1981). Nevertheless, cascade hydropower development in the Anning River blocks the river continuity and destroys the ecological integrity, which might seriously impacts the propagative migration of adults and the feeding migration of juveniles. Therefore, G. firmispinatus had been listed as key protected animals in Sichuan province because of the decreased population.
In a word, the population of G. firmispinatus had gradually reduced with several anthropogenic activities, but some sound population management strategy has been hindered by lack of its basic biological studies. The available reports refer mostly to taxonomic characters and distribution (Chen and Cao, 2000), and acute toxicity (Xu et al., 2017). There have been few studies on the biology of G. firmispinatus. Ma et al. (2017a) compared three types of age identification material, and established the periodicity of annulus formation in otoliths and vertebrae. Ma et al. (2017b) reported the lengthweight relationship and fecundity of the fish. G. firmispinatus seemed to be a small Schizothoracinae fish with some life-history characteristics of slow growing, low fecundity, and late maturity (Chen and Cao, 2000).
Accurate estimates of age, growth, and mortality are very important for understanding population dynamics and provide fundamental data for fishery management (Campana and Thorrold, 2001). Many reports have pointed out that ages of Schizothoracinae fishes (including G. firmispinatus) can be determined reliably by otolith sections (Ma et al., 2011, 2017a; Huo et al., 2012; Zhou et al., 2017). Although the population of G. firmispinatus had decreased gradually mainly owing to the dam construction for over ten years, the growth parameters and mortality rates of the species were not estimated up to now. Therefore, the main goals of this study were to 1) estimate the age and growth parameters of G. firmispinatus by sectioned otoliths, and compare growth characters of the species with other congeneric fishes; 2) estimate the mortality rates by several empirical equations; 3) discuss the implications of our findings for conservation management of the G. firmispinatus population.
2 MATERIAL AND METHOD 2.1 Study area and sample collectionThis study was conducted in the Anning River, China, which is the biggest tributary in the lower reach of the Yalong River, with 326 km long at mainstream, 11 150 km2 of the area and 975 m of the average drop. The river belongs to the subtropical monsoon climate, with the mean annual air temperature of 17−19℃ and the annual rainfall of above 1 240 mm/a. The vegetation coverage along the river bank is very high (more than 80%), especially in spring and summer (Ning, 2009).
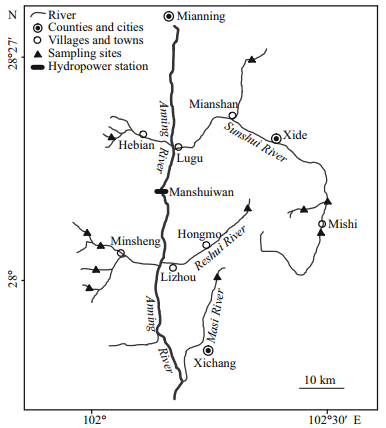
Our field investigates showed that G. firmispinatus seldom reside in the mainstream nowadays; instead, they mostly occur in the tributaries. Specimens were collected in the tributaries of the Anning River (Fig. 1), from 1 879.2 m to 2 509.0 m ASL. Usually, G. firmispinatus was the dominant species in these tributaries. Specimens (n=582) were collected monthly within the period between July 2015 and June 2016. Each month more than 30 specimens were captured using backpack electro-fishing gear (HailiBao, China, 12 V). The catches were transported in icebox to the laboratory for the subsequent analysis. Fish was euthanized with MS-222 and eviscerated immediately, and then total length (TL) and total weight (W) were measured to the nearest 1 mm and 0.1 g. Specimens were sorted separately by male, female, or undetermined by visual inspection of gonads. Lapilli were removed from each fish, cleaned with distilled water, and stored dry in labeled tubes.
|
| Fig.1 Sampling locations of G. firmispinatus from July 2015 to June 2016 in the Anning River, China |
A lapillus was mounted on a glass slide using transparent colorless nail polish, ground manually using wet sandpaper (1 000−2 500 grit) and polished with alumina paste (3 μm) until the core and most annuli were observed under a compound microscope. Then the mounting medium was dissolved with acetone, the otolith was remounted with the polished side facing the glass slide, and then ground and polished to expose the core and the growth increments (Ma et al., 2011). Age determination was made with an image analysis system (Jiseki ARP/W version 5.20, Ratoc System Engineering Company, Tokyo, Japan) using a microscope (Olympus BX51) and a computer connected with a CCD (Charge-coupled Device).
