Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Yu, FU Xiaoting, DUAN Delin, XU Jiachao, GAO Xin
- Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata
- Journal of Oceanology and Limnology, 37(3): 815-824
- http://dx.doi.org/10.1007/s00343-019-8129-6
Article History
- Received May. 1, 2018
- accepted in principle Jul. 10, 2018
- accepted for publication Aug. 17, 2018
2 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 State Key Lab of Seaweed Bioactive Substances, Qingdao 266400, China
Ahnfeltia plicata is an Agarophytes belonging to Family Ahnfeltiaceae and widely distributes in Russia, Japan, and Korean Peninsula. Agar has been considered as a mixture of a neutral fraction of agarose and a charged fraction of agaropectin (Duckworth and Yaphe, 1971). Agar and agarose have wide applications, especially for agarose, which is a high-value material extensively used in biotechnology and molecular biology fields (Meena et al., 2007). Numerous technologies and studies have been reported in the literature on the agar and agarose preparation from agarophytes. Gelidium species was the world's first source for the manufacture of agar at the beginning of the seventeenth century; however, after the 20th century, Gracilaria became a preferred seaweed for making food-grade agar and it was successfully cultivated in Chile and Indonesia (Bixler and Porse, 2011). However, a high concentration of alkali pre-treatment was necessary for Gracilaria species in order to produce agar with qualified gel strength. As a result, a large number of studies have been carried out to optimize the process of alkali treatment (Lee et al., 2017). Meena et al. (2011) reported that alkali treatment significantly improved the properties of Gelidium agar, which the yield, sulfate and ash contents greatly decreased and the gel strength greatly improved. Freile-Pelegrín and Murano (2005) revealed the optimal property of agar was obtained with a high NaOH concentration of 7% to 10%. Although Gracilaria species is widely used in agar production until now, environmental pollution caused by excessively consumed alkali is a disadvantage for this species of alga. Thus, our work is to develop an eco-friendly extraction method using less amount of alkali to produce agar from A. plicata.
Agarose purified from agar should have applications in rapid electrophoresis, a sulfate content of less than 0.2% (Kirkpatrick et al., 1991). Numerous studies have been reported on agarose preparation with a basic principle of separation the agarose from the agaropectin depending on their different ionic charges (Polson, 1967). The quaternary ammonium salt was used as a selective reagent to precipitate agaropectin and thus to leave agarose in the solution. However, it was reported to be difficult to remove the precipitate effectively from the agarose solution (Hjertén, 1964). Blethen (1966) discovered that addition of polysaccharides such as carrageenan, which had sulfate content higher than that of agaropectin, formed a separable flocculation of agaropectin with the quaternary ammonium salt, thus the precipitation could be removed from agarose solution much easier. Provonchee (1991) reported the adoption of low molecular weight alkylene glycol for purification of agarose. Polson (1967) reported the agarose purification method by using polyethylene glycol, which selective by precipitate charged molecules from un-charged polysaccharides. Ion exchange chromatography was to separate agarose from other charged polysaccharides (Cook and Witt, 1981). In our researches, three kinds of methods were compared for purification of agarose from the agar of A. plicata.
The fraction of agaropectin is a complex sulfated polysaccharide. Kloareg and Quatrano (1988) reported that sulfated polysaccharides characteristically was contained in the cell walls from marine algae, which are not found in land plants and which may have specific functions in ionic regulation. In recent years, sulfated polysaccharides isolated from seaweeds have demanded a potential biological activity in potential medicinal value, such as antioxidant, anticoagulant, antitumor and antiinflammatory (Nishino et al., 1991; Rupérez, 2002; Lins et al., 2009; Costa et al., 2010). Our work herein was to provide its structure information for further study of its function.
At present, there were only a few studies about the polysaccharides of species from Ahnfeltia genus. Rheological and thermal properties of agar extracted from A. plicata were studied. However, its extraction condition has not been studied in detail (Watase and Nishinari, 1986). Whyte et al. (1984) screened the phycocolloid of British Columbia red algae including A. plicata and reported the monosaccharide composition of A. plicata agar. Sukhoverkhov et al. (2000) reported that A. tobuchiensis was an excellent material for the production of agar and agarose, whose polysaccharide has only slight deviations from the structure of agarose. However, there is no report on a comprehensive study of phycocolloid of A. plicata. Our research was to establish a procedure for the production of superior qualified agar from A. plicata, and to characterize the chemical properties of agar, agarose, and agaropectin from A. plicata to reveal its further application.
