Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Bojian, NIU Cuijuan, YUAN Lin, ZHANG Wenyi
- Physiological responses in vitamin C system during hibernation in juvenile Chinese soft-shelled turtle Pelodiscus sinensis
- Journal of Oceanology and Limnology, 37(2): 767-776
- http://dx.doi.org/10.1007/s00343-019-7345-4
Article History
- Received Dec. 13, 2017
- accepted in principle Apr. 11, 2018
- accepted for publication May. 10, 2018
2 Ministry of Education Key Laboratory for Biodiversity Science and Ecological Engineering, College of Life Sciences, Beijing Normal University, Beijing 100875, China
Animals that live at high latitudes must be able to escape or tolerate extended periods of freezing temperatures in winter. Freshwater turtles escape these freezing periods by hibernating aquatically, taking shelter in the mud underneath water (Zhang et al., 2016). Adaptive metabolic depression, lactic acid buffering via shell exploitation, and many other physiological processes help turtles to withstand long periods of hibernation under water (Reese et al., 2001, 2004; Jackson and Ultsch, 2010). However, the reduced cardiovascular circulation, suspended air breathing, and reduced extra-pulmonary oxygen uptake during hibernation may result in ischemic and hypoxic conditions in tissues (Ultsch, 1989; Jackson, 2000). When rewarming from hibernation in spring, the upsurge in oxygen consumption and aerobic metabolism, together with the ischemia-reperfusion, may result in a burst of reactive oxygen species (ROS), which lead to oxidative stress in tissues (Hermes-Lima et al., 2001; Storey, 2006; Bickler and Buck, 2007). However, empirical studies have reported that freshwater turtles can tolerate this severe ROS generation without significant oxidative damage (Rice et al., 2002; Baker et al., 2007). Hence, the regulation of the antioxidant system that balances ROS in turtles has become an area of research interest (Storey, 2007). Most previous studies on this topic have focused on gene and enzyme regulation under extreme conditions, i.e. freezing tolerance during hibernation in higher-latitude species (Storey, 2006; Krivoruchko and Storey, 2010). Relatively few studies have focused on Southeast Asian originated turtles, such as the Chinese soft-shelled turtle Pelodiscus sinensis, which usually hibernates at temperatures above freezing (approximately 5℃; Zhang et al., 2016, 2017b).
Low molecular weight antioxidants, which consist of ROS scavengers such as glutathione, uric acid, and ascorbic acid (vitamin c, Vc) serve as major components of animals' antioxidant defense system (Davies, 2000; Venditti et al., 2010). Among these antioxidants, Vc has been reported to play a vital role in protecting tissues from ischemia-reperfusion during hibernation in euthermic animals (Drew et al., 2002; Ma et al., 2004). Vitamin C is abundant in turtles, suggesting that it is a key component of their antioxidant system (López-Torres et al., 1993; Rice et al., 1995). In our previous study on soft-shelled turtle P. sinensis, we found that the synergistic effects of the extraordinary Vc synthesis capacity, strong plasma Vc transport potential, and high-level tissue-specific Vc storage enabled these turtles to endure severe acute thermal fluctuations without apparent tissue oxidative damage (Chen et al., 2015). Zhang et al. (2016, 2017b) examined stress responses of the antioxidant enzyme system and glutathione system of the soft-shelled turtle hatchings during hibernation. However, how the Vc system responds to environmental stress during natural hibernation remained unclear.
In China, P. sinensis is a popular farmed species with a high economic value. More and more turtle farms are raising turtles according to their natural habit, that is, letting the turtles hibernate in field ponds during the winter instead of being reared in a greenhouse (hibernation could last for more than five months in northern area). Mortality can be high (above 20% or even higher than 40% in some conditions; Liu and Tan, 1997; Zhang et al., 2017a) during arousal of the turtles in the spring, and is thought to be due to weakness resulting from an extended period of no food intake, stress, and suppression of the immune system during hibernation (Jing et al., 2011). Knowledge of the physiological responses of turtles during hibernation will provide important data for turtle farming. Furthermore, the synergistic effort of Vc system response protected the Chinese soft-shelled turtle from acute cold stress, however the fast and significant decrease of Vc concentration in brain (Vc concentration decreased 30.1% in 8 h; Chen et al., 2015) suggests such coping regulation might not be sufficient for prolonged cold conditions, i.e., winter hibernation (though hibernation might influence more comprehensively than merely work as a simple prolonged cold exposure for turtles). Thus, we tend to predict that the Vc system in Chinese soft-shelled turtle may response differently from acute cold stress during long term hibernation.
