Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SILVA J. Paulo, ALVES Celso, PINTEUS Susete, SILVA Joana, VALADO Ana, PEDROSA Rui, PEREIRA Leonel
- Antioxidant and antitumor potential of wild and IMTA-cultivated Osmundea pinnatifida
- Journal of Oceanology and Limnology, 37(3): 825-835
- http://dx.doi.org/10.1007/s00343-019-8110-4
Article History
- Received May. 21, 2018
- accepted in principle Jul. 31, 2018
- accepted for publication Oct. 15, 2018
2 MARE-Marine and Environmental Sciences Centre, School of Tourism and Maritime Technology, Polytechnic Institute of Leiria, Peniche 2520-641, Portugal;
3 Instituto Politécnico de Coimbra, ESTESC-Coimbra Health School, Biomedical Sciences Laboratory, R. 5 de Outubro, S. Martinho do Bispo, Ap. 7006, Coimbra 3046-854, Portugal;
4 Centre of Pharmacology and Chemical Biopathology, Faculty of Medicine, University of Porto, Porto 4200-319, Portugal;
5 MARE-Marine and Environmental Sciences Centre, Department of Life Sciences, Faculty of Sciences and Technology, University of Coimbra, Coimbra 3000-456, Portugal
Cancer figures among the leading causes of mortality worldwide, being the second leading cause of death behind cardiovascular diseases and causing about 8.8 million deaths in 2015 (GBD 2015 Mortality and Causes of Death Collaborators, 2016). The global cancer burden is growing at an alarming pace, reaching about 21.6 million new cancer cases and 13 million cancer deaths by 2030 (American Cancer Society, 2018). Several approaches on targeted therapies have significantly changed the treatment of cancer over the last years (Tsimberidou, 2015). Cisplatin, carboplatin, and oxaliplatin are still involved in nearly 50% of all anticancer therapies worldwide (Liu et al., 2014). However, problems of platinum resistance and undesirable side effects are limiting their future use, emphasizing the urgent need for new anticancer agents.
The marine environment harbors a great diversity of organisms that can synthesize secondary metabolites to deal with ecological pressures. Furthermore, it has been found that these natural compounds are biologically active, offering a great scope for the discovery and development of new drugs (Pomponi, 2001). In fact, some natural products have made it into clinical routines, mainly in the cancer therapeutic area (ziconotide and trabectedin, for instance), and many more are in all phases of clinical testing (e.g. pliditepsin, PM060184, marizomib and bryostatin I) (Martins et al., 2014).
In the particular case of marine algae, seaweedderived products shown potential as sources of potent anticancer drugs when tested in vitro and/or in vivo (Murphy et al., 2014). Additionally, studies on the bioactivities of seaweed components revealed many other health-promoting effects including antiviral, antibiotic, antithrombotic, anticoagulant, antiinflammatory and immunostimulatory (Smit, 2004). Seaweeds are either rich sources of antioxidants and there are some indications for the potential to mediate a cancer chemopreventive effect (Park and Pezzuto, 2013) since antioxidants are capable of protecting cells by modulating the harmful effects of oxidative stress (Lee et al., 2013).
Seaweeds are consumed on a daily basis in some parts of the world and are now being spread to the western cousins, where they are considered healthy delicacies. In fact, seaweeds are known to be rich in polysaccharides and other constituents as minerals, proteins, essential amino acids, polyunsaturated fatty acids, fiber, pigments and vitamins (Cardoso et al., 2014). For their various bioactivities, compounds derived from marine algae are important ingredients in many products, such as cosmetics (Bedoux et al., 2014) and functional food (Holdt and Kraan, 2011).
Osmundea pinnatifida (Hudson) Stackhouse (phylum Rhodophyta, order Ceramiales, family Rhodomelaceae, tribe Laurenciae) is an edible seaweed traditionally consumed in several countries of Europe. This aromatic seaweed is dried and used as a pepper- or curry-flavored spice in Scotland and Ireland. In Azores (Portugal) it is collected, prepared, and pickled in vinegar and later eaten with fried food (Pereira, 2016). It is quite abundant throughout the year in the intertidal rocky shores on the north and center of Portugal, which turns it in a potential candidate for biotechnological applications. Indeed, there are some investigation regarding bioactive assessment (Barreto et al., 2012; Paiva et al., 2012; Rodrigues et al., 2015a, 2016; Pereira, 2018) and nutritional value (Patarra et al., 2013; Paiva et al., 2014; Rodrigues et al., 2015b; Pereira, 2016) of O. pinnatifida. The present study evaluates the antioxidant and antitumor potential of O. pinnatifida, establishing a comparison between wild samples collected from continental Portuguese coast and those cultivated in an integrated multi-trophic aquaculture (IMTA) system in a sustainable way at ALGAplus facilities (www.algaplus.pt). Until now, there are no records of cultivation of this specie, which turns this work a pilot study.
2 MATERIAL AND METHOD 2.1 Seaweed collection and cultivationOsmundea pinnatifida was collected at the rocky shore of Cape Mondego, Figueira da Foz (Portugal) and split into two portions. One of them was used to undergo a small-scale cultivation trial at ALGAplus facilities located in Ria de Aveiro, Portugal. The other portion was washed to remove epiphytes, detritus, and encrusting material, and then was hermetically stored at -80℃ until further use.
The seaweed farm ALGAplus is integrated with a seabream and seabass production and uses the nutrient enriched water resultant from the fish production as its cultivation medium (IMTA concept). Osmundea pinnatifida was grown by vegetative propagation in a batch-culture mode (in 15-L fiber-glass tanks), using the nutrient-enriched fish water (renewed every 3–4 days). This land-based cultivation assay was conducted for five weeks and performed in quadruplicate. The biomass produced, as well as frozen samples of the initial inoculum (wild biomass from Cape Mondego), were used to obtain extracts.