2 MATERIAL AND METHOD 2.1 MaterialAhnfeltia plicata was purchased from Russia, which was collected from Vladivostok (43°09′N, 131°53′E). The sample was sun-dried and stored at room temperature. Commercial agarose BWA was purchased from Bio-west Company (Hercules, CA, USA), Commercial agarose of S-0576 and S-6877 were purchased from Sigma Company (St. Louis, MO, USA). Dextran was from China Institute of Metrology (Beijing, China). Human serum albumin was from Octapharma Co. Ltd. (Oberlaaer, AUT). DNA ladder of BM15000 and BM2000 was from Bomeike Biotechnology (Tianjin, China). Dialysis membranes were from Solarbio (Beijing, China).
2.2 Preparation of agar from A. plicataThe alga A. plicata was pre-treated by ultrasound of 20 KHz in the Citric acid-sodium citrate buffer solution (pH 5.0) in a ratio of 1:30 (w/v) for 2 min. The alga was hydrolysis by cellulase of 10 U/kg at the temperature of 50℃ for 4 h. Then the alga was treated by 0.18% of NaClO with a pH value of 5.39 for 10 min. The solutions were discarded and the alga was washed to neutral with water. The alga was then treated in an autoclave with 1.2% alkali solution in a ratio of 1:30 w/v for 2 h at 121℃. The solution was collected by filtration and followed by gelation at room temperature, frozen at -20℃ for 20 h, and thawed. Finally, the gel was dried at 60℃.
2.3 Characterization of agar from A. plicata(a) Physical properties of agar
A 1.5% (w/v) of agar solution was prepared by using an autoclave at 120℃ for 30 min. After gelation at room temperature, it was maintained at 20℃ for 15 h. Gel strength was measured by the type of TMSPRO Texture Analyzer with a 1-cm2 probe at 20℃, as described by Hurtado-Ponce and Umezaki (1988). Gelling and melting temperatures were measured by Sample stage of MCR101 Rheometer (Anton Paar, Physica, Austria) as reported by Zhang et al. (2009).
(b) Chemical properties
Ash content was determined as described in the reference of the Rupérez et al. (2002). Sulfate content was analyzed by the turbidimetric method as described by Dodgson and Price (1962). 3, 6-AG content was estimated by the method described by Matsuhiro and Zanlungo (1983).
2.4 Purification of agarose from A. plicataThree methods were applied to purify agarose from agar A. plicata, i.e. polyethylene glycol (PEG) precipitation, DEAE-cellulose chromatography, and PEG combined with DEAE-cellulose chromatography.
(a) Purification of agarose by PEG 6000
Agarose was prepared according to the method reported by Duckworth and Yaphe (1971) with some modification. An amount of 600-g PEG was added gently into hot agar solution with a concentration of 3% in 0.05 mol/L sodium chloride at 70℃ with constant stirring for 10 min. The precipitate was separated from the hot solution by centrifugation and washed twice with a hot solution of 25% of PEG in 0.1 mol/L of NaCl. The remaining PEG was removed from the precipitate by extensively washing by 0.1 mol/L NaCl solution until the washing could not form dark brown with I-KI solution. The well-washed flocculent was further washed by 70% of ethanol and dried at 60℃. The fraction obtained by this method was named PA.
(b) Purification of agarose by DEAE-Cellulose chromatography
One percent 250-mL agar solution was added into 100-mL DEAE-Cellulose suspension and agitated at 70℃ for 2 h. Before this process, DEAE-Cellulose was preheated at 70℃ in order to ensure a homogeneous solution avoiding a small amount of precipitation appeared. The solution was collected by filtration, freezing, and thawing, and the gel was dried at 60℃. The fraction obtained by this method was named DA.
(c) Purification of agarose by PEG combined with DEAE-cellulose
PA was dissolved and further purified by DEAEcellulose as described above. The agarose obtained by this method was named PDA.
2.5 Characterization of agarose from A. plicataThe agarose and its physicochemical qualities of yield, gel strength, gelling and melting temperature, ash, sulfate and 3, 6-anhydro-L-galactose (3, 6-AG) content, determined as described above. Electroendosmosis (EEO) was followed the analyzed by agarose electrophoresis using dextran and human serum albumin as standards (Kirkpatrick et al., 1991).
The purified agarose of PA, DA, and PDA was applied to DNA agarose electrophoresis using DNA ladders of 2 kbp and 15 kbp to estimate the separation performance. Commercial agarose of S-0576 was used as a control. Solutions (0.8%) of PA, DA, and PDA were prepared and electrophoresis was carried out under the conditions of 85 V for 20 min.
2.6 Separation of agaropectin from A. plicataAfter filtration to obtain the fraction of DA as described above, the remaining DEAE-cellulose media was eluted with 300-mL 0.5 mol/L NaCl (Fraction 1) followed by 300-mL 1.0-mol/L NaCl (Fraction 2), respectively. The two fractions were collected, concentrated by a rotary vacuum evaporator with the water bath at 50℃, and dialyzed against distilled water by a dialysis membrane with a molecular weight cut-off of 1kDa to remove NaCl. The dialyzed fractions were dried by a lyophilizer. The two fractions were named APP-1 (Fraction 1) and APP-2 (Fraction 2).