The purpose of this study was to investigate physiological responses of the Vc system in Chinese soft-shelled turtles during hibernation. Protein synthesis is considered one of the most energy consuming activities in all cells (Hochachka et al., 1996), hence keeping a decreased metabolism (global suppression of transcription and translation is a critical part of the metabolic rate depression; see in Storey, 2004; Storey and Storey, 2004) and reduced energy expenditure may contribute greatly for long term survival in turtles do not experience freezing during hibernation. Hence we predicted that the mRNA and/or enzyme activity level of key enzymes in Vc system may decrease during hibernation (prediction 1). Thusly, we measured the mRNA level of the gene encoding L-gulonolactone oxidase (GLO), the key enzyme catalyzing the last step of Vc biosynthesis (Nandi et al., 1997; Ching et al., 2014), and its corresponding enzyme activity in the kidney (the specific organ for Vc synthesis in P. sinensis; Qian et al., 2008) during the entire hibernation process (before, during and after hibernation). Turtles are ectotherms, and so ambient temperature may affect enzyme activity beyond its internal regulation on a reaction kinetic level (Mæland and Waagbø, 1998; Sizer, 2006). Therefore, we measured the GLO activity at two incubation temperatures that would be experienced during hibernation. Furthermore, we predicted that there might be a pre-storage of Vc in different tissues before hibernation (prediction 2). During hibernation in the cold winter, Vc level might decrease because of the lowered production rate in the kidney, the lowered transfer rate to other tissues owing to the lowered metabolic rate at cold temperature, and the potential increased consumption of Vc under the winter stress. In recovery, both the Vc synthesis capability and transport ability through the blood will increase as the temperature rise up, thus, Vc level in tissues will increase and may recover to the before hibernation level. Therefore, we determined the Vc concentrations in the brain, liver, kidney, spleen, and muscle of juvenile Chinese soft-shelled turtle. Finally, in order to investigate whether oxidative stress occurred in tissues during hibernation, the concentration of malondialdehyde (MDA), which is the stable final product of lipid peroxidation, was determined as an index of tissue oxidative damage. Our work will provide basic data to stress physiology of the turtles and to Vc requirement for turtle culture.
2 MATERIAL AND METHODThis study was approved by the Ethic and Animal Welfare Committee (EAWC) of Beijing Normal University (Approval No. CLS-EAW-2013-005).
2.1 Turtle collection and samplingJuvenile Chinese soft-shelled turtles, P. sinensis (n=27, w: 116.97±2.41 g) were collected from a semiecological turtle hatchery facility (39°53.422'N, 117°33.612'E; Hebei Province, China), in which the turtles can hibernate freely under water in the mud as they do in nature. Generally, Chinese soft-shelled turtles hibernate in late autumn. Feeding was withheld until their arousal the next spring.
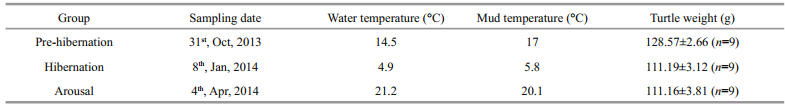
According to the results of empirical studies (Shao, 2012; Chen et al., 2015), observations of the workers in the facility over the years, and the temperature dynamics of the year, we selected three sampling points: 1) pre-hibernation (before massive hibernation of the turtle occurred, i.e. > 90% of turtles could not be observed at the water surface); 2) hibernation (the mid-term of an average hibernation course); and 3) arousal (one day after massive arousal of the turtle, i.e. > 90% of turtles had emerged from the mud and started to feed) (Zhang et al., 2016). Nine juvenile Chinese soft-shelled turtles were sampled at each sampling time. On account of long term starvation during hibernation, the turtles sampled in hibernation and arousal were lighter in weight than the ones in pre-hibernation (one-way ANOVA, P=0.001; see details in Table 1 and Fig. 1). All animals were sacrificed by fast decapitation at the sampling site immediately after they were captured from the water or dug out from the mud. The brain, liver, kidney, spleen, and muscle were quickly excised, frozen in liquid nitrogen, and stored at -80℃ in the laboratory at Beijing Normal University. Due to suppressed cardiovascular circulation of the turtle during hibernation, very little blood can be collected under field conditions, and so plasma was not collected.
|
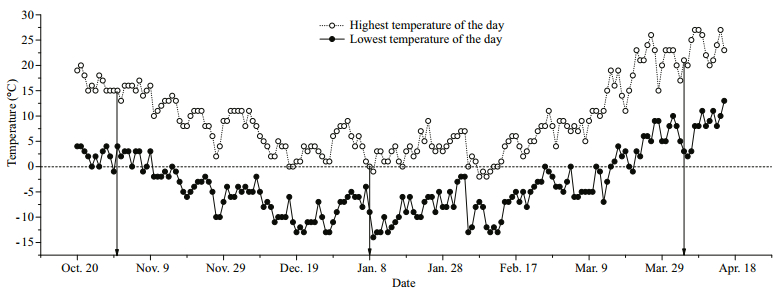
| Fig.1 Atmosphere temperature data of the sampling region during the experiment The arrow points sampling date (pre-hibernation: Oct. 31st, 2013; hibernation: Jan. 8th, 2014; arousal: Apr. 4th, 2014). The climate data were obtained from the Chinese meteorological data network (http://data.cma.cn/). |
A method combining the NucleoSpin RNA Ⅱ Kit (Macherey-Nagel, Germany) and Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from kidney tissues (Munang'andu et al., 2013; for details, see Chen et al., 2015). For each sample, we obtained 40 μL total RNA. The quantity and quality of the isolated RNA were evaluated using a NanoDrop 2000 spectrophotometer (Thermo, USA) and electrophoresis, respectively (A260/280>1.9, c: 0.2–0.8 μg/μL).