Each fish was assigned to an age class assuming 1 April as the birth date, which approximately corresponds to the peak spawning season and the annuli formation time (Granada et al., 2004; Qiu and Chen, 2009; Ma et al., 2017a; unpublished data). Annuli were interpreted without knowing the capture date, sex or length of all specimens. Each otolith was read twice, if the two counts differed, the lapillus was recounted, and two readings should be consistent at the end. The index of the average percent error (APE) was computed to evaluate the precision of the age estimations between two counts (Beamish and Fournier, 1981):
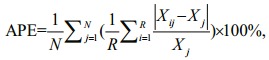
where N is the number of fish aged, R is the number of times each fish is aged, Xij is the ith age estimation of the jth fish, Xj is the mean age computed for the jth fish.
2.3 Growth modelingRelationships between weight and total length were evaluated using a power regression, expressed by W=aTLb. Differences of the slopes of the W-TL functions between sexes were detected by analysis of covariance (ANCOVA). Prior to analysis, all data were log-transformation (Cazorla and Sidorkewicj, 2008). The allometric index value (b) calculated from the equation was compared to the expected value "3" with a t-test for detecting the allometry of G. firmispinatus (Duan and Sun, 1999).
The von Bertalanffy growth function (VBGF) was applied to fit the otolith readings and observed length data of G. firmispinatus (von Bertalanffy, 1938). The formula was described as follow:

where Lt is the length at age t, L∞ is the theoretical maximum length, K is the growth coefficient, t is the age (a), and t0 is hypothetical age at length 0. The growth performance index (Ø) was computed by the formula: Ø=log10K+2 log10L∞ (Munro and Pauly, 1983). Differences of growth parameters between G. firmispinatus and other Gymnocypris species were performed by comparing their Ø calculated in this study and other literature reported.
2.4 Mortality estimationThe mortality rates (Z) were estimated using agebased catch curve analysis (Beverton and Holt, 1957; Chapman and Robson, 1960), where the fish number of each age class was log-transformed and plotted against their corresponding age. Then Z was evaluated from the descending slope (b) of a linear regression fitted to the data of the age frequency distribution.
The natural mortality (M) was evaluated by two empirical equations: Ralston's (1987) regression method M=0.0189+2.06K and M=-0.0021+2.5912/tmax (Zhan et al., 1986), where tmax is the maximum age in the catch.
The fishing mortality (F) was calculated by F=Z–M.
The exploitation ratio was estimated as:
E=F/Z.
According to Gulland (1971), the most suitable exploitation ratio for a population should be 0.5, i.e., the fishing mortality of the population should be equal its natural mortality.
2.5 Data analysisThe data are expressed as means±standard deviation (S.D.) and differences were regarded as 0.05 significant level. Analysis and image process were performed using Microsoft Excel 2010, SPSS 16.0, Origin Pro 2016, and Adobe Photoshop cc2015.