2.2 Preparation of seaweed extractsLyophilized seaweed was ground with a mixer grinder and sequentially extracted at a proportion of 1:4 (biomass: solvent) with methanol and dichloromethane for 12 h at constant stirring. Methanolic extract was subjected to a liquid-liquid extraction with n-hexane (1:1) in 15 min during which methanol and n-hexane fractions were obtained. The organic extract and the two fractions were filtered with Whatman filter paper and the solvents evaporated in a rotary evaporator at 40℃. After that, dried extracts were dissolved in dimethyl sulfoxide (DMSO; 2% final concentration) and maintained at -20℃ until further use. The percentage extraction yield (%) was calculated according to Foo et al. (2017), as follows:

The concentration of polyphenols in seaweed extracts was determined with the Folin-Ciocalteu method adapted to microscale (Zou et al., 2011) with minor modifications. Gallic acid was used as a standard phenolic compound. Briefly, 2 μL of extract or standard gallic acid solutions (10, 30,100,300, and 1 000 μg/mL) were added to 158 μL of distilled water and 10 μL of Folin-Ciocalteu reagent. After 2 min it was added 30 μL of 20% Na2CO3 and incubated 1 h in the dark. The absorbance was measured at 755 nm in a microplate reader compared to the blank solution (prepared by the same procedure described above, but replacing Folin-Ciocalteu reagent for water). The TPC is expressed as mg gallic acid equivalents per g of dry extract (mg GAE/g).
2.4 Estimation of antioxidant activity 2.4.1 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activityDPPH radical scavenging activity was assessed by the DPPH decolorization assay adapted to microscale (Herald et al., 2012) with slight modifications. DPPH radical was dissolved in absolute ethanol (0.1 mmol/L). The reaction of 2 μL of extract with 198 μL of DPPH solution occurred for 30 min at room temperature in the dark, and the absorbance measured at 517 nm. Butylated hydroxytoluene (BHT), a synthetic compound commonly used as an antioxidant, was used as the standard (Sudha et al., 2017; Lesjak et al., 2018). The antioxidant capacity was expressed as a percentage of control and determined according to Pinteus et al. (2017), as follows:
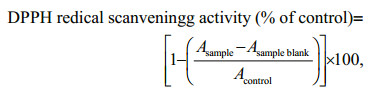
in which Acontrol is the absorbance of the control (DPPH solution with dimethyl sulfoxide), Asample is the absorbance of the test sample (DPPH solution plus test sample), and the Asample blank is the absorbance of the sample in ethanol (sample without DPPH solution).
2.4.2 Oxygen Radical Scavenging Activity (ORAC)Oxygen Radical Absorbance Capacity (ORACfluorescein) assay was performed as described by Dávalos et al.(2004). The results are presented as μmol Trolox equivalents/g of dry extract (μmol TE/g).
2.5 Evaluation of antitumor activityThe experiments were performed in different human cancer cell lines: a model of neuroblastoma (SH-SY5Y cells) (ACC 209) and a breast adenocarcinoma model (MCF-7) (ACC 115) acquired from DMSZ bank, and a model of a human hepatocellular cancer (HepG-2) acquired from ATCC bank (ATCC: HB-8065). SH-SY5Y cells were cultured in DMEM supplemented with 20% (v/v) of fetal bovine serum (FBS) and 1% of the antibiotic/ antimycotic commercial solution. HepG-2 and MCF- 7 cells were cultured in RPMI-1640 medium supplemented with 10% of FBS and 1% of antibiotic/ antimycotic. The medium used for MCF-7 cells was the same used for HepG-2 cells, however, supplemented with 1% of MEM non-essential amino acids (includes L-alanine, L-asparagine, L-aspartic acid, L-glycine, L-serine, L-proline, and L-glutamic acid), 1 mmol/L of sodium pyruvate and 10 μg/mL of human insulin cells.
For subculture, cells were dissociated with trypsinEDTA, split into a 1:3 (HepG-2) and 1:4 (MCF-7 and SH-SY5Y) ratio and maintained in controlled conditions (37℃, 5% CO2, 95% of moisture).
Cytotoxicity and antiproliferative experiments were performed after the cells reached total confluence and after 24 h of the seeding, respectively. Crude extracts previously filtered (0.2 μm, Whatman, UK) were incubated with the cells for 24 h at 1 mg/mL. Dose-response studies were accomplished for the samples that exhibited the highest activities (10– 1 000 μg/mL; 24 h). The extract effects were revealed in the MTT method as described by Alves et al. (2016), in which Cisplatin (Sigma, USA) was used as a chemotherapeutic standard drug, and results are expressed in IC50.
2.6 Statistical analysisAll experiments were performed at least in triplicate (n ≥3), except for the extraction yield. Results are expressed as mean±SD or IC50. IC50 values were calculated from non-linear regression analysis using the GraphPad Prism (v6.01) program with the equation Y = 100 /(1+10(X–logIC50)). Two-way analysis of variance (ANOVA) followed by Fisher's Least Significant Difference (LSD) test was performed to test for differences between samples (wild and IMTAcultivated) and extracts/fractions (dichloromethane, methanol, and n-hexane) using the software IBM SPSS v21.0. The significance of the differences was defined at the 5% level (P < 0.05). The correlation between TPC, DPPH radical scavenging activity, and ORAC was assessed by Pearson correlation test in GraphPad Prismsoftware (v5.0). Principal components analysis (PCA) was also performed using CANOCO software (v4.5).