2.3 First-strand cDNA synthesis and real-time PCRThe extracted total RNA was reverse-transcribed into cDNA using a PrimeScript Ⅱ first strand cDNA synthesis kit (TaKaRa, Japan) following the manufacturer's instructions. In brief, each 30 μL reaction system contained 2 μg total RNA template with oligo dT primers. The acquired cDNA was diluted six times before use in quantitative real-time PCR analyses.
The online software prime3 (http://primer3.ut.ee/) was used to design real-time PCR primers. We verified all primers via sequencing after assembling the PCR products into cloning vectors. Table 2 summarizes the gene names, accession numbers, primer sequences, amplicon sizes, and real-time PCR efficiency.

|
Real-time PCRs were performed on a 7500 realtime PCR system (Applied Biosystems, Foster City, CA, USA) with default settings. Each 20 μL reaction system contained 3 μL diluted cDNA template, 0.25 μL each specific forward and reverse primers (20 mol/L), 10 μL of 2× SYBR Green PCR Master Mix (Applied Biosystems), and 6.5 μL Milli-Q H2O. We selected β-actin as the internal control gene and calculated relative gene expression using the comparative CT method (Schmittgen and Livak, 2008).
2.4 GLO activity assessmentThe activity of GLO in kidneys was determined according to Dabrowski (1990), Mæland and Waagbø (1998) and Ching et al. (2001) with modifications (for details, see Chen et al., 2015). Briefly, 96–113 mg kidney tissue was used in each determination. The GLO activity in each kidney sample was assessed at two incubation temperatures (5℃ and 20℃; close to the actual temperatures that the turtles experienced during hibernation period). The mixture was incubated for 1 h and GLO activity was calculated as μg Vc synthesized/(h·g) tissue.
2.5 Vc concentration assessmentWe analyzed the total Vc concentration (ascorbate and dehydroascorbate) in each tissue using a HPLC method with a 5-μm analytical column (4.6 mm × 250 mm, Agilent, Palo Alto, CA, USA) and electrochemical detection (Waters 2695/2487, USA). For each determination, the injection volume was 20 μL. The mobile phase, which was filtered and degassed before use, consisted of methyl alcohol and phosphoric acid (6: 94 v/v, pH: 2.63). The flow rate was 1 mL per min and ultraviolet detection was set at λ=243 nm.
Eight working standards containing 1–250 μg Vc/ mL (NIFDC, PR China) were prepared to establish a standard curve. One standard Vc sample (10–20 μg Vc/mL) was prepared and estimated alongside each batch of the sample determinations.
Approximately 100 mg muscle, 70 mg liver, 40 mg brain, kidney, and spleen were weighed and mixed with 15% meta-phosphoric acid at a ratio of 1:9 (w/v, muscle and liver) or 1:19 (w/v, brain, kidney and spleen) on ice using a homogenizer (Model Pro 200. PRO Scientific Inc. Oxford GT, USA). The homogenate was centrifuged at 20 000×g for 20 min (4℃) and the supernatant was collected. For each sample, the Vc concentration was determined for three replicates, and the mean value was calculated (Chen et al., 2015).
2.6 Protein and MDA concentration assessmentFor each sample, approximately 40 mg tissue was weighed and mixed with 0.8% ice-cold saline at a ratio of 1:9 (w/v), and then the mixture was homogenized on ice using a rotor-stator homogenizer. The homogenates were centrifuged following the operating instruction of the assay kits (Protein/ MDA assay kits, Nanjing Jiancheng Bioengineering Institute, China) and the supernatants were collected for protein and MDA concentration assessments.
For soluble protein determination, the tissue homogenates (10%) were first diluted to 1% and then protein concentration was determined using the Coomassie blue dye-binding method (Bradford, 1976) using an assay kit (Nanjing Jiancheng Bioengineering Institute, China). To determine the MDA concentration, the TBARS method (Baker et al., 2007) was applied with an assay kit (Nanjing Jiancheng Bioengineering Institute, China). Each assay contained 20 μL crude tissue homogenate (10%), and absorbance (λ=532 nm) was read on a Synergy H1 Hybrid Reader (BioTek, USA). Protein and MDA contents were determined for three replicates of each sample. In each determination, triplicate readings were recorded and the values were averaged. The MDA concentration was expressed as nmol per mg tissue protein in the crude homogenate.
2.7 StatisticsAll data are presented as means±S.E. Firstly, the data were examined for homogeneity of variance (Levene's test) and normality (Kolmogorov-Smirnov test). If appropriate, one-way ANOVA followed by Duncan post-hoc tests was used to assess the statistical significance between different groups. If heterogeneity of variance existed between groups, the KruskalWallis test followed by Mann-Whitney U post-hoc test was used to compare the difference between them (Buckley, 2006). Pre-hibernation group was used as the control group for comparison. All data were analyzed using the SPSS statistical package (version 19.0, SPSS Inc., USA). P < 0.05 was set as the level of statistical significance.