2.7 Characterization of agaropectin from A. plicata(a) Sulfate content and purity
Sulfate contents of APP-1 and APP-2 were analyzed by the turbidimetric method as described by Dodgson and Price (1962). To check the purity of agaropectin, 1mg/mL of their solutions were scanned by UV-spectrophotometer in the range of 200– 400 nm.
(b) Monosaccharide analysis
APP-1 and APP-2 were hydrolyzed, acetylated according to the method of Kang and Qu (2006), and analyzed by a 6890N GC (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and a DB-225 fused silica capillary column (30 m×0.32 mm×0.25 mm). The column temperature was kept at 120℃ for 2 min and increased to 220℃ at 8℃/min.
(c) Molecular weight
The molecular weights of APP-1 and APP-2 were determined by HPLC analysis. The standard curve was performed using Dextran (521 000,289 000,110 000, 60 600, 12 600, and 4 320 g/mol) as the standards. APP-1, APP-2, and Dextran standards were dissolved and diluted to 1 mg/mL, loaded into an Agilent HPLC system equipped with TSK-GEL G3000PWXL column (7.8 mm×300 mm), eluted with 0.2-mol NaCl solution and detected by refractive index detector.
(d) FT-IR
The structures of APP-1 and APP-2 were identified by FT-IR. The infrared spectrum was recorded and scanned on Nicolet is10 FT-IR (Thermo, USA) in a range of 400–4 000 cm-1.
2.8 Statistical analysisIndependent experiments were carried out in triplicate. All mean values were analyzed by one-way ANOVA (SPSS V17.0). Data were expressed as the mean±standard deviation (SD). Group means were considered to be significantly different at P < 0.05.
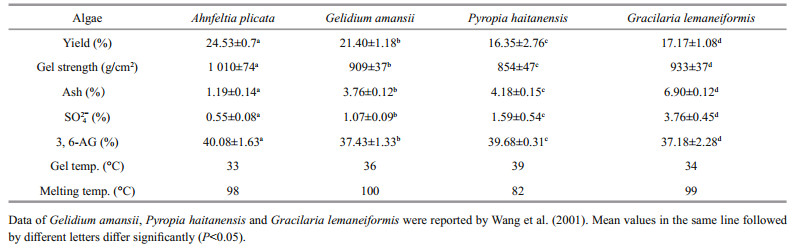
3 RESULT 3.1 Extraction and characterization of agarThe physicochemical properties of agar from A. plicata were summarized and compared with those of red algae commonly used in China for agar production (Table 1). Significant differences (P < 0.01) were observed in ash, sulfate, and 3, 6-AG contents among four kinds of seaweeds (Table 1). The agar from A. plicata exhibited higher yield, gel strength, and 3, 6-AG contents, while lower ash and sulfate than those of others.
|
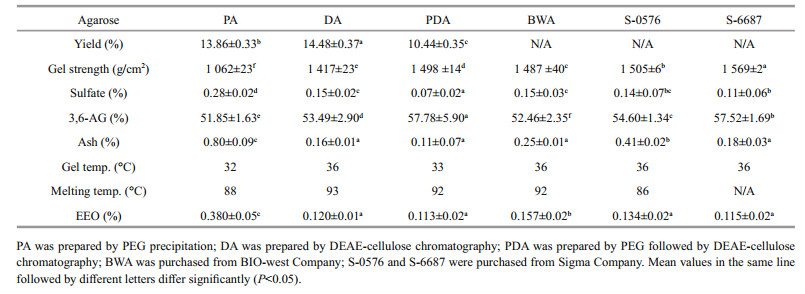
The yields of agarose prepared by three methods are listed and their physicochemical properties compared with those of commercially available agarose (Table 2). The yield of DA was 14.48%±0.37%, which is higher than those of PA and PDA. The gel strength of PDA samples was 1 498±14 g/cm2, which is significantly higher than those of PA, DA, and BWA and slightly lower than those of commercial agarose from Sigma Company (P = 0.008). Significant differences (P = 0.000) were observed in the content of sulfate. The sulfate content of PDA was the lowest, while that of DA was similar to those of BWA and S-0567. PDA had the highest 3, 6-AG contents of 57.78%±5.90%, which is even higher than those of three kinds of commercial agarose. It was observed that the ash content of DA and PDA was 0.16%±0.01% and 0.11%±0.07%, which is much lower than that of PA (0.80%±0.09%). From the crossover of G' and G" traces in the process of cooling and heating (not shown in figure), the gelling temperatures of the PA, DA, and PDA were 32℃, 36℃, and 33℃, while the melting temperatures of those were 88℃, 93℃, and 92℃, respectively. Significance (P = 0.000) was observed in the value of EEO, while those of DA and PDA samples were as low as those of commercial agaroses from Sigma Company.