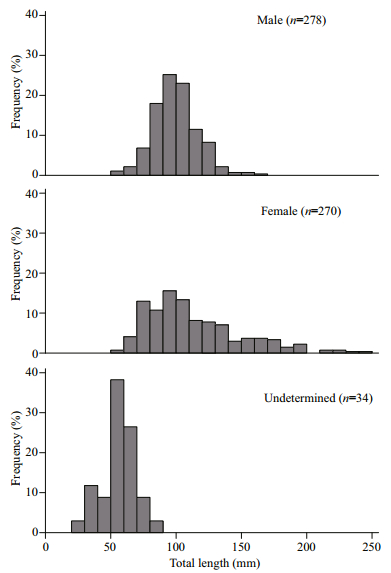
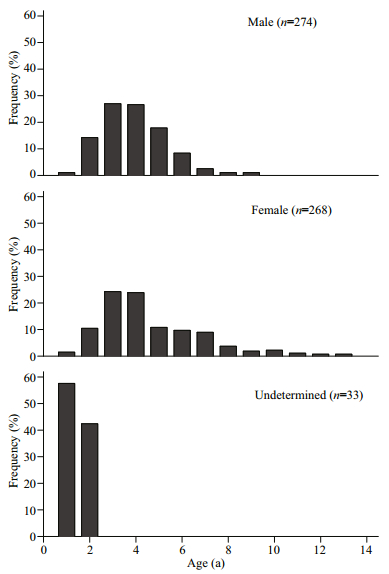
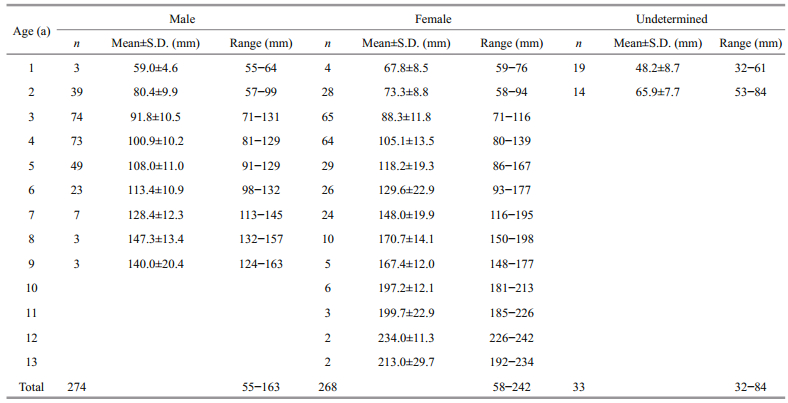
3 RESULT 3.1 Size distribution and W-TL relationshipThe TL ranged 27−242 mm, and W ranged 0.2−148.2 g. Of the 582 G. firmispinatus collected, 278 were males with 55–163 mm TL, 270 were females with 58−242 mm TL, and 34 were undetermined specimens with 27−84 mm TL. The proportion of males in the 80−120 mm TL group was substantially higher than that of females, whereas there were almost females in the >160 mm TL group (Fig. 2).
|
| Fig.2 Distributions of the total length frequency of G. firmispinatus from July 2015 to June 2016 in the Anning River |
The regression functions of W-TL relationships (Fig. 3) were expressed as W=1.010×10-5TL2.979 (R2=0.940, n=278) for males, W=7.605×10-6TL3.037 (R2=0.977, n=270) for females and W=8.051×10-5TL3.017 (R2=0.955, n=34) for the undetermined. No significant differences were found for W-TL relationships between males and females (ANCOVA, F=1.810, P>0.05), so the regression equation achieved from all specimens was described as W=7.754×10-6TL3.034 (n=582, R2=0.977). The allometric index value (b) did not significantly differ from 3 (t-test, t=1.758, P>0.05), indicating a tendency for isomeric growth in G. firmispinatus.

|
| Fig.3 Weight-length relationships of G. firmispinatus from July 2015 to June 2016 in the Anning River |
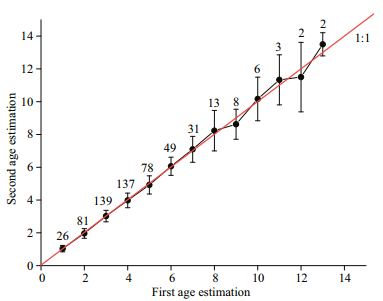
Because of otolith fragmentation and unidentifiable annulus deposition, there were 7 otoliths discarded (accounting for 1.2%). Overall, 274 males, 268 females, and 33 undetermined specimens were successfully aged (Fig. 4). The APE between 2 readings was 4.58%. The age bias for pairwise age comparisons between 2 readings was provided in Fig. 5. The age range observed in all specimens varied from 1 to 13 a (corresponding to 27–242 mm TL). The fish within 1–10 a were very common, accounting for 97.6%. The maximum ages of males and females were 9 a (140 mm TL) and 13 a (213 mm TL), respectively (Fig. 6).