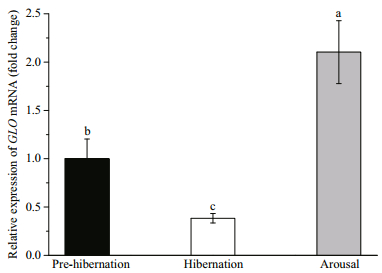
3 RESULT 3.1 Relative gene expressionThe relative expression of β-actin mRNA remained stable during all the three (pre-hibernation, hibernation and arousal) periods (internal control gene, P=0.824, data not presented). On the other hand, the expression of GLO mRNA changed significantly (P < 0.001). Compared with pre-hibernation (control group), the mRNA expression level decreased to 0.38 fold in hibernation, but increased to 2.1 fold in arousal (Fig. 2).
|
| Fig.2 Relative expression of GLO mRNA in kidney of the turtle at pre-hibernation, hibernation and arousal Values are mean±S.E. Data with different superscript letters indicate a significant difference (n=9 per group, Kruskal-Wallis test, P<0.05). |
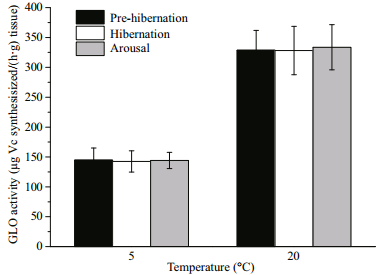
There was no significant difference between the pre-hibernation (control), hibernation and arousal groups (5℃: P=0.996; 20℃: P=0.993), regardless of the incubation temperature in the enzyme activity determinations (5℃ or 20℃; Fig. 3). The GLO activity of the control group at 5℃ was 144.76 ± 20.14 μg Vc synthesized/(h·g) tissue, the enzyme activity at 20℃ was 328.67±33.22 μg Vc synthesized/(h·g) tissue. The Q10 of the GLO activity for each sampling group from 20℃ to 5℃ was 1.73 (pre-hibernation), 1.74 (hibernation) and 1.75 (arousal).
|
| Fig.3 GLO activities in kidney of the turtle at prehibernation, hibernation and arousal |
Turtles are ectotherms that ambient temperature may affect the enzyme activity beyond its internal regulation on a reaction kinetic level, therefore, the GLO activity was measured at two incubation temperatures (5℃ and 20℃), which were close to the temperatures they actually experienced during the entire hibernation period. Values are mean±S.E. No significant difference in GLO activity was found between the pre-hibernation (control), hibernation and arousal group at either incubation temperature (n=9 per group, One-way ANOVA, 5℃: P=0.996; 20℃: P=0.993).
3.3 Vc concentrationThere were three patterns of Vc variations among tissues during the entire hibernation period (Fig. 4).
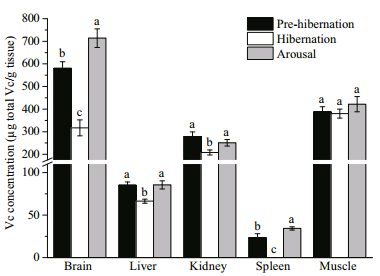
|
| Fig.4 Vc concentration in different tissues at prehibernation, hibernation and arousal Values are mean±S.E. Data with different superscript letters indicate a significant difference (n=9 per group, Kruskal-Wallis test was used to access differences in spleen while One-way ANOVA was used in other tissues, P<0.05). |
1) The Vc concentration in muscle kept stable during all three hibernation periods (P=0.497).
2) The Vc concentration in liver and kidney decreased significantly in hibernation and restored to pre-hibernation levels in arousal (liver, P=0.003; kidney, P=0.018).
3) The Vc concentration in brain and spleen dropped significantly (decreased 45% and 100% of tissue Vc, respectively) in hibernation. The Vc level increase in arousal was also significant, and in which, it exceeded the Vc level in pre-hibernation (brain, P < 0.001; spleen, P=0.001).
3.4 Oxidative statusThe MDA concentration did not change significantly in most tissues at all three sampling points (brain, P=0.632; liver, P=0.708; kidney, P=0.96, muscle, P=0.808), except spleen (P < 0.001) (Fig. 5). The MDA concentration in spleen increased significantly to approximately 1.3 fold in hibernation, and dropped to 0.81 fold in arousal compared with pre-hibernation.
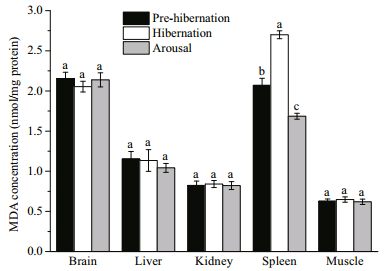
|
| Fig.5 MDA concentration in different tissues during at prehibernation, hibernation and arousal Values are mean±S.E. Data with different superscript letters indicate a significant difference (n=9 per group, Kruskal-Wallis test was used to access differences in spleen while One-way ANOVA was used in other tissues, P<0.05). |
The GLO mRNA level decreased significantly during hibernation, but increased to a level more than 2-fold higher during arousal when compared with pre-hibernation conditions (partly fulfilled prediction 1; Fig. 2). However, GLO activity was not consistent with the mRNA expression pattern, and remained stable throughout the entire examined hibernation period, regardless of the incubation temperature (Fig. 3). The Vc concentration varied among tissues and showed tissue-specific changes during the entire examined hibernation period (partly fulfilled prediction 2; Fig. 4).
