Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CARVALHO G. Loïc, SILVA Raquel, GONÇALVES J. Maria, BATISTA T. Maria, PEREIRA Leonel
- Extracts of the seaweed Bifurcaria bifurcata display antifungal activity against human dermatophyte fungi
- Journal of Oceanology and Limnology, 37(3): 848-854
- http://dx.doi.org/10.1007/s00343-019-8118-9
Article History
- Received May. 2, 2018
- accepted in principle Aug. 22, 2018
- accepted for publication Oct. 12, 2018
2 Institute of Mechanical, Process and Energy Engineering, School of Engineering and Physical Sciences, Heriot Watt University, Edinburgh EH14 4AS, Scotland, UK;
3 MARE (Marine and Environmental Sciences Centre), Department of Life Sciences, Faculty of Sciences and Technology, University of Coimbra, Coimbra 3030-790, Portugal;
4 CIEPQPF, Department of Chemical Engineering, Faculty of Science and Technology, University of Coimbra, Coimbra 3030-790, Portugal
Nowadays, seaweeds are a blooming subject in the field of marine biotechnology, be it for direct consumption as food or as ingredients in food, agriculture, pharmaceutical or cosmetic industries. They can have significant effects as biostimulants for plant growth (Cardoso et al., 2014), as cosmetic ingredients and their bioproducts, mainly polysaccharides, have been shown to have a range of bioactivities (Cardoso et al., 2014; Carvalho and Pereira, 2015). New drug discovery is a very active field of research in seaweed biotechnology with evidence for antimicrobial, antitumoral, antiinflammatory and immunomodulation effects (Cardoso et al., 2014; Carvalho and Pereira, 2015). There has been work on antibacterial and antiviral effects of seaweed extracts, but in comparison, there is much less published regarding antifungal (specifically human fungal pathogens) and anti-protozoal activities. A variety of seaweed extracts show interesting results as antifungal agents. For example, Turbinaria conoids, Padina gymnospora, Sargassum tenerrimum (Phaeophyceae) revealed antifungal activities against Candida albicans, Penicillium sp., Aspergillus flavus, Aspergillus tetreus, Candida glabrata, and Cryptococcus neoformans (Manivannan et al., 2011). Extracts from Codium decorticatum, Caulerpa scalpelliformis (Chlorophyta), Turbinaria conoides, Sargassum swartzii (formerly Sargassum wightii) (Phaeophyceae) and Acanthophora spicifera (Rhodophyta) have also been reported as an antifungal (Lavanya and Veerappan, 2012).
Bifurcaria bifurcata is a macroalga from the Order Fucales (Ochrophyta, Phaeophyceae) that can be found all along the European Atlantic shores (Pereira, 2015, 2018a). It is perennial, fixed in the rocky substrate that, depending on local hydrodynamics, can be found all year around from the mid-littoral zone. Research has focused on the biochemical composition on B. bifurcata with reports of pigments (e.g. carotenoids, chlorophylls e xanthophyll), fatty acids, polar compounds (e.g. phlorotannins), as well as sterols and diterpenes (Hellio et al., 2001; Maréchal et al., 2004; Abboud et al., 2009; Gallé et al., 2013). The potential economic value of this seaweed is unknown, although there is work pointing to biological activity, such as anti-proliferative, antioxidant, antitumor, antifouling, heavy metal bioremediation and even industrial use for hydroseeding (Pereira, 2015). Although not consumed in most countries where it is native, this alga is considered edible (Pereira, 2016) which means that it has generally regarded as safe to handle and use for food and product development.
The main of this work is to explore the antifungal potential of B. bifurcata against human dermatophyte fungi.
2 MATERIAL AND METHOD 2.1 Sampling location, sample acclimatization, and preparationSamples were harvested from two different geographical locations in Portugal. The first was at North Baleal Beach, from Baleal Island (Peniche, Portugal), in winter (BbPe). It is a mixed substrate location with rocky zones (calcareous rocks) and sand zones, very exposed to hydrodynamics of sea waves (Pereira, 2018b). However, the exact spot of the harvest was protected against waves and showed a local low gradient slope. The second harvest location was at Aguda Beach (Arcozelo, Vila Nova de Gaia, Portugal), in autumn, samples termed BbAg. This place consists of a large rocky shore area, with low gradient slope but subjected to strong hydrodynamics of sea waves (Pereira, 2018c).
All harvested samples were washed with seawater on site and were transported in insulated sealed iceboxes for temperature stress reduction until arrival to the laboratory where they were cleansed of sand, mollusks, crustaceans, and other seaweeds before freezing (-22℃) and subsequent freeze-drying.