|
| Fig.4 Sectioned lapillus under a compound microscope with transmitted light, from a 4-a-old G. firmispinatus with 120 mm TL Dots indicate annuli. |
|
| Fig.5 Age bias plot for pairwise age comparisons between two readings |
|
| Fig.6 Age frequency composition of G. firmispinatus from July 2015 to June 2016 in the Anning River |
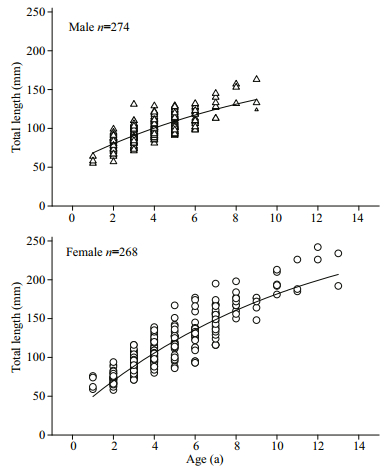
The mean length-at-age (±S.D.) values derived from otolith observations, classified as males, females, and the undetermined, are given in Table 1. For age class 1, the mean lengths-at-age had no significant difference between sexes (unpaired t-test, P>0.05); while in age classes 2–9, the mean lengths-at-age had significant differences between sexes (unpaired t-test, P < 0.05). The von Bertalanffy growth models fitted to the observed length and age data were provided as follows (Fig. 7):
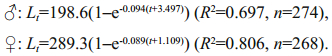
|
|
| Fig.7 Von Bertalanffy growth curve fitted to total length-atage for G. firmispinatus from samples captured |
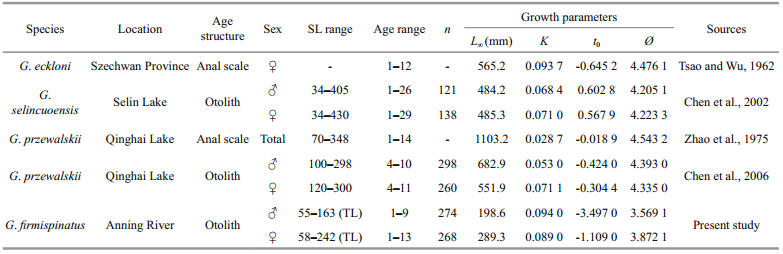
Growth parameters suggested that males grew at a relatively faster rate than females. The growth performances (Ø) of male and female G. firmispinatus were 3.569 1 and 3.872 1, respectively.
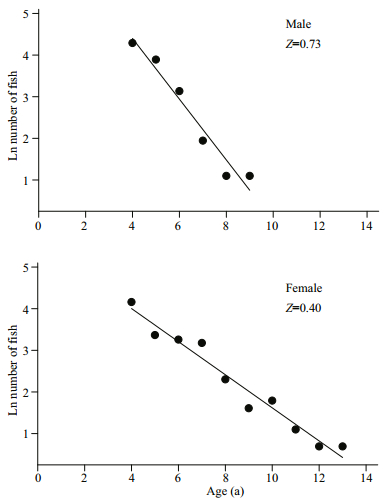
3.3 Mortality estimationThe estimates of Z from the descending slope of the catch curve were 0.73/a for males and 0.40/a for females (Fig. 8). The values of M evaluated from the equation of Ralston and Zhang were 0.21 and 0.29/a for males and 0.20 and 0.20/a for females. Therefore, M was assumed to be 0.25/a for males and 0.20/a for females, the corresponding F was estimated as 0.48/a for males and 0.20/a for females. The exploitation ratio was 0.66/a for males and 0.50/a for females, respectively.
|
| Fig.8 Catch-curve based on observed age for G.firmispinatus samples captured between July 2015 and June 2016 Ages 1–3 were excluded (outliers), because they were considered as not fully recruited to the electro-fishing gear. |
In this study, our results showed that the maximum ages estimated for G. firmispinatus were 9 a for males and 13 a for females, near with Gymnocypris przewalskii and Gymnocypris eckloni, but smaller than Gymnocypris selincuoensis (Table 2). When compared to other genus of Schizothoracinae fishes, G. firmispinatus is a shorter-lived fish [♂: 37, ♀: 40 for Schizothorax waltoni (Zhou et al., 2017), ♂: 24, ♀: 45 for Ptychobarbus dipogon (Li and Chen, 2009), ♂: 40, ♀: 50 for Schizothorax oconnori (Ma et al., 2010)] based on otolith observations. These longlived Schizothoracinae fishes only inhabit in the Yarlung Zangbo River, Tibet, where fishes were preserved relatively well due to religious sentiments. Nevertheless, the fishing intensity in the Anning River was much heavier than that in the Tibetan rivers. Thus, the exploitation level might be also a reason affecting the ecological longevity of the fish.