Previous studies on hibernating North American turtles have reported that, under conditions of comprehensive depression of transcription and translation, antioxidant defense-related genes are among a small group of up-regulated genes that encode important components of the freezing tolerance mechanism during hibernation (Krivoruchko and Storey, 2010; Storey, 2006). Our result found that the GLO mRNA level decreased during hibernation and increased during arousal seemed to contradict these reports. In our related study on the antioxidant response in hibernating Chinese soft-shelled turtle hatchlings, the transcript levels of Nrf2 (encoding nuclear factor erythroid 2-related factor) and genes for most of the downstream antioxidant enzymes also decreased or did not change during hibernation (Zhang et al., 2016), suggesting there are major differences in the regulation of the antioxidant system during hibernation between North American turtles and Southeast Asian turtles. Storey (2006) suggested that whether freezing tolerance strategies are employed is dependent on the environmental conditions. Thus, it would be interesting to determine whether North American turtles would use similar antioxidant regulation strategies as Southeast Asian species do when they do not encounter freezing temperatures during hibernation period.
The mismatch between GLO mRNA levels and GLO enzyme activity during the entire examined hibernation period differed from the results of our previous study on the turtle antioxidant system during short-term cold exposure. In our previous study, the transcript levels of GLO and GLO enzyme activity both remained stable during short-term cold exposure (Chen et al., 2015). The similar Q10 value of GLO activity from 20℃ to 5℃ (pre-hibernation: 1.73, hibernation: 1.74 and arousal: 1.75) indicated that GLO activity during the entire hibernation period is a temperature-dependent kinetic reaction, similar to our observations of its activity during short-term cold exposure. However, absolute enzyme activity was significantly lower in hibernation than at higher temperatures. There were huge differences in GLO activity levels between hibernation (the present study) and short-term cold exposure (our previous study; Chen et al., 2015): the GLO activity in hibernation tested at 5℃ (the present study) only reached approximately half of the activity level at 8℃ in short term cold exposure. The large-scale difference in GLO activity suggest that there are different regulation strategies between long term hibernation and shortterm cold exposure, although there will also be a temperature effect on enzyme kinetics. One possible explanation for the huge differences in GLO activities could be that ROS are majorly generated from mitochondrial respiration (Skjærven et al., 2013), which decreased with the depression of aerobic metabolism during hibernation. The respiratory rate and metabolic rate decreased in turtles during hibernation, indicating reduced oxidative stress conditions (Hermes-Lima and Zenteno-Savín, 2002). Therefore, whereas high antioxidant enzyme activity would be retained during short-term thermal changes, the decreased gene transcription and lower enzyme activity during hibernation might be due to reduced oxidative stress. This would save energy for prolonged survival of soft-shelled turtle during long term hibernation. In North American turtles, even though their metabolism is depressed during hibernation, subzero temperatures can induce ice penetration throughout the body cavity that can block blood circulation and cause severe oxidative damage in tissues. Hence, their genes and enzymes related to anti-freeze and antioxidant functions are up-regulated during hibernation as counteracting measures (Storey, 2006).
Because of the depressed metabolism and low GLO activity in soft-shelled turtles during hibernation, the Vc synthesis capacity during hibernation may have decreased accordingly. In addition, Vc is produced only in the kidney in soft-shelled turtles, and is then transported to other tissues via blood flow, which is severely reduced under low temperature during hibernation period (Nandi et al., 1997; Galli and Richards, 2012). Our previous study showed that cold exposure significantly impaired Vc transport via the bloodstream. However, the Vc transport potential would be fully functional when the turtles are restored to higher temperatures (Chen et al., 2015). Hence, greater Vc stocking before hibernation might be a practical measure for turtles to counteract low Vc synthesis and circulation during hibernation. This would explain our findings that the Vc concentration in the liver was 1.75-fold higher and that in the kidney was 4.89-fold higher before hibernation than at 28℃ (Chen et al., 2015). Since Vc is only synthesized in the kidney, the substantial amount of Vc stored before hibernation might also be used to supply other tissues during arousal before the Vc synthesis capacity has been fully restored (partly fulfilled prediction 2).
The dynamic pattern of Vc in the brain and spleen was different to that in other tissues (Fig. 4). No Vc pre-stocking was found in the brain. Even so, the Vc concentration was still markedly higher in the brain than in other tissues (Fig. 4). Vc serves as one of the most important low molecular weight antioxidants in turtle brain (Pérez-Pinzón and Rice, 1995; Rice, 2000), its cellular levels are maintained by active uptake though plasma (Rice, 1999). A study on seasonal variation in cerebral Vc distribution revealed that the Vc concentration in turtle brain was significantly lower in winter than that in summer (Pérez-Pinzón and Rice, 1995). The depressed cardiovascular circulation (Nandi et al., 1997; Galli and Richards, 2012) and insufficient plasma Vc transportation (Chen et al., 2015) at low temperatures made the turtle maintain its cerebral Vc level more difficult during long term hibernation period. However, compared with our previous study in which the turtle's cerebral Vc concentration decreased more than 30% within 8 h cold exposure (Chen et al., 2015), our current results showed that even after 5 months of hibernation, the Vc concentration in the brain only decreased by 45.3% (Fig. 4), with no apparent oxidative damage detected (Fig. 5). The mechanism behind the storage and consumption of Vc in the brain is still elusive, and further research is required to fully understand the role of Vc in the brain in antioxidant protection (Garbarino et al., 2015).