Part of the sample harvested in Aguda Beach was also submitted for aquaculture at the company AlgaPlus (ĺlhavo, Portugal—www.algaplus.pt) using a batch culture system in laboratory conditions with controlled light intensity, temperature, and photoperiod and using enriched water from a fish aquaculture grown for 4-5 weeks. After culture, this material was freeze-dried to produce the B. bifurcata laboratory cultured sample (BbLC).
2.2 Extraction procedure 2.2.1 Sample preparation for extractionAfter freeze-drying, the samples of B. bifurcata (BbPe, BbAg, and BbLC) were pulverized to a diameter < 0.5 mm.
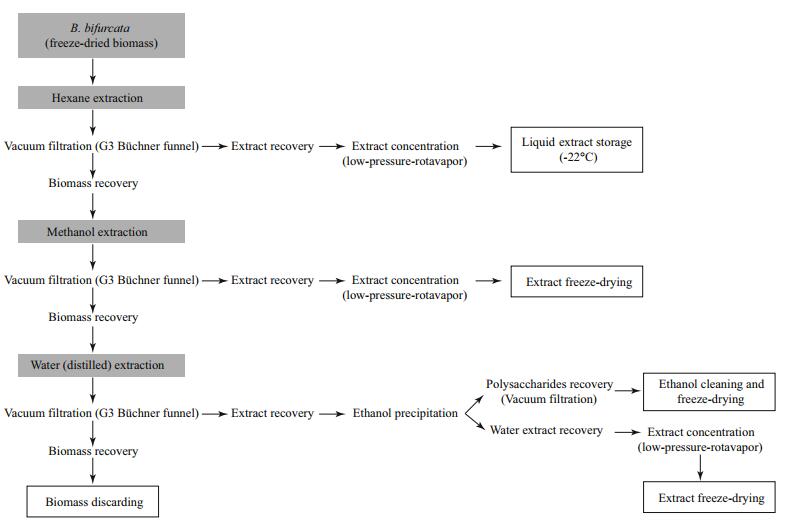
2.2.2 Extraction methodThe extraction method for this work was designed to extract the widest variety of compounds with different polarities from the B. bifurcata biomass. The method (Fig. 1) used a sequential extraction with organic solvents, hexane then methanol (MeOH) in the proportion of 1:20 (m/v) and at room temperature, for the extraction of apolar and polar compounds, respectively. Following the organic solvents, water (H2O) in the proportion of 1:100 (m/v) at 80–100℃ was used to extract the remaining polar compounds and polysaccharides. Polysaccharides in this extract were precipitated by ethanol (EtOH) addition and recovered by filtration. Four distinct extracts: hexane extract, MeOH extract, polysaccharides extract and final (H2O extract (after polysaccharides recovery) were obtained.
|
| Fig.1 Experiment design of the sequenced extraction procedure |
The MeOH and (H2O extracts were then concentrated at low pressure by rotary evaporation then freezedried. Hexane extracts were stored as liquids and the polysaccharides were stored in EtOH (99%) for 24 h at 4℃ before vacuum filtration, using G3 Büchner funnels, and finally freeze-dried (Fig. 1).
2.3 Antifungal assayFor evaluation of antifungal activity, a broth macrodilution assay was developed, according to M38-A2 (CLSI, 2008), for the determination of the minimum inhibitory concentration (MIC) and minimum lethal concentration (MLC) of the extracts obtained from the 3 different B. bifurcata (BbPe, BbAg, and BbLC) samples.
For this assay, the Roswell Park Memorial Institute (RPMI 1640, Biochrom) medium was used, at the concentration of 1.04 g/L with an addition of the 3-(n-morpholino) propanesulfonic acid (MOPS) buffer at a final concentration of 0.165 mol/L. All the components were dissolved in distilled water and the pH was adjusted to pH 7 before filtering the medium for sterilization.
2.3.1 Fungal strainsThe dermatophyte fungi used in this work were: three strains of clinical dermatophyte isolated from nails and skin (Epidermophyton floccosum FF9, Microsporum canis FF1 and Trichophyton mentagrophytes FF7) and four dermatophyte type strains from the Colección Espanõla de Cultivos Tipo (Microsporum gypseum CECT 2908, Trichophyton mentagrophytes var. interdigitale CECT 2958, Thrichophyton rubrum CECT 2794, Trichophyton verrucosum CECT 2992). These isolates stored in a Sabouraud broth with glycerol at -70℃. Before the assays, the fungi were inoculated in Sabouraud dextrose agar (SDA) or in potato dextrose agar (PDA) to ensure the optimal growth, quality, and purity of the cultures.
2.3.2 Seaweed extracts preparation for the assayDue to the difficulty of solubilizing seaweed hexane extracts in a way that would comply with the antifungal assay, these were not used for this work. Seaweed MeOH extracts were re-dissolved in 40% aqueous EtOH solution and dried (H2O and polysaccharides extracts were re-dissolved in sterilized distilled (H2O. Subsequently, these extracts were homogenized by vortex and ultrasonic treatment.