|
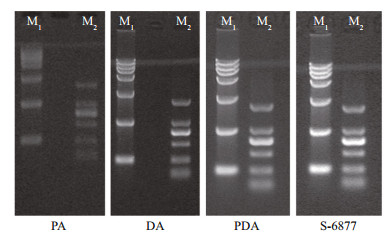
Images of DNA agarose gel electrophoresis are shown in Fig. 1. The image of PA was blurred, and the DNA ladder bands were not completely separated from each other, while those of DA and PDA were clear with well-separated DNA ladder bands.
|
| Fig.1 Agarose gel electrophoresis by different agarose gel M1: DNA Marker BM 15000; M2: DNA Marker BM 2000; PA was agarose prepared by PEG precipitation; PDA was agarose prepared by PEG precipitation followed by DEAE-cellulose chromatography; DA was agarose prepared by DEAE-cellulose chromatography; S-6687 was agarose obtained from Sigma Company. |
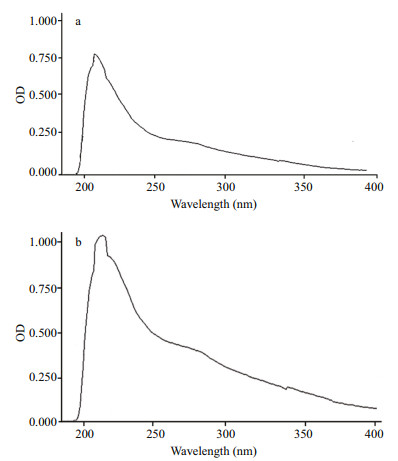
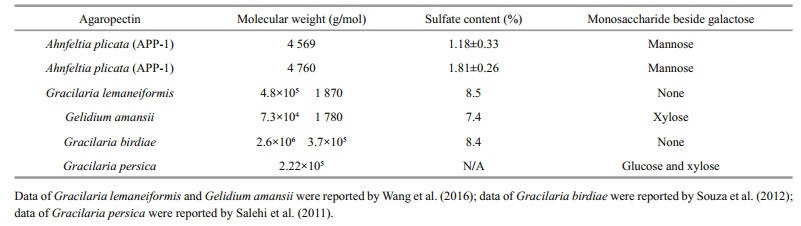
Monosaccharide composition of fractions was assayed and contrasted with the retention time of the standard monosaccharide (Fig. 2). No difference was seen in monosaccharide composition between two agaropectin fractions of APP-1and APP-2. Furthermore, APP-1 and APP-2 fractions appeared to be composed of two kinds of monosaccharide of mannose and galactose. The average molecular weights of APP-1, APP-2 calculated by HPLC, according to the calibration curve with standard dextran (Y = -0.494 8X+12.917, R2 = 0.990 6), were 4 569 Da and 4 760 Da, respectively, with a retention time of 18.725 min and 18.689 min, respectively. UV spectrum of agaropectins is shown in Fig. 3. There are no peaks between the wavelengths from 250 to 280 nm, which reveals that no protein and DNA remained in the substances of APP-1 and APP-2. The characteristics of two fractions of A. plicata agaropectin are listed and compared with those of agaropectin from other red algae (Table 3). The A. plicata agaropectin showed relatively lower molecular weight and sulfate content than those of other agaropectins.

|
| Fig.2 GC graphs of Monosaccharide composition analysis a. monosaccharide standard; b. the fraction of APP-1; c. the fraction of APP-2. |
|
| Fig.3 UV-spectrogram of agaropectin from Ahnfeltia plicata a. the fraction of APP-1; b. the fraction of APP-2. |
|
The FT-IR spectrograms of APP-1 and APP-2 are similar as shown in Fig. 4. Absorption around 3 200– 3 400 cm-1 revealed a typical major broad stretching peak of –OH. A weak absorption around 3 000–2 800 and 1 400–1 200 cm-1 was caused by C–H stretching vibration and bending vibration, respectively, which is the typical absorption of carbohydrates (He et al., 2006). Adsorptions around 1 640 and 1 540 cm-1 indicated there was an acylamino group in the carbohydrate (He et al., 2006). Absorption around 1 100 cm-1 was caused by C–O stretching vibration, which indicates the ring structure of the carbohydrate (He et al., 2006). Absorption around 1 070 and 930 cm-1was caused by 3, 6-AG (Ji, 1997). Absorption around 1 250 cm-1 indicating the total sulphuric residue (Ji, 1997), and the sulfate content was determined to be 1.18% for APP-1 and 1.81% for APP-2, respectively, by the turbidimetric method. Absorption around 850 and 820 indicated the sulfate located in C6 and C4 of galactose. Absorption around 870 and 800 were typical peaks of D-mannose (Huo et al., 1999), which was consisted of the above result of monosaccharide composition.