The spleen which serves as a major component of lymphoid tissues, possesses key functional importance in reptile immune system, including turtles (see review in Zimmerman et al., 2010a). However, its stored Vc level was the lowest among all the tissues tested. It was the only tissue that showed Vc depletion and accompanying oxidative damage during hibernation in our study (Figs. 4, 5). The complete consumption of Vc in the spleen during hibernation was associated with a significantly elevated MDA level; when the Vc concentration was restored during arousal, the MDA level decreased accordingly. Such matching patterns between Vc and MDA indicate a key role of Vc in protection against lipid peroxidation in the spleen. The function of immune system in reptiles is dramatically affected by the seasonal cycle, during which the involution of lymphoid tissue and decrease of lymphocyte numbers occur in winter times (Leceta and Zapata, 1985; Munoz et al., 2004). A study on red-eared slider (Trachemys scripta) showed that the immune defense level was relatively lower at the beginning of the active season (after hibernation), but increased rapidly in late spring (Zimmerman et al., 2010b). Pathogen-induced mortality of soft-shelled turtle during hibernation has also been reported (Liu et al., 2004; Wang et al., 2008). Though it is suggested that the higher immune defense capability in late spring and summer times may be due to higher activity level and infection risks in these seasons (Munoz et al., 2004; Zimmerman et al., 2010b), the upsurge of ambient temperature accompanied with internal ROS burst and lower immune defense capability laid more pressure for turtles to survive during arousal. Given oxidative stress may be detrimental in immune functions (high percent of polyunsaturated fatty acids in immune cells which are sensitive to oxidative stress; see review in Knight, 2000), whether the oxidative damage in the spleen during hibernation poses impairment on structure integrity of the spleen and has potential connection with decreased immune function during and soon after hibernation deserve further study.
5 CONCLUSIONThe GLO mRNA level significantly decreased during hibernation and increased during arousal; however, the GLO enzyme activity remained at a relatively lower but stable level during hibernation. The dynamic pattern of Vc was tissue-specific, with the highest stored Vc concentration in the brain. The liver and kidney exhibited a suggestive Vc prestocking pattern before hibernation. Most tissues showed a comprehensive decrease in Vc content during hibernation, with the most extreme depletion in the spleen, where the Vc reserves were completely depleted. Most tissues did not suffer from oxidative damage during the entire hibernation period, suggesting that the antioxidant defense system provided effective protection for Chinese soft-shelled turtle during hibernation. However, the depleted Vc and elevated MDA levels in the spleen during hibernation indicated potential impairment of tissue integrity and immune function, which warrants further investigation.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENTThis study was approved by the Ethic and Animal Welfare Committee (EAWC) of Beijing Normal University. We are very grateful to Mr. CAO Zhendong, Dr. LIU Kai and Dr. ZHANG Zuobing for their help (technical assistance) in this study.
Baker P J, Costanzo J P, Lee Jr R E. 2007. Oxidative stress and antioxidant capacity of a terrestrially hibernating hatchling turtle. J. Comp. Physiol. B, 177(8): 875-883.
DOI:10.1007/s00360-007-0185-0 |
Bickler P E, Buck L T. 2007. Hypoxia tolerance in reptiles, amphibians, and fishes:life with variable oxygen availability. Ann. Rev. Physiol., 69(1): 145-170.
DOI:10.1146/annurev.physiol.69.031905.162529 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Buckley R. 2006. Choosing and using statistics:a biologist's guide, 2nd edition. Austral Ecol., 31(3): 425.
DOI:10.1111/j.1442-9993.2006.01623.x |
Chen B J, Niu C J, Yuan L. 2015. Ascorbic acid regulation in stress responses during acute cold exposure and following recovery in juvenile Chinese soft-shelled turtle(Pelodiscus sinensis). Comp. Biochem. Phys. A, 184: 20-26.
DOI:10.1016/j.cbpa.2015.01.018 |
Ching B Y, Ong J L, Chng Y R, Chen X L, Wong W P, Chew S F, Ip Y K. 2014. l-gulono-γ-lactone oxidase expression and vitamin C synthesis in the brain and kidney of the African lungfish, Protopterus annectens. FASEB J., 28(8): 3506-3517.
DOI:10.1096/fj.14-249508 |
Ching S, Mahan D C, Dabrowski K. 2001. Liver L-gulonolactone oxidase activity and tissue ascorbic acid concentrations in nursing pigs and the effect of various weaning ages. J. Nutr., 131(7): 2002-2006.
DOI:10.1093/jn/131.7.2002 |
Dabrowski K. 1990. Gulonolactone oxidase is missing in teleost fish. The direct spectrophotometric assay. Biol.Chem. Hoppe-Seyler., 371(1): 207-214.
DOI:10.1515/bchm3.1990.371.1.207 |
Davies K J. 2000. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB life, 50(4): 279-289.
DOI:10.1080/713803728 |
Drew K L, Tøien Ø, Rivera P M, Smith M A, Perry G, Rice M E. 2002. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp. Biochem. Phys. C, 133(4): 483-492.
DOI:10.1016/S1532-0456(02)00118-7 |
Galli G L J, Richards J G. 2012. The effect of temperature on mitochondrial respiration in permeabilized cardiac fibres from the freshwater turtle, Trachemys scripta. J. Therm.Biol., 37(3): 195-200.