3 RESULT 3.1 Extraction yieldTable 1 shows the extraction yields using methanol, dichloromethane, and n-hexane as organic solvents to obtain extracts with a different polarity from wild and IMTA-cultivated samples of Osmundea pinnatifida. The highest yield was achieved by the extraction with methanol. The amount of extract obtained with the other solvents was very low, however, the extraction with dichloromethane, on average, displayed a slightly higher yield than n-hexane.

|
TPC values are presented in Table 1 and expressed as mg gallic acid equivalents per gram of dry extract (mg GAE/g). When TPC values for all three extracts are totaled, there are no statistically significant differences (P > 0.05) between phenolic contents of wild and IMTA-cultivated samples (87.57 and 65.89 mg GAE/g, respectively). On the other hand, when analyzing the effects at the extractant level, the extraction performed with dichloromethane had the highest value. The value of dichloromethane extract from the wild sample (Dw) was 46.82 mg GAE/g while those of the remaining extracts were between 31.83 (methanol fraction) and 8.92 mg GAE/g (n-hexane fraction). In the case of the IMTA-cultivated sample, dichloromethane extract (Dc) registered a TPC value of 43.48 mg GAE/g while methanol (Mc) and n-hexane (Hc) fractions presented 15.55 and 6.86 mg GAE/g, respectively. The amount of phenols extracted either with dichloromethane and n-hexane solvents did not differ significantly (P > 0.05) between wild and IMTA-cultivated samples. However, significant differences (P < 0.05) were found in TPC among methanol fractions of these samples.
Regarding antioxidant activity, the methanol fraction of wild sample (Mw), as well as n-hexane fractions (both samples), showed the highest DPPH radical scavenging activity. No statistically significant differences (P > 0.05) were found among these extracts. Their ability to scavenge DPPH radical in comparison to control were almost the same, ranging from 51.17 (Hc) to 55.23% (n-hexane fraction of wild sample, Hw), being followed by dichloromethane extracts (42.03% and 44.82% for IMTA-cultivated and wild samples, respectively). The lowest DPPH radical scavenging activity (32.57) was observed in the Mc. No significant correlation (P > 0.05) was found between DPPH radical scavenging activity and TPC. Additionally, EC50 values (μg/mL) were determined for the samples which displayed over 50% reduction on the DPPH radical. Mw, Hw and Hc scored, respectively, 911.1 (855.6–970.2), 1 114 (873.1– 1 421) and 1 346 (1 149–1 577) μg/mL. BHT was used as a positive reference (EC50: 40.55 (27.39– 60.05) μg/mL).
Lastly, when ORAC values for all three extracts are totaled for each sample, there are no statistically significant differences (P > 0.05) among them. The peroxyl radical scavenging activity of seaweed extracts ranged from 61.9 to 499.9 μmol of TE/g of dry extract, depending on sample and extractant used (Table 1). Dichloromethane extracts showed higher scavenging activity against peroxyl radicals than methanol and n-hexane fractions, being the mean difference statistically significant at the 0.05 level. The highest ORAC value was obtained for the Dc (499.9 μmol of TE/g extract), followed by Dw (414 μmol of TE/g extract) and Mw (271.32 μmol of TE/g extract), whereas Hw (61.9 μmol of TE/g extract) presented the lowest. There must note that ORAC values obtained with dichloromethane extraction do not differ significantly (P > 0.05) among the wild and IMTA-cultivated samples. Interestingly, a significantly strong correlation (P < 0.01, R = 0.949 9, R2 = 0.902 4) was found between ORAC and TPC.
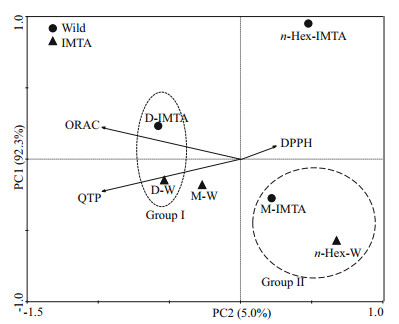
3.1.2 Principal components analysisPrincipal components analysis (PCA) was performed to acquire an overview of the antioxidant potential of all samples (6 fractions (methanol, dichloromethane, and n-hexane) from wild and IMTAcultivated seaweeds), accessed by all methodologies (QTP, DPPH, and ORAC). Principal components 1 and 2 explain 92.3% and 5.0% of the total variance of the data set, respectively (Fig. 1). The analysis of the second principal component (PC2) (Fig. 1), the vertical axis expressed an opposition between DPPH radical scavenging activity (left) and TPC (right). Moreover, TPC showed a negative correlation with DPPH radical scavenging activity. In fact, the fractions that showed high phenolic content presented low ability to scavenge DPPH radical (for example, n-hexane fraction of wild sample, and methanol fraction of the IMTA-cultivated, Group II). On the other hand, dichloromethane fractions of both samples (Group I) showed high levels of TPC and weak DPPH radical scavenging activity, because it has located on the opposite side of PC2 (Fig. 1). Additionally, through the PC2 analysis, it is also possible to observe that TPC has a positive correlation with ORAC (Fig. 1). The first principal component (PC1), explain the variance between the samples in relation to the ability to neutralize peroxyl radicals (ORAC). Dichloromethane extracts showed the highest ability to neutralize peroxyl radicals, being the same extracts where the highest phenolic content was found (Fig. 1).