According to the length range of all catches, the asymptotic length (L∞) of G. firmispinatus was not attained, and L∞ for females was much higher than that of males. In addition, the results indicated that males grew smaller than females, while the K value for males (0.094) was slightly higher than that of females (0.089). G. firmispinatus grows slowly in a pattern that is shared with other Gymnocypris fishes (Table 2); this genus has K values ranging from 0.03 to 0.09. The L∞ of G. firmispinatus obtained in this study was the smallest, while k values derived here were higher than other congeneric fishes (except for G. eckloni). The growth performance indices (Ø) of the fish were smaller than those of other congeneric fishes (Table 2), revealing that the growth ofG. firmispinatus is relatively slower than others. The growth of G. firmispinatus might be affected by many factors such as food deficiency and environmental conditions in the small tributaries of the Anning River (Beamish and McFarlane, 1983).
Based on empirical evidence from comparative life history information, many methods have been reported in the last 70 a to evaluate the natural mortality rate (M) of a stock (Then et al., 2014). In the present study, we chose two methods to avoid the one-sided scheme of a single tmax-based estimator or single growth-based estimator. The exploitation ratio of G. firmispinatus was 0.66/a for males and 0.50/a for females, respectively. Therefore, the G. firmispinatus population may be over-exploited under the current harvesting strategy. The exploitation ratio of the species was similar with Oxygymnocypris stewarti in the Yarlung Zangbo River (Huo et al., 2015), but higher than those of P. dipogon in the Lhasa River (Li and Chen, 2009). At present, many Schizothoracinae fishes have been seriously threatened owing to overfishing, damming and biological invasions. Therefore, scientist and fishery department have both paid great attentions to the conservation of their natural populations (Huo et al., 2015).
Many researchers found that the anthropogenic activities mainly contributed to the declined population of G. firmispinatus in the Anning River (Wang, 2008; Ru et al., 2016). It seems that the species mostly inhabited in the tributaries, rather than mainstream, because of the river fragmentation induced by the construction of hydropower stations at the mainstream. Furthermore, the populations of slow-growing fish (such as G. firmispinatus) would be difficult to be restored if they were expired (Musick, 1999). It is therefore essential to establish effective management strategies for G. firmispinatus focused on a tolerable fishing intensity and prohibiting fishing during the spawning season, to allow for its sustainability. Specifically, some small-size hydropower stations in the mainstream of the Anning River should be reduced as much as possible, to insure more suitable habitats for G. firmispinatus.
5 CONCLUSIONIt was concluded that G. firmispinatus seem to be a small-size fish with relatively shorter longevity, when compared to other Schizothoracinae fishes. The growth of the fish is relatively slower than other congeneric fishes. Our results also indicated that the population of G. firmispinatus might be in overexploitation under the current harvesting strategy.
6 DATA AVAILABILITY STATEMENTAll data supporting the findings of this study are available within the article.
7 ACKNOWLEDGEMENTWe gratefully acknowledge LIANG Meng for help with the collection of the survey data. The authors also thank Prof. XIE Songguang (Institute of Hydrobiology, Chinese Academy of Sciences) for providing the otolith image analysis system.
Beamish R J, Fournier D A. 1981. A method for comparing the precision of a set of age determinations. Can. J. Fish.Aquat. Sci., 38(8): 982-983.
DOI:10.1139/f81-132 |
Beamish R J, McFarlane G A. 1983. The forgotten requirement for age validation in fisheries biology. Trans. Am. Fish.Soc., 112(6): 735-743.
DOI:10.1577/1548-8659(1983)112<735:TFRFAV>2.0.CO;2 |
Beverton R J H, Holt S J. 1957. On the Dynamics of Exploited Fish Populations. Fisheries Investigation Series 2, Volume 19, UK Ministry of Agriculture. Fisheries and Food, London, UK.
|
Campana S E, Thorrold S R. 2001. Otoliths, increments, and elements:keys to a comprehensive understanding of fish populations?. Can. J. Fish. Aquat. Sci, 58(1): 30-38.