2.3.3 Inoculum preparationFungi were cultivated in tubes for 7 days at 30℃ in SDA medium and suspensions of spores and mycelia were obtained by adding physiological saline solution and vortexing with glass pearls (3-mm diameter). The resulting mixture of conidia and hyphal fragments were transferred to a sterile tube and adjusted to the desired concentration by hemocytometer counting.
2.3.4 Antifungal testSerial dilutions of the extract (0.8, 0.4, 0.2, 0.1, 0.05 mg/mL) were prepared in distilled water. In each test tube, 100 μL of the diluted extract and 900 μL of inoculated RPMI media containing (1–2)×104 cells/mL was added. The tubes were incubated aerobically at 30℃ for 7 days and MICs were determined from negative growth tubes. To evaluate MLCs, aliquots (20 μL) of broth were taken from each negative tube and cultured in SDA plates. Plates were then incubated for 7 days at 30℃. In addition, a reference antifungal compound, fluconazole (Pfizer) was used as a standard control to assess the sensitivity of tested microorganisms. For each strain tested, the growing conditions and the sterility of the medium were checked in two control tubes. All experiments were performed in duplicate for a total of three independent assays.
3 RESULT AND DISCUSSIONBefore testing against the seven dermatophyte strains, the extracts were screened for antifungal potential using the same methodology but applied to only Epidermophyton floccosum FF9, Microsporum canis FF1 and Trichophyton mentagrophytes FF7 (data not shown). Only MeOH extracts of all B. bifurcata samples (BbPe, BbAg, BbLC) demonstrated antifungal activity so only those were tested against all seven strains and the polysaccharides and (H2O extracts were not tested.
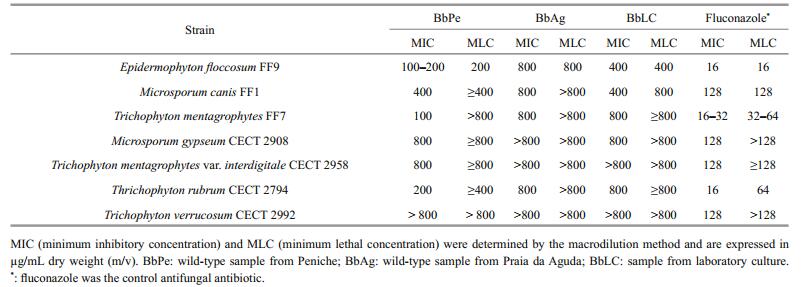
MIC and MLC data was gathered for each extract and the respective dermatophyte strain (Table 1).
|
Of the tested seaweed samples, BbPe was the most active against dermatophyte strains with BpAg being the less active (Table 1). Significant inhibitory and lethal activities were verified for the Epidermophyton floccosum FF9. Moreover, BbPe presented the highest inhibitory capacity against Trichophyton mentagrophytes FF7 (100 μg/mL MIC) and Trichophyton rubrum CECT 2794 (200 μg/mL MIC) (Table 1).
Fluconazole shows much lower MIC and MLC values but in terms of fungi resistance it can be observed the same pattern with BbPe where E. floccosum FF9, T. mentagrophytes FF7, and T. rubrum CECT 2794 are the less resistant and Microsporum gypseum CECT 2908, Trichophyton mentagrophytes var. interdigitale CECT 2958 and T. verrucosum CECT 2992 the most resistant strains (Table 1).
To our knowledge, this is the first study to demonstrate the antifungal capacity of B. bifurcata against human dermatophyte fungi. The fact that only the MeOH extracts showed antifungal activity is quite in line with other published work where polar fractions or extracts reveal antifungal activity (Hellio et al. (2001), Manivannan et al. (2011) and Lavanya and Veerappan (2012). As described by Oumaskour et al. (2012), dichloromethane/MeOH extracts of Laminaria ochroleuca (Phaeophyceae) had activity against Cryptococcus neoformans, and MeOH extracts of Colpomenia sinuosa and Saccorhiza polyschides (formerly Saccorhiza bulbosa) (Phaeophyceae) had activity towards Candida tropicalis. The greater effectiveness of polar extracts was reported with ethyl acetate and MeOH extracts of Cystoseira sedoides, Cystoseira crinita and Cystoseira compressa against 5 yeast species (Candida albicans, Candida glabrata, Candida krusei, Candida dubliniensis, and Candida kefyr) (Mhadhebi et al., 2012).
Antifungal activity against human dermatophyte fungi strains was found in MeOH extracts produced from Ulva rigida (Chlorophyta) and