|
| Fig.4 Infrared spectrums of agaropectin from Ahnfeltia plicata |
The majority of the agarophytes having an agar yield of 20%–30% (Mclachlan and Bird 1986). However, the agar yield varies greatly among species and under different environmental conditions or developmental stages (Armisen, 1995). Agar yield is important for seaweed farmers, as the economic return on the seaweed crop largely depends on the yield of seaweed per dollar of production cost and yield of agar per kilogram of seaweed (Cordover, 2007). Compared to those of other agarophytes commonly used in China, the yield of agar from A. plicata was the maximum (Table 1), which reveals that A. plicata was a potentially excellent material for agar production.
Alkaline treatment plays a vital role in the manufacture of agar from most agarophytes in order to get a high yield and enough gel strength, however, a high concentration of alkaline treatment will cause environmental pollution. Nowadays, Gracilaria is the main source for agar-producing industry. Alkali treatment such as a pretreatment of 8% alkaline was necessary to produce superior quality agar from Gracilaria (Zhao et al., 1996). Wang et al. (2001) also reported that alkali treatment increased the gel strength and decreased the sulfate content of agar from Gracilaria. Freile-Pelegrín and Murano (2005) revealed that the optimal property of agar from Gracilariawas obtained by a high NaOH concentration of 7% to 10%. The environmental pollution caused by highly consumed alkali is a disadvantage for agarproducing by Gracilaria species. It is necessary to find some agar-producing resource with less alkaline treatment demand. Our results indicated that a low alkali consumption of 1.2% was enough for extraction of agar from A. plicata with superior qualities. As shown in Table 1, both the yield and the gel strength of agar from A. plicata were significantly higher than those from other agarophytes (P < 0.05), and the alkaline consumption was much lower than that of Gracilaria. Less alkaline consumption indicated that the A. plicata agar should be a potentially good resource for agar production.
Gel strength is the important characteristic for agar quality, and a gel strength greater than 700 g/cm2 (1.5% gel concentration) was referred to as a highquality agar (Armisen, 1995). As shown in Table 1, A. plicata agar produced in this study showed a qualified gel strength of 1 010 g/cm2. The gelling properties of an agar depend on the three equatorial hydrogen atoms on the 3, 6-AG residues, which constrain the molecule to form a helix and the interaction of these helices resulting in gel formation (Rees, 1961). When the 3, 6-AG residue is replaced with galactose-6-sulphate, kinks are formed in the helix, thus inducing decreasing in gel strength. Superior gel strength of A. plicata agar attributed to its lowest sulfate and ash contents as well as the highest 3, 6-AG contents. Huang et al. (2010) reported that 1.17% of sulfate content was achieved under the optimum conditions of alkali treatment for G. lemaneiformis agar production. In this study, 0.55% of sulfate content was achieved for A. plicata agar. The high gel strength indicated that A. plicata was a potential resource for agar production. Meanwhile, the low sulfate and ash content revealed its great potential for agarose preparation.
Lower gelling temperature is desired for bactoagar because the thermal-sensitive materials such as antibiotics favor a lower temperature when they are added to the agar solution (McHugh, 2003). As shown in Table 1, the gelling temperature of A. plicata agar was similar to that of G. lemaneiformis and lower than those of the other two. Thus, the A. plicata agar showed potential application for bacteria culture medium.
4.2 Purification and characterization of agarose from A. plicataThree kinds of methods were compared for preparation of agarose from A. plicata. For PEG precipitation, 70℃ was chosen as eluting temperature because the higher temperature of 80 to 100℃ caused the co-precipitation of agaropectin while the lower temperature of 40 to 60℃ resulted in the precipitation of agar (Hegenauer and Nace, 1965). PEG precipitation method was reported for the preparation of agarose. However, no data of product quality was available (Hegenauer and Nace, 1965; Duckworth and Yaphe, 1971). Our research indicated that sulfate and ash contents of PA were significant higher (P < 0.01) than those of DA and PDA, which revealed that the single-step method of PEG precipitation could not produce agarose with satisfactory quality.
As comparing of properties of A. plicata agar (Table 1) and agarose purified by three methods (Table 2), a significant difference was observed. The ash content, sulfate content, and melting temperature noticeably decreased during purification (P < 0.05), meanwhile the 3, 6-AG content and the gel strength greatly increased (P < 0.01). The result indicated that the purification procedure greatly improved the purity of agarose.