|
| Fig.1 Principal component analysis (PCA) scatter plot of total phenolic content (TPC) and antioxidant activities (DPPH radical scavenging activity and ORAC) of dichloromethane extract (D), methanol (M) and n-hexane (n-Hex) fractions of O. pinnatifida from the Portuguese coast |
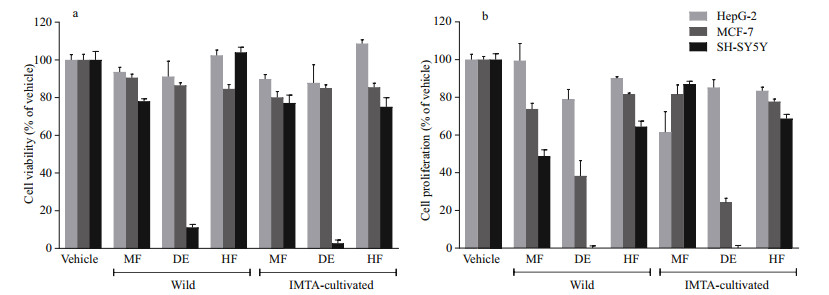
Cell viability and proliferation effects on HepG-2, MCF-7 and SH-SY5Y cell lines after exposure to extracts of O. pinnatifida (1 000 μg/mL; 24 h) were firstly quantified as a percentage of control (Fig. 2). As shown in Fig. 2a, after 24 h of incubation, all the tested extracts presented a very low level of cytotoxicity both in HepG-2 and MCF-7 cell lines. In fact, none of the extracts could reduce cell viability by over 20%. However, except for the Hw, SH-SY5Y cells were more susceptible to the extracts than HepG-2 and MCF-7 cells. In fact, dichloromethane extracts (Dw and Dc) were responsible for reducing SH-SY5Y cell viability above 88%. There are no statistically significant differences at the 0.05 level between the effects caused by Dw and Dc in SH-SY5Y cells. The effects of these extracts were dose-dependent and Dc presented a lower IC50 value than Dw (431.2 and 656.8 μg/mL, respectively).
|
| Fig.2 Effect of Osmundea pinnatifida extracts on viability (a) and proliferation (b) of HepG-2, MCF-7, and SH-SY5Y cells Cells were treated with seaweed fractions (MF: methanol fraction; HF: n-hexane fraction; DE: dichloromethane extract) at 1 000 μg/mL for 24 h. Results are the mean±SEM. |
On the other hand, effects on cell proliferation were also observed. While the extracts inhibited from near 10% (Hw) to 38% (Mc) of the HepG-2 cell proliferation, in MCF-7the percentage of inhibition ranged from 18% (Hw and Mc) to 75% (Dc), whereas in SH-SY5Y it was between 13% (Mc) and 100% (Dw and Dc). Only dichloromethane extracts (Dw and Dc) have shown the ability to reduce cell proliferation by more than 50% both in MCF-7 and SH-SY5Y cells. It is important to emphasize that these extracts also exhibited dose dependency in MCF-7 and SH-SY5Y cell proliferation. There are no statistically significant differences (P > 0.05) between the effects caused by Dw and by Dc in MCF-7 and SH-SY5Ycells proliferation. As shown in Table 2, the Dc presented a lower IC50 value (923.6 μg/mL) than Dw (945.8 μg/ mL) in MCF-7 cells. Contrariwise, Dw was more effective than Dc in SH-SY5Y cells (IC50: 508.8 and 525.9 μg/mL, respectively).

|
The isolation of compounds from natural sources frequently begins with the evaluation of their extracts properties. Solvent extractions, due to their ease of use, efficiency, and wide applicability, are the most commonly used procedures to recover bioactive compounds from foodstuffs. The efficiency of extraction depends on several factors including solvent chemical properties (such as polarity), extraction time and temperature, the sample-tosolvent ratio as well as on the chemical composition and physical characteristics of the samples (Dai and Mumper, 2010; Bae et al., 2012). As shown in Table 1, and in agreement with that obtained by Barreto and co-workers (2012), methanol (polarity index 5.1) was the most effective solvent to extract soluble compounds from O. pinnatifida. Similar yield values (2.85%–5.01%) are given in methanol extracts from selected Indian red seaweeds (Ganesan et al., 2008). Curiously, in our work, while the yield of extraction with dichloromethane and n-hexane registered very little variation, the one obtained with methanol in wild sample was 2-fold higher than IMTA-cultivated. The chemical composition of seaweeds varies with individuals, species, habitats, maturity and environmental conditions (Ito and Hori, 1989). Wild and IMTA-cultivated samples grown in different environmental conditions (light, temperature, salinity) and were harvested in distinct seasons. As referred by Abreu et al. (2011), temperature and light are usually the most important environmental factors affecting growth and nutrient uptake of seaweeds. Furthermore, the observed yield variation may also be due to lack of ecological pressures, both biotic (e.g. predation, competition) and abiotic (e.g. nutrients) from the controlled environment in which the algae were grown. In turn, this may have led to a suppression of the production of certain polar chemical compounds in the IMTA-cultivated sample.
4.2 Antioxidant activityAs suggested by Huang et al. (2005), to comprehensively study different aspects of antioxidants, validated and specific assays, such as DPPH radical scavenging activity, are needed in addition to the total phenol assay using FolinCiocalteau reagent and ORAC assay. Briefly, depending on the reactions involved, the antioxidant capacity assays can be classified into two types: assays based on hydrogen atom transfer reactions (HAT), where the antioxidant's reducing capacity is measured, and assays based on electron transfer (ET), where the hydrogen atom donating capacity is measured. ORAC assay is based on HAT, and according to most HAT-based assays, a competitive reaction scheme is applied, in which antioxidant and substrate compete for thermally generated peroxyl radicals through the decomposition of azo compounds. In the other hand, DPPH and TPC assays are based on ET, in which the capacity of an antioxidant in the reduction of an oxidant is measured (the color changes when the oxidant is reduced) (Huang et al., 2005).