DOI:10.1016/j.jtherbio.2011.12.012 |
Garbarino V R, Orr M E, Rodriguez K A, Buffenstein R. 2015. Mechanisms of oxidative stress resistance in the brain:Lessons learned from hypoxia tolerant extremophilic vertebrates. Arch. Biochem. Biophys., 576: 8-16.
DOI:10.1016/j.abb.2015.01.029 |
Hermes-Lima M, Storey J M, Storey K B. 2001. Antioxidant defenses and animal adaptation to oxygen availability during environmental stress. Cell Mol. Response Stress, 2: 263-287.
DOI:10.1016/S1568-1254(01)80022-X |
Hermes-Lima M, Zenteno-Savín T. 2002. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Phys. C, 133(4): 537-556.
DOI:10.1016/S1532-0456(02)00080-7 |
Hochachka P W, Buck L T, Doll C J, Land S C. 1996. Unifying theory of hypoxia tolerance:molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc.Natl. Acad. Sci. USA, 93(18): 9493-9498.
DOI:10.1073/pnas.93.18.9493 |
Jackson D C, Ultsch G R. 2010. Physiology of hibernation under the ice by turtles and frogs. J. Exp. Zool. A, 313(6): 311-327.
DOI:10.1002/jez.603 |
Jackson D C. 2000. Living without oxygen:lessons from the freshwater turtle. Comp. Biochem. Phys. A, 125(3): 299-315.
DOI:10.1016/S1095-6433(00)00160-4 |
Jing R Z, Niu C J, Qian M Y, Huang C X, Zhang Z B, Qian Y Q. 2011. Effect of dietary Vitamin C and hibernation on energy metabolism in juvenile soft-shelled turtle(Pelodiscus sinensis). J. Beijing Normal Univ. (Nat. Sci.), 47(2): 185-191.
(in Chinese with English abstract) |
Knight J A. 2000. Review:free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci., 30(2): 145-158.
|
Krivoruchko A, Storey K B. 2010. Activation of antioxidant defenses in response to freezing in freeze-tolerant painted turtle hatchlings. Biochim. Biophys. Acta, 1800(7): 662-668.
DOI:10.1016/j.bbagen.2010.03.015 |
Leceta J, Zapata A. 1985. Seasonal changes in the thymus and spleen of the turtle, Mauremys caspica. A morphometrical, light microscopical study. Dev. Comp. Immunol., 9(4): 653-668.
DOI:10.1016/0145-305X(85)90030-8 |
Liu Q, Yang H, Wang S, Ma J, You J. 2004. The prevention and treatment of overwintering death in Chinese soft-shelled turtle Pelodiscus sinensis. Fishery Guide, (22): 53.
(in Chinese) |
Liu X N, Tan B P. 1997. Preliminary study on prevention methods in overwintering death disease of Chinese softshelled turtle. Reservoir Fisheries, (1):26-27, (1): 26-27, 49.
(in Chinese) |
López-Torres M, Pérez-Campo R, Cadenas S, Rojas C, Barja G. 1993. A comparative study of free radicals in vertebrates-Ⅱ. Non-enzymatic antioxidants and oxidative stress. Comp. Biochem. Phys. B, 105(3-4): 757-763.
DOI:10.1016/0305-0491(93)90117-N |
Ma Y L, Rice M E, Chao M L, Rivera P M, Zhao H W, Ross A P, Zhu X W, Smith M A, Drew K L. 2004. Ascorbate distribution during hibernation is independent of ascorbate redox state. Free Radical Bio. Med., 37(4): 511-520.
DOI:10.1016/j.freeradbiomed.2004.04.025 |
Mæland A, Waagbø R. 1998. Examination of the qualitative ability of some cold water marine teleosts to synthesise ascorbic acid. Comp. Biochem. Phys. A, 121(3): 249-255.
DOI:10.1016/S1095-6433(98)10125-3 |
Munang'andu H M, Fredriksen B N, Mutoloki S, Dalmo R A, Evensen Ø. 2013. Antigen dose and humoral immune response correspond with protection for inactivated infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L). Vet. Res., 44: 7.
DOI:10.1186/1297-9716-44-7 |
Munoz F J, de La Fuente M, Beaupre S J. 2004. Seasonal changes in lymphoid distribution of the turtle Mauremys caspica. Copeia, 2004(1): 178-183.
DOI:10.1643/CP-02-058R2 |
Nandi A, Mukhopadhyay C K, Ghosh M K, Chattopadhyay D J, Chatterjee I B. 1997. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radical Bio. Med., 22(6): 1047-1054.
DOI:10.1016/S0891-5849(96)00491-1 |
Pérez-Pinzón M A, Rice M E. 1995. Seasonal-and temperaturedependent variation in CNS ascorbate and glutathione levels in anoxia-tolerant turtles. Brain Res., 705(1-2): 45-52.
DOI:10.1016/0006-8993(95)01136-6 |
Qian M Y, Niu C J, Jing R Z, Qian Y Q, Zhang Z B. 2008. Effect of dietary Vc and hibernation on biosynthesis of Vc and liver Vc concentration in juvenile soft-shelled turtles Pelodiscus sinensis. Acta Zool. Sin., 54(2): 309-316.