DOI:10.1139/f00-177 |
Cao W X, Chen Y Y, Wu Y F, Zhu S Q. 1981. Origin and evolution of schizothoracine fishes in relation to the upheaval of the Xizang Plateau. In: Tibetan Expedition Team of the Chinese Academy of Science ed. Studies on the Period, Amplitude and Type of the Uplift of the Qinghai-Xizang Plateau. Science Press, Beijing. p.118-130. (in Chinese)
|
Cao W X. 2017. Issues of ecological protection facing cascade hydroelectric dam development in the upper reaches of the Yangtze River. In: Zhang C H, Wang G Q eds. The Frontiers of Water Science and Engineering (Part I).Science Press, Beijing. p.284-301. (in Chinese)
|
Cazorla A L, Sidorkewicj N. 2008. Age and growth of the largemouth perch Percichthys colhuapiensis in the Negro River, Argentine Patagonia. Fish. Res., 92(2-3): 169-179.
DOI:10.1016/j.fishres.2008.01.016 |
Chapman D G, Robson D S. 1960. The analysis of a catch curve. Biometrics, 16(3): 354-368.
DOI:10.2307/2527687 |
Chen D Q, Zhang X, Xiong F, Liu S P, Tang H Y. 2006. Studies on growth characteristics of Gymnocypris przewalskii przewalskii. Acta. Zool. Sin., 30(2): 173-179.
(in Chinese with English abstract) |
Chen Y F, Cao W X. 2000. Schizothoracinae. In: Yue PQ ed.Fauna Sinica Osteicthtyes Cypriniformes Ⅲ. Science Press, Beijing. p.273-388 (in Chinese).
|
Chen Y F, He D K, Cao W X, Duan Z H. 2002. Growth of Selincuo Schizothoracini (Gymnocypris selincuoensis) in Selincuo Lake, Tibeten Platean. Acta. Zool. Sin., 48(5): 667-676.
(in Chinese with English abstract) |
Duan Z H, Sun J Y. 1999. Studies on the age and growth of Pelteobagrus vachelli (Richardson). Acta Hydrobiol. Sin., 23(6): 617-623.
(in Chinese with English abstract) |
Granada V P, Masuda Y, Matsuoka T. 2004. Age and growth of the yellowbelly threadfin bream Nemipterus bathybius in Kagoshima Bay, southern Japan. Fish. Sci., 70(3): 497-506.
DOI:10.1111/fis.2004.70.issue-3 |
Gulland J A. 1971. The fish resources of the ocean. Fishing News, West Byfleet.Huo B, Ma B S, Xie C X, Duan Y J, Yang X F, Huang H P. 2015. Stock assessment and management implications of an endemic fish, Oxygymnocypris stewartii, in the Yarlung Zangbo River in Tibet, China. Zool. Stud., 54: 53.
|
Huo B, Xie C X, Ma B S, Yang X F, Huang H P. 2012. Age and growth of Oxygymnocypris stewartii (Cyprinidae:Schizothoracinae) in the Yarlung Tsangpo River, Tibet, China. Zool. Stud., 51(2): 185-194.
|
Li X Q, Chen Y F. 2009. Age structure, growth and mortality estimates of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae:Schizothoracinae) in the Lhasa River, Tibet. Environ. Biol. Fish., 86(1): 97-105.
DOI:10.1007/s10641-008-9371-5 |
Ma B S, Nie Y Y, Wei K J, Xu B, Gan W X, Zhu X Y, Xu J, Deng L J, Yao Y H. 2017a. Precision of age estimations from otolith, vertebra, and opercular bone of Gymnocypris firmispinatus (Actinopterygii:Cypriniformes:Cyprinidae)in the Anning River, China. Acta Ichthyol. Piscat., 47(4): 321-329.