The total phenolic content evaluated through FolinCiocalteu reagent has become a routine assay in studying phenolic antioxidants once it is a convenient, simple and reproducible assay (Huang et al., 2005). Seaweed may contain phenolics varying from simple (e.g. phenolic acids) to more complex substances (e.g. phlorotannins) in different quantities. The fact that phenolic compounds can be combined with other components (such as carbohydrates and proteins) means that there is no universal extraction method for removal of all phenolic compounds from plant matrices. Nevertheless, solvents such as methanol, ethanol, acetone and their combinations have been widely used for the extraction of phenolics (Dai and Mumper, 2010). Furthermore, it has been found that the phenolics extraction efficiency increase with increasing polarity of the extractant (Airanthi et al., 2011). Contrariwise, in the present study, phenolic compounds presented higher solubility in dichloromethane (polarity index, P', of 3.1) than in methanol (P' 5.1) solvent. In the case of the O. pinnatifida collected in the Azores, the amount of phenolics extracted with methanol did not differ significantly from that extracted with dichloromethane (34.67 versus 33.26 mg GAE/g, respectively) (Barreto et al., 2012). As occurs with other chemical constituents, the composition of phenolic compounds both qualitatively and quantitatively might vary depending on the specie and many other variables such as habitat, season of harvesting, geographical distribution and environmental conditions (salinity, light and temperature) (De Quirós et al., 2010; Ibañez et al., 2012).
In the present study, despite being insignificant (P > 0.05), TPC was negatively correlated (R = -0.228 8) to their DPPH radical scavenging activity. This feature is in agreement with the previous evaluation of antioxidant activities of 2 selected Indian red seaweeds—Acanthophora spicifera and Gracilaria edulis (Rhodophyta)—where DPPH radical scavenging activity increased with decreasing TPC (Ganesan et al., 2008). Thus, we presume that antioxidant compounds other than polyphenols are involved in the observed DPPH radical scavenging activity. Mw, Hw and Hc fractions are responsible for reducing the DPPH radical by more than 50% (52.85 and 51.17, respectively). In the case of n-hexane fractions, the radical scavenging activity could be related to less polar compounds, such as fatty acids, carotenoids (e.g. β-carotene, lutein), tocopherols, sterols, and terpenoids, as referred by Burtin (2003). To measure the extracts' potency, the analysis of concentration-effect curves through the measurement of the extract concentration necessary to give 50% of the maximum response (EC50) was made. The antioxidant activity of these 3 fractions exhibited dose dependency, increasing with increasing concentration of extract (data not shown). The determined EC50 values are higher than BHT (EC50: 40.55 μg/mL), used as positive control. These results are in agreement with that obtained by Barreto et al. (2012), revealing that O. pinnatifida extracts are weak DPPH radical scavengers.
ORAC assay involves the absorption of peroxyl radical absorption by antioxidant compounds. This method is particularly interesting when the sample is a mixture of compounds since the entire reaction is accompanied throughout time (Wang et al., 2009; Zulueta et al., 2009). The presence of a correlation between TPC and the ORAC of seaweed extracts has been previously reported (Wang et al., 2009). In this study, ORAC values were positively, highly (R = 0.949 9; R2 = 0.902 4), and significantly (P < 0.01) correlated with TPC values. This could indicate that phenolic compounds present may be mostly responsible for the verified peroxyl radical absorption capacity of O. pinnatifida extracts. In fact, among the myriad of bioactivities related to phenolic compounds, antioxidant activity seems to be the main one (Ibañez et al., 2012). The multifunctional antioxidant activity of polyphenols is highly related to phenol rings which act as electron traps to scavenge peroxyl, superoxide anions and hydroxyl radicals (Sathya et al., 2017). However, due to the nature of the extracts, we cannot discard a synergistic or antagonistic interaction between phenolic and non-phenolic compounds that could be affecting the bioactivity.
Wojcikowski et al. (2007) studied the in vitro antioxidant capacity of 55 medicinal plants through ORAC method using a sequential multi-solvent extraction process (ethyl acetate, methanol and 50% aqueous methanol). Of them, only six species have registered higher total (sum of fractions) ORAC values than both the wild (747.22 μmol of TE/g extract) and IMTA-cultivated (771.59 μmol of TE/g extract) O. pinnatifida. Even Camelia sinensis leaf and Sylibum marianum seed, which were included due to its known high antioxidant activity, showed lower total ORAC values (627.14 and 553.91, respectively) than both O. pinnatifida samples. Up to now, ORAC data on seaweeds is very limited. Indeed, to our knowledge, this is the first time that this methodology is applied for the screening of Osmundea pinnatifida antioxidant activity. Even so, the ORAC values obtained in this study for dichloromethane extracts (414 and 499.9 for wild and IMTA-cultivated sample, respectively) are higher than 70% acetone extracts of Chondrus crispus, Palmaria palmata (Rhodophyta), Ulva lactuca (Chlorophyta) (numerical data not shown) and Laminaria digitata (Phaeophyceae; 4 μmol of TE/g extract); and comparable to those obtained for Saccharina latissima and Alaria esculenta (Phaeophyceae) (Wang et al., 2009). As reported by Nogueira et al. (2014), amongst the red seaweeds, those belonging to the family Rhodomelaceae (order Ceramiales) are the most promising as potential producers of antioxidants; and this feature seems to be related to the ability to synthesize polyphenols and their derivatives, as bromophenols.
Therefore, O. pinnatifida can be obtained in an economical and environmentally sustainable way, through IMTA, maintaining bioactive properties with high potential for further nutraceutical purposes. It is known also that cerebral ischemia is the most common cause of neurological incapacitation in adults, causing inflammation, oxidative stress, and neuronal apoptosis. Thus, the ingestion of algae rich in antioxidants may stimulate the mechanisms of endogenous protection, promoting a neuroprotective effect (Pangestuti and Kim, 2011; Silva et al., 2017).