(in Chinese with English abstract) |
Reese S A, Crocker C E, Carwile M E, Jackson D C, Ultsch G R. 2001. The physiology of hibernation in common map turtles (Graptemys geographica). Comp. Biochem. Phys.A, 130(2): 331-340.
DOI:10.1016/S1095-6433(01)00398-1 |
Reese S A, Ultsch G R, Jackson D C. 2004. Lactate accumulation, glycogen depletion, and shell composition of hatchling turtles during simulated aquatic hibernation. J. Exp. Biol., 207(16): 2889-2895.
DOI:10.1242/jeb.01124 |
Rice M E, Forman R E, Chen B T, Avshalumov M, Cragg S J, Drew K L. 2002. Brain antioxidant regulation in mammals and anoxia-tolerant reptiles:balanced for neuroprotection and neuromodulation. Comp. Biochem. Phys. C, 133(4): 515-525.
DOI:10.1016/S1532-0456(02)00116-3 |
Rice M E, Lee E J, Choy Y. 1995. High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J.Neurochem., 64(4): 1790-1799.
DOI:10.1046/j.1471-4159.1995.64041790.x |
Rice M E. 1999. Ascorbate compartmentalization in the CNS. Neurotox. Res., 1(2): 81-90.
DOI:10.1007/BF03033272 |
Rice M E. 2000. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci., 23(5): 209-216.
DOI:10.1016/S0166-2236(99)01543-X |
Schmittgen T D, Livak K J. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc., 3(6): 1101-1108.
DOI:10.1038/nprot.2008.73 |
Shao Q. 2012. Soft-shelled turtles. 460. In: Lucas J S, Southgate P C eds. Aquaculture (Second edition): Farming Aquatic Animals and Plants. Blackwell Publishing Ltd., West Sussex, UK.
|
Sizer I W. 2006. Effects of temperature on enzyme kinetics. In: Nord F F, Werkman C H eds. Advances in Enzymology and Related Areas of Molecular Biology. Interscience Publishers, Inc., New York. p.35-62.
|
Skjærven K H, Penglase S, Olsvik P A, Hamre K. 2013. Redox regulation in Atlantic cod (Gadus morhua) embryos developing under normal and heat-stressed conditions. Free Radical Biol. Med., 57: 29-38.
DOI:10.1016/j.freeradbiomed.2012.11.022 |
Storey K B, Storey J M. 2004. Metabolic rate depression in animals:transcriptional and translational controls. Biol.Rev. Camb. Philos. Soc., 79(1): 207-233.
DOI:10.1017/S1464793103006195 |
Storey K B. 2004. Strategies for exploration of freeze responsive gene expression:advances in vertebrate freeze tolerance. Cryobiology, 48(2): 134-145.
DOI:10.1016/j.cryobiol.2003.10.008 |
Storey K B. 2006. Reptile freeze tolerance:metabolism and gene expression. Cryobiology, 52(1): 1-16.
DOI:10.1016/j.cryobiol.2005.09.005 |
Storey K B. 2007. Anoxia tolerance in turtles:metabolic regulation and gene expression. Comp. Biochem. Phys. A, 147(2): 263-276.
DOI:10.1016/j.cbpa.2006.03.019 |
Ultsch G R. 1989. Ecology and physiology of hibernation and overwintering among freshwater fishes, turtles, and snakes. Biol. Rev., 64(4): 435-515.
DOI:10.1111/j.1469-185X.1989.tb00683.x |
Venditti P, Di Stefano L, Di Meo S. 2010. Oxidative stress in cold-induced hyperthyroid state. J. Exp. Biol., 213(17): 2899-2911.
DOI:10.1242/jeb.043307 |
Wang X Z, Wang M B, Sun X Z, Xin X Y. 2008. The investigation on overwintering death in Chinese softshelled turtles and preventive measures. Shandong Fisheries, (11): 40.
(in Chinese) |
Zhang J, Wang F, Jiang Y L, Hou G J, Cheng Y S, Chen H L, Li X. 2017a. Modern greenhouse culture of juvenile softshelled turtle, Pelodiscus sinensis. Aquacult. Int., 25(4): 1607-1624.
DOI:10.1007/s10499-017-0137-y |
Zhang W Y, Niu C J, Chen B J, Yuan L. 2016. Antioxidant responses in hibernating Chinese soft-shelled turtle Pelodiscus sinensis hatchlings. Comp. Biochem. Phys. A, 204: 9-16.
DOI:10.1016/j.cbpa.2016.10.014 |
Zhang W Y, Niu C J, Liu Y K, Chen B J. 2017b. Glutathione redox balance in hibernating Chinese soft-shelled turtle Pelodiscus sinensis hatchlings. Comp. Biochem.Phys. B, 207: 9-14.
DOI:10.1016/j.cbpb.2017.02.003 |
Zimmerman L M, Paitz R T, Vogel L A, Bowden R M. 2010b. Variation in the seasonal patterns of innate and adaptive immunity in the red-eared slider (Trachemys scripta). J.Exp. Biol., 213(9): 1477-1483.
DOI:10.1242/jeb.037770 |
Zimmerman L M, Vogel L A, Bowden R M. 2010a. Understanding the vertebrate immune system:insights from the reptilian perspective. J. Exp. Biol., 213(5): 661-671.
DOI:10.1242/jeb.038315 |
 2019, Vol. 37
2019, Vol. 37