DOI:10.3750/AIEP/02219 |
Ma B S, Xie C X, Huo B, Yang X F, Huang H P. 2010. Age and growth of a long-lived fish Schizothorax o'connori in the Yarlung Tsangpo River, Tibet. Zool. Stud., 49(6): 749-759.
|
Ma B S, Xie C X, Huo B, Yang X F, Li P. 2011. Age validation, and comparison of otolith, vertebra and opercular bone for estimating age of Schizothorax o'connori in the Yarlung Tsangpo River, Tibet. Environ. Biol. Fish., 90(2): 159-169.
DOI:10.1007/s10641-010-9727-5 |
Ma B S, Xu B, Wei K J, Deng L J, Gan W X, Zhu X Y, Yao Y H. 2017b. Length-weight and length-length relationships of four native fish species from the Yalong River, China. J. Appl. Ichthyol., 33(4): 839-841.
DOI:10.1111/jai.2017.33.issue-4 |
Munro J D, Pauly D. 1983. A simple method for comparing the growth of fishes and invertebrates. Fishbyte, 1(1): 5-6.
|
Musick J A. 1999. Ecology and conservation of long-lived marine animals. In: Life in the Slow Lane: Ecology and Conservation of Long-lived Marine Animals: Proceedings of the Symposium Conservation of Long-Lived Marine Animals. California, USA.
|
Ning X. 2009. Studies on characteristics of annual runoff in Anning River. Sichuan Normal University, Chengdu, China. (in Chinese with English abstract)
|
Qiu H, Chen Y F. 2009. Age and growth of Schizothorax waltoni in the Yarlung Tsangpo River in Tibet, China. Ichthyol. Res., 56(3): 260-265.
DOI:10.1007/s10228-009-0096-z |
Ralston S. 1987. Mortality rates of snappers and groupers. In: Polovina J J, Ralston S eds. Tropical Snappers and Grouper: Biology and Fisheries Management. Westview Press, Boulder, CO. p.375-404.
|
Ru H J, Zhang Y, Li Y F, Wang H M, Shen Z W, Wu X X, Li R, Sheng Q, Ni Z H. 2016. Community Composition and Status of Fish Resources in Anning River. J. Hydroecol., 37(5): 68-74.
(in Chinese with English abstract) |
Then A Y, Hoenig J M, Hall N G, Hewitt D A. 2014. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci., 72(1): 82-92.
|
Tsao W X, Wu X W. 1962. An investigation of the fish biology and fishery problems in Ganze-Apa region of western Szechwan Province. Acta Hydrobiol. Sin., (2): 79-111.
(in Chinese) |
von Bertalanffy L. 1938. A quantitative theory of organic growth (inquiries on growth laws Ⅱ). Human Biol., 10(2): 181-213.
|
Wang Q. 2008. Investigation on the fisheries resources in the Yalongjiang River within Liangshan Yi Autonomous Prefecture section. Sichuan Agricultural University, Chengdu, China. (in Chinese with English abstract)
|
Xian W. 2011. Analysis of ecological environment based on remote sensing and assessment of ecological vulnerability.Graduate School of Chinese Academy of Sciences, Beijing, China. (in Chinese with English abstract)
|
Xu B, Nie Y Y, Wei K J, Deng L J, Gan W X, Ma B S, Zhu X Y, Xu J. 2017. Acute toxicity of four pesticides to Gymnocypris potanini firmispinatus. Freshw. Fish., 47(2): 86-90, 106.
(in Chinese with English abstract) |
Zhan B Y, Lou D C, Zhong J S. 1986. An assessment of the filefish population and rational exploitation of the resource. J. Fish. China, 10(4): 409-418.
(in Chinese) |
Zhao L H, Wang S H, Zhao T Q. 1975. The age and growth of Gymnocypris przewalskii przewalskii (Kessler). In: Institute of Biology of Qinghai Province ed. The fish fauna of Qinghai Lake Region and Biology of Gymnocypris przewalskii przewalskii (Kessler). Science Press, Beijing. p.37-45. (in Chinese)
|
Zhou X J, Xie C X, Huo B, Duan Y J, Yang X, Ma B S. 2017. Age and growth of Schizothorax waltoni (Cyprinidae:Schizothoracinae) in the Yarlung Tsangpo River, China. J.Appl. Anim. Res., 45(1): 346-354.
DOI:10.1080/09712119.2016.1194842 |
 2019, Vol. 37
2019, Vol. 37