4.3 Antitumoral activityCurrently, several research institutions have concentrated their efforts in the search for effective and safe novel drugs for the treatment of cancer. As reported by Abu-Dahab and Afifi (2007) and in agreement with the US NCI plant screening program, a pure compound is generally considered to have in vitro active cytotoxic effect if the IC50 value in carcinoma cells, following incubation between 48 to 72 h, is less than 4 μg/mL, while for crude extracts it is less than 20 μg/mL.
Despite many in vitro tests did not differentiate between cytotoxic and cytostatic effects, previous studies reported that seaweeds of Laurenciae tribe are a good source of metabolites that have displayed cytotoxic activity. Chamigren sesquiterpenoid metabolites isolated from algae of the genus Laurencia were found to possess cytotoxic activity, especially against colon tumor cell lines (Juagdan et al., 1997). In addition, two polyether squalene derivatives (thyrsenol A and thyrsenol B) isolated from Laurencia viridis has been demonstrated to exhibit cytotoxic activity in a panel of cancer cell lines (P-388, A-549, MEL 28, and HT 29; from DBA/2 mouse lymphoid neoplasm, human lung carcinoma, human colon carcinoma and human melanoma, respectively) (Norte et al., 1997).
In the present study, the tested extract and fractions have selective cytotoxic and antiproliferative effects, since, in general, SH-SY5Y cells were more susceptible than MCF-7 cells, being the latter more susceptible than HepG-2 cells (Fig. 2). Additionally, a cytostatic effect has occurred in the MCF-7 cell line. The dichloromethane extracts of both samples did not induce cell mortality but kept them from proliferating in a 24-h long in vitro colorimetric assay using MTT. However, the IC50 values obtained for these extracts (945.8 and 923.6 μg/mL for wild and IMTA-cultivated samples, respectively) in the MCF-7 antiproliferative activity do not fit in the NCI criteria for considering a crude extract as active. The cytotoxic and antiproliferative activity shown by both dichloromethane extracts (Dw and Dc) in SH-SY5Y cells do not fit in these criteria as well. It is necessary to evaluate the effects in a longer trial time, so to carry out fractionation of the dichloromethane crude extract to isolate and identify the compound responsible for the observed effect on MCF-7 and SH-SY5Y cell lines.
Nevertheless, previous studies also screened cytotoxic effects of Osmundea extracts in tumor cell lines. In agreement with our work, Barreto et al. (2012) also found that among methanol and n-hexane fractions and dichloromethane extracts, the latter had the strongest activity. Thus, O. pinnatifida dichloromethane extract also had selective and weak cytotoxicity, scoring an IC50 value of 129.3 μg/mL in HeLa (human cervix carcinoma) cell line, whereas it was over than 200 μg/mL for Vero non- tumor cell line. In addition, O. hybrida and O. pinnatifida extracts had no cytotoxicity towards L-6, another non- tumor cell line.
Several authors have already investigated the nutritional composition of numerous seaweed, including Osmundea pinnatifida (Patarra et al., 2013; Paiva et al., 2014). They strikeout that the tested seaweed species are a good source of protein, polyunsaturated fatty acids, fiber, vitamins, minerals and also contain acceptable amounts of 9 out of 10 essential amino acids as compared to terrestrial foodstuffs. In addition, it was also found to contain dietary essential fatty-acids, namely linoleic acid, which prevents deficiency symptoms and cannot be synthesized by humans, as referred by Patarra et al. (2013). The fatty acid profiles, the ω-3/ ω-6 and h/H ratios and also the non-animal nature of the seaweed nutrients suggests that they can have potential health benefits and interest to the food supplement and/or pharmaceutical industries, whose products might augment a nutritionally balanced diet.
5 CONCLUSIONThis is the first study screening the antioxidant and antitumor potential of the edible seaweed O. pinnatifida collected from the Portuguese coast. Additionally, the biotechnological potential of cultivated samples of this specie is compared with wild ones for the first time. Here, we report that dichloromethane extract was the best source of antioxidants, had weak cytotoxicity in SH-SY5Y cells, affected weakly the SH-SY5Y and MCF-7 cell proliferation, and that phenolic content may be responsible for the high peroxyl radical absorption capacity observed. Among the extracts/fractions that have shown to possess biological activities, there were no statistically significant differences between samples of wild and IMTA-cultivated seaweed. This means that O. pinnatifida can be obtained in an economic and environmentally sustainable way, through IMTA, not experiencing a loss of their biological potential in relation to its potential in the wild state and ensuring a valorization and preservation of natural resources. This species has the potential to turn into a great ingredient for the nutraceutical area. Additionally, with the knowledge, the most common cause of neurological disability in the human adults is cerebral ischemia promoting the inflammatory reaction, oxidative stress, and neuronal apoptosis. The deficiency of antioxidants or the inhibition of antioxidant enzymes causes oxidative stress. Thus, the ingestion of antioxidants has a neuroprotective effect.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available in the repository "Estudo Geral" from the University of Coimbra, with the identifier http://hdl.handle.net/10316/31713.
7 ACKNOWLEDGEMENTThis work had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic project (No. UID/MAR/04292/2013) granted to MARE. This work had also the support from the European Union through EASME Blue Labs project AMALIA—Algae-to-MArket Lab IdeAs (No. EASME/EMFF/2016/1.2.1.4/03/SI2.750419). Received funding from European Structural & Investment Funds through the COMPETE Programme and from National Funds through FCT—Fundação para a Ciência e a Tecnologia under the Programme (No. SAICTPAC/0019/2015).
Abreu M H, Pereira R, Yarish C, Buschmann A H, Sousa-Pinto I. 2011. IMTA with Gracilaria vermiculophylla:productivity and nutrient removal performance of the seaweed in a landbased pilot scale system. Aquaculture, 312(1-4): 77-87.