EEO is one of the important quality standards of agarose. High EEO causes a net flow of water through the gel away from the positive electrode, which will disrupt the accurate migration of nucleic acid and protein. Agarose suitable for analytical and preparative electrophoresis, blotting, immunodiffusion should present enough low EEO (Kirkpatrick et al., 1991). As comparing the commercial products from different chemical companies, EEO value as low as 0.2 was necessary for all-purpose applications. As shown in Table 2, PA possessed an EEO value of 0.380, which was higher than the demanded EEO, thus its agarose gel electrophoresis pattern was fuzzy (Fig. 1). As shown in Table 2, EEO values of DA and PDA were 0.120 and 0.113, respectively, which was similar to those of Sigma products. Because of their low EEO, their agarose gel electrophoresis patterns were clear and the DNA ladders migrated accurately (Fig. 1). As comparing the sulfate and EEO value in Table 2, the product of DA was similar to the sigma product of S-6877 that was defined as the medium EEO product, and the product of PDA was even better than the sigma product of S-0576 that was defined as the low EEO product.
4.3 Purification and characterization of agaropectin from A. plicataThe molecular weight of APP-1and APP-2 was similar to that of each other and lower than those of others. The composition was complex for agaropectin of G. lemaneiformis, G. amansii, and G. birdiae, because there were two peaks in their HPLC spectra, while there was only one peak in those of APP-1, APP-2, and G. persica.
After purification, the sulfate content increased from 0.55% (agar) to 1.18% (APP-1) and 1.81% (APP-2), respectively. Though it was increased by purification, the sulfate content of APP-1and APP-2 was much lower than those of agaropectin from other red algae, which was due to the low sulfate content of A. plicata agar. As compared in Table 1, the sulfate content of A. plicata agar was much lower than those of others. Agaropectin with high sulfate content was reported for having anticoagulant activity by Qi et al. (2008). G. amansii agar was fractionated on DEAEcellulose, and agaropectin fractions with sulfate content of 22.8% and 32.5% were purified and exhibited anticoagulant activity in a dose-dependent manner in vitro to prolong the coagulation time in rats. On the account of the low sulfate content, anticoagulant activity of A. plicata agaropectin might be weak, however, the biological activity of sulfated polysaccharides from marine algae is related to their molecular weight, type of sugar, sulfate content, sulfate position, type of linkage and molecular geometry (Shanmugam and Mody, 2000).
It was quite peculiar that besides galactose, mannose was found in APP-1 and APP-2 fractions in monosaccharide composition analysis (Fig. 2). Agar is known to compose of repeating agarobiose units of alternating 1, 3-linked-D-galactose and 1, 4-linked-3, 6-anhydro-L-galactose residues (Rebello et al., 1997). Only a few research found that besides galactose, there were other kinds of monosaccharides existing in the agar carbohydrate chain. Wang et al. (2016) reported that besides galactose, xylose was found in the agar of G. amanssi. Salehi et al. (2011) reported that besides galactose, there were 4.1% of glucose and 1.1% of xylose in the agar of G. persica.
As reviewed by Nishinari and Fang (2017), most typical absorption bands for sulphuric residue in agar were 1 250 cm-1 and 850 cm-1, which is also found in the IR spectrum of APP-1 and APP-2 (Fig. 4). The fraction of APP-2 was eluted with a higher concentration of NaCl than APP-1. Though the sulfate content of APP-2 (1.81%) was higher than that of APP-1 (1.18%) as determined by turbidimetric method, no significant differences were found in their IR spectra in absorption bands of 1 250 cm-1 and 850 cm-1. Except for the absorbance around 870 and 800 that are typical peaks of D-mannose (Fig. 4), there was no significant difference when comparing the IR spectrum with typical agar fractions from other agarophytes.
5 CONCLUSIONIt was the first time to comprehensively study agar and its fractions in the red alga of A. plicata. The agar with the yield of 24.53% and the gel strength of 1 010 g/cm2 and the agarose with the gel strength of 1 498 g/cm2 and the sulfate content of 0.07% were obtained from A. plicata by the established process in this research. The result indicated that A. plicata could be used for the production of agar and agarose with superior qualities, thus it is a potential supplementary resource for agar and agarose production besides the alga of Gracilaria and Gelidium.
Armisen R. 1995. World-wide use and importance of Gracilaria. Journal of Applied Phycology, 7(3): 231-243.
DOI:10.1007/BF00003998 |
Bixler H J, Porse H. 2011. A decade of change in the seaweed hydrocolloids industry. Journal of Applied Phycology, 23(3): 321-335.