DOI:10.1016/j.aquaculture.2010.12.036 |
Abu-Dahab R, Afifi F. 2007. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Scientia Pharmaceutica, 75(3): 121-136.
DOI:10.3797/scipharm.2007.75.121 |
Airanthi M K W A, Hosokawa M, Miyashita K. 2011. Comparative antioxidant activity of edible Japanese brown seaweeds. Journal of Food Science, 76(1): C104-C111.
DOI:10.1111/j.1750-3841.2010.01915.x |
Alves C, Pinteus S, Horta A, Pedrosa R. 2016. High cytotoxicity and anti-proliferative activity of algae extracts on an in vitro model of human hepatocellular carcinoma. SpringerPlus, 5(1): 1339.
DOI:10.1186/s40064-016-2938-2 |
American Cancer Society. 2018. Cancer facts & figures 2018.https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed on 2018-02-10.
|
Bae H, Jayaprakasha G K, Crosby K, Jifon J L, Patil B S. 2012. Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods for Human Nutrition, 67(2): 120-128.
DOI:10.1007/s11130-012-0290-4 |
Barreto M C, Mendonça E, Gouveia V, Anjos C, Medeiros J S, Seca A M L, Neto A I. 2012. Macroalgae from S. Miguel Island as a potential source of antiproliferative and antioxidant products. Arquipelago Life Mar. Sci., 29: 53-58.
|
Bedoux G, Hardouin K, Burlot A S, Bourgougnon N. 2014. Bioactive components from seaweeds:cosmetic applications and future development. Advances in Botanical Research, 71: 345-378.
DOI:10.1016/B978-0-12-408062-1.00012-3 |
Burtin P. 2003. Nutritional value of seaweeds. Electron. J.Environ. Agric. Food Chem., 2: 498-503.
|
Cardoso S M, Carvalho L G, Silva P J, Rodrigues M S, Pereira O R, Pereira L. 2014. Bioproducts from seaweeds:a review with special focus on the Iberian Peninsula. Current Organic Chemistry, 18(7): 896-917.
DOI:10.2174/138527281807140515154116 |
Dai J, Mumper R J. 2010. Plant phenolics:extraction, analysis and their antioxidant and anticancer properties. Molecules, 15(10): 7313-7352.
DOI:10.3390/molecules15107313 |
Dávalos A, Gómez-Cordovés C, Bartolomé B. 2004. Extending applicability of the oxygen radical absorbance capacity(ORAC-fluorescein) assay. Journal of Agricultural and Food Chemistry, 52(1): 48-54.
DOI:10.1021/jf0305231 |
De Quirós A R B, Lage-Yusty M A, López-Hernández J. 2010. Determination of phenolic compounds in macroalgae for human consumption. Food Chemistry, 121(2): 634-638.
DOI:10.1016/j.foodchem.2009.12.078 |
Foo SC, Yusoff F M, Ismail M, Basri M, Yau S K, Khong M H, Chan K W, Ebrahimi M. 2017. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. Journal of Biotechnology, 241: 175-183.
DOI:10.1016/j.jbiotec.2016.11.026 |
Ganesan P, Kumar C S, Bhaskar N. 2008. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresource Technology, 99(8): 2717-2723.
DOI:10.1016/j.biortech.2007.07.005 |
GBD 2015 Mortality and Causes of Death Collaborators. 2016. Global, regional, and national life expectancy, allcause mortality, and cause-specific mortality for 249 causes of death, 1980-2015:a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053): 1459-1544.
DOI:10.1016/S0140-6736(16)31012-1 |
Herald T J, Gadgil P, Tilley M. 2012. High-throughput micro plate assays for screening flavonoid content and DPPHscavenging activity in sorghum bran and flour. Journal of the Science of Food and Agriculture, 92(11): 2326-2331.
DOI:10.1002/jsfa.5633 |
Holdt S L, Kraan S. 2011. Bioactive compounds in seaweed:functional food applications and legislation. Journal of Applied Phycology, 23(3): 543-597.
DOI:10.1007/s10811-010-9632-5 |
Huang D J, Ou B X, Prior R L. 2005. The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53(6): 1841-1856.
DOI:10.1021/jf030723c |
Ibañez E, Herrero M, Mendiola JA, Castro-Puyana M. 2012.Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M ed. Marine Bioactive Compounds. Springer US, Boston, MA. p.55-98.
|
Ito K, Hori K. 1989. Seaweed:chemical composition and potential food uses. Food Reviews International, 5(1): 101-144.
DOI:10.1080/87559128909540845 |
Juagdan E G, Kalidindi R, Scheuer P. 1997. Two new chamigranes from an hawaiian red alga, Laurencia cartilaginea. Tetrahedron, 53(2): 521-528.
DOI:10.1016/S0040-4020(96)01002-2 |
Lee J C, Hou M F, Huang H W, Chang F R, Yeh C C, Tang J Y, Chang H W. 2013. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell International, 13: 55.
DOI:10.1186/1475-2867-13-55 |
Lesjak M, Beara I, Simin N, Pintać D, Majkić T, Bekvalac K, Orčić D, Mimica-Dukić N. 2018. Antioxidant and antiinflammatory activities of quercetin and its derivatives. Journal of Functional Foods, 40: 68-75.
DOI:10.1016/j.jff.2017.10.047 |
Liu Z, Romero-Canelón I, Qamar B, Hearn J M, Habtemariam A, Barry N P E, Pizarro A M, Clarkson G J, Sadler P J. 2014. The potent oxidant anticancer activity of organoiridium catalysts. Angew Chemie, 53(15): 3941-3946.
DOI:10.1002/anie.201311161 |
Martins A, Vieira H, Gaspar H, Santos S. 2014. Marketed marine natural products in the pharmaceutical and cosmeceutical industries:tips for success. Marine Drugs, 12(2): 1066-1101.