DOI:10.1007/s10811-010-9529-3 |
Blethen J. 1966-10-25. Method for the separation of agaropectin from agarose: US, US3281409A.
|
Cook R B, Witt H J. 1981-09-22. Agarose composition, aqueous gel and method of making same: US, US4290911A.
|
Cordover R. 2007. Seaweed Agronomy: Cropping in Inland Saline Groundwater Evaporation Basins. Rural Industries Research and Development Corporation, Tasmania.
|
Costa L S, Fidelis G P, Cordeiro S L. 2010. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomedicine & Pharmacotherapy, 64(1): 21-28.
|
Dodgson K S, Price R G. 1962. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochemical Journal, 84(1): 106-110.
DOI:10.1042/bj0840106 |
Duckworth M, Yaphe W. 1971. Preparation of agarose by fractionation from the spectrum of polysaccharides in agar. Analytical Biochemistry, 44(2): 636-641.
|
Freile-Pelegrín Y, Murano E. 2005. Agars from three species of Gracilaria (Rhodophyta) from Yucatán Peninsula. Bioresource Technology, 96(3): 295-302.
DOI:10.1016/j.biortech.2004.04.010 |
He B Y, Ou G N, Li X M, Chen W J, Zhang X R. 2006. Primary study on the composition and structure of polysaccharides from Bangia fusco-purpurea. Food Science, 27(11): 210-214.
(in Chinese with English abstract) |
Hegenauer J C, Nace G W. 1965. An improved method for preparing agarose. Biochimica et Biophysica Acta (BBA)-General Subjects, 111(1): 334-336.
DOI:10.1016/0304-4165(65)90503-9 |
Hjertén S. 1964. The preparation of agarose spheres for chromatography of molecules and particles. Biochimica et Biophysica Acta (BBA)-Specialized Section on Biophysical Subjects, 79(2): 393-398.
DOI:10.1016/0926-6577(64)90020-8 |
Huang T T, Ye L Y, Sha Y, Tu S, Xiao Z Y, Li X M, Li Y Z, Li J B. 2010. Optimization of alkali treatment technological conditions for agar prepared from Gracilaria lemaneiformis. Chemical Engineering & Equipment, 28(10): 12-15, 28.
(in Chinese with English abstract) |
Huo P, Zhang K C, XU R. 1999. Studies on using stillage producing polysaccharide by submerged fermentation of lentinus edodes. Journal of Wuxi University of Light Industry, 18(1): 47-49.
(in Chinese with English abstract) |
Hurtado-Ponce A Q, Umezaki I. 1988. Physical properties of agar gel from Gracilaria (Rhodophyta) of the Philippines. Botanica Marina, 31(2): 171-174.
|
Ji M H. 1997. IR Spectra of Agar. Seaweed Chemistry. Science Press, Beijing, China. p.89-92.
|
Kang X J, Qu J S. 2006. Analysis of Angelica dahurica polysaccharide by gas chromatography. Chinese Journal of Pharmaceutical Analysis, 26(7): 891-894.
(in Chinese with English abstract) |
Kirkpatrick F H, Guiseley K, Provonchee R, Nochumson S. 1991-01-08. High gel strength low electroendosmosis agarose: US, US4983268A.
|
Kloareg B, Quatrano R S. 1988. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanography and Marine Biology:An Annual Review, 26: 259-315.
|
Lee W K, Lim Y Y, Leow A T C, Namasivayam P, Abdullah J O, Ho C L. 2017. Factors affecting yield and gelling properties of agar. Journal of Applied Phycology, 29(3): 1527-1540.
DOI:10.1007/s10811-016-1009-y |
Lins K O A L, Bezerra D P, Alves A P N N, Alencar N M N, Lima M W, Torres V M, Farias W R L, Pessoa C, De Moraes M O, Costa-Lotufo L V. 2009. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). Journal of Applied Toxicology, 29(1): 20-26.
DOI:10.1002/jat.v29:1 |
Matsuhiro B, Zanlungo A. 1983. Colorimetric determination of 3, 6-anhydrogalactose in polysaccharides from red seaweeds. Carbohydrate Research, 118: 276-279.
DOI:10.1016/0008-6215(83)88056-2 |
McHugh D J. 2003. A Guide to the Seaweed Industry. FAO Fish Tech Pap 441, Rome, Italy. 105p.
|
Mclachlan J, Bird C J. 1986. Gracilaria (Gigartinales, Rhodophyta) and productivity. Aquatic Botany, 26: 27-49.
DOI:10.1016/0304-3770(86)90004-5 |
Meena R, Prasad K, Siddhanta A K. 2011. Preparation of superior quality products from two Indian agarophytes. Journal of Applied Phycology, 23(2): 183-189.