DOI:10.3390/md12021066 |
Murphy C, Hotchkiss S, Worthington J, McKeown S R. 2014. The potential of seaweed as a source of drugs for use in cancer chemotherapy. Journal of Applied Phycology, 26(5): 2211-2264.
DOI:10.1007/s10811-014-0245-2 |
Nogueira C C, Paixão I C, Teixeira V L. 2014. Antioxidant activity of natural products isolated from red seaweeds. Natural Product Communications, 9(7): 1031-1036.
|
Norte M, Fernández J J, Souto M L, Gavín J A, García-Grávalos M D. 1997. Thyrsenols A and B, two unusual polyether squalene derivatives. Tetrahedron, 53(9): 3173-3178.
DOI:10.1016/S0040-4020(97)00028-8 |
Paiva L S, Patarra R F, Neto A I, Emc L, Jab B. 2012. Antioxidant activity of macroalgae from the Azores. Arquipelago Life Mar. Sci., 29: 1-6.
|
Paiva L, Lima E, Patarra R F, Neto A I, Baptista J. 2014. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chemistry, 164: 128-135.
DOI:10.1016/j.foodchem.2014.04.119 |
Pangestuti R, Kim S K. 2011. Neuroprotective effects of marine algae. Marine Drugs, 9(5): 803-818.
DOI:10.3390/md9050803 |
Park E J, Pezzuto J M. 2013. Antioxidant marine products in cancer chemoprevention. Antioxidants & Redox Signaling, 19(2): 115-138.
DOI:10.1089/ars.2013.5235 |
Patarra R F, Leite J, Pereira R, Baptista J, Neto A I. 2013. Fatty acid composition of selected macrophytes. Natural Product Research, 27(7): 665-669.
DOI:10.1080/14786419.2012.688048 |
Pereira L. 2016. Edible Seaweeds of the World. Science Publishers' (SP), an Imprint of CRC Press/Taylor & Francis Group, Boca Raton, FL. 448p.
|
Pereira L. 2018. Therapeutic and Nutritional Uses of Algae.CRC PressBoca Raton, FL. 640p.
|
Pinteus S, Silva J, Alves C, Horta A, Thomas O P, Pedrosa R. 2017. Antioxidant and cytoprotective activities of fucus spiralis seaweed on a human cell in vitro model. International Journal of Molecular Sciences, 18(2): 292.
DOI:10.3390/ijms18020292 |
Pomponi S A. 2001. Roger revelle commemorative lecture-the oceans and human health:the discovery and development of marine-derived drugs. Oceanography, 14(1): 78-87.
DOI:10.5670/oceanog |
Rodrigues D, Freitas A C, Pereira L, Rocha-Santos T A P, Vasconcelos M W, Roriz M, Rodríguez-Alcalá L M, Gomes A M P, Duarte A C. 2015b. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chemistry, 183: 197-207.
DOI:10.1016/j.foodchem.2015.03.057 |
Rodrigues D, Sousa S, Silva A, Amorim M, Pereira L, RochaSantos T A P, Gomes A M P, Duarte A C, Freitas A C. 2015a. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the central west coast of Portugal. Journal of Agricultural and Food Chemistry, 63(12): 3177-3188.
DOI:10.1021/jf504220e |
Rodrigues D, Walton G, Sousa S, Rocha-Santos T A P, Duarte A C, Freitas A C, Gomes A M P. 2016. In vitro fermentation and prebiotic potential of selected extracts from seaweeds and mushrooms. LWT, 73: 131-139.
DOI:10.1016/j.lwt.2016.06.004 |
Sathya R, Kanaga N, Sankar P, Jeeva S. 2017. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arabian Journal of Chemistry, 10(S2): S2 608-S2 614.
DOI:10.1016/j.arabjc.2013.09.039 |
Silva P J, Alves C, Pinteus S, Silva J, Valado A, Pedrosa R, Pereira L. 2017. Screening of Biotechnological Potential of wild and IMTA-Cultivated Osmundea Pinnatifida(Rhodophyta). P1. 33, 7th International Conference on Algal Biomass, Biofuels & Bioproducts. 18-21 June 2017, Miami, FL.
|
Smit A J. 2004. Medicinal and pharmaceutical uses of seaweed natural products:a review. Journal of Applied Phycology, 16(4): 245-262.
DOI:10.1023/B:JAPH.0000047783.36600.ef |
Sudha A, Jeyakanthan J, Srinivasan P. 2017. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resource-Efficient Technologies, 3(4): 506-515.
DOI:10.1016/j.reffit.2017.07.002 |
Tsimberidou A M. 2015. Targeted therapy in cancer. Cancer Chemotherapy and Pharmacology, 76(6): 1113-1132.
DOI:10.1007/s00280-015-2861-1 |
Wang T, Jónsdóttir R, Ólafsdóttir G. 2009. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chemistry, 116(1): 240-248.
DOI:10.1016/j.foodchem.2009.02.041 |
Wojcikowski K, Stevenson L M, Leach D N, Wohlmuth H, Gobe G. 2007. Antioxidant capacity of 55 medicinal herbs traditionally used to treat the urinary system:a comparison using a sequential three-solvent extraction process. Journal of Alternative and Complementary Medicine, 13(1): 103-109.
DOI:10.1089/acm.2006.6122 |
Zou Y P, Chang S K C, Gu Y, Qian S Y. 2011. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. Journal of Agricultural & Food Chemistry, 59(6): 2268-2276.
DOI:10.1021/jf104640k |
Zulueta A, Esteve M J, Frígola A. 2009. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chemistry, 114(1): 310-316.
DOI:10.1016/j.foodchem.2008.09.033 |
 2019, Vol. 37
2019, Vol. 37