DOI:10.1007/s10811-010-9523-9 |
Meena R, Siddhanta A K, Prasad K, Ramavat B K, Eswaran K, Thiruppathi S, Ganesan M, Mantri V A, Rao P V S. 2007. Preparation, characterization and benchmarking of agarose from Gracilaria dura of Indian waters. Carbohydrate Polymers, 69(1): 179-188.
DOI:10.1016/j.carbpol.2006.09.020 |
Nishinari K, Fang Y P. 2017. Relation between structure and rheological/thermal properties of agar. A mini-review on the effect of alkali treatment and the role of agaropectin. Food Structure, 13: 24-34.
|
Nishino T, Nagumo T, Kiyohara H, Yamada H. 1991. Structural characterization of a new anticoagulant fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydrate Research, 211(1): 77-90.
DOI:10.1016/0008-6215(91)84147-7 |
Polson A. 1967-08-08. Fractionation of mixtures of agarose and agaropectin: US, US3335127A.
|
Provonchee R B. 1991-02-05. Agarose purification method using glycol: US, US4990611A.
|
Qi H M, Li D X, Zhang J J, Liu L, Zhang Q B. 2008. Study on extraction of agaropectin from Gelidium amansii and its anticoagulant activity. Chinese Journal of Oceanology and Limnology, 26(2): 186-189.
DOI:10.1007/s00343-008-0186-1 |
Rebello J, Ohno M, Ukeda H, Kusunose H, Sawamura M. 1997. 3, 6 anhydrogalactose, sulfate and methoxyl contents of commercial agarophytes from different geographical origins. Journal of Applied Phycology, 9(4): 367-370.
DOI:10.1023/A:1007954220257 |
Rees D A. 1961. Enzymic synthesis of 3:6-anhydro-L-galactose within porphyran from L-galactose 6-sulphate units. Biochemical Journal, 81(2): 347-352.
DOI:10.1042/bj0810347 |
Rupérez P, Ahrazem O, Leal J A. 2002. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. Journal of Agricultural and Food Chemistry, 50(4): 840-845.
DOI:10.1021/jf010908o |
Rupérez P. 2002. Mineral content of edible marine seaweeds. Food Chemistry, 79(1): 23-26.
DOI:10.1016/S0308-8146(02)00171-1 |
Salehi P, Dashti Y, Tajabadi F M, Safidkon F, Rabei R. 2011. Structural and compositional characteristics of a sulfated galactan from the red alga Gracilariopsis persica. Carbohydrate Polymers, 83(4): 1570-1574.
DOI:10.1016/j.carbpol.2010.10.017 |
Shanmugam M, Mody K H. 2000. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Current Science, 79(12): 1672-1683.
|
Souza B W S, Cerqueira M A, Bourbon A I, Pinheiro A C, Martins J T, Teixeira J A, Coimbra M A, Vicente A A. 2012. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocolloids, 27(2): 287-292.
DOI:10.1016/j.foodhyd.2011.10.005 |
Sukhoverkhov S V, Kadnikova I A, Podkorytova A V. 2000. Production of agar and agarose from the red alga Ahnfeltia tobuchiensis. Applied Biochemistry and Microbiology, 36(2): 201-203.
DOI:10.1007/BF02737922 |
Wang L, Liu L, Wang Y M, Yuan Q Y, Li Z E, Xu Z H. 2001. Comparative research on the structures and physicalchemical properties of agars from several agarophyta. Oceanologia et Limnologia Sinica, 32(6): 658-664.
(in Chinese with English abstract) |
Wang X Y, Duan D L, Fu X T. 2016. Enzymatic desulfation of the red seaweeds agar by Marinomonas arylsulfatase. International Journal of Biological Macromolecules, 93: 600-608.
DOI:10.1016/j.ijbiomac.2016.08.031 |
Watase M, Nishinari K. 1986. Rheological and thermal properties of polysaccharide gels extracted from Ahnfeltia plicata. Colloid and Polymer Science, 264(10): 877-882.
DOI:10.1007/BF01410638 |
Whyte J N C, Foreman R E, DeWreede R E. 1984. Phycocolloid screening of British Columbia red algae. Hydrobiologia, 116-117(1): 537-541.
DOI:10.1007/BF00027741 |
Zhang L, Xu J Z, Xue C H, Gao X, Zhang D. 2009. Rheological properties and gel properties of agar. Chinese Journal of Marine Drugs, 28(2): 11-17.
(in Chinese with English abstract) |
Zhao M M, Liu T X, Wu H, Peng Z Y, Gao K R. 1996. A study of the alkali-treatment effect on the extraction yield, properties and chemical composition of agar. Food and Fermentation Industries, (6): 1-7.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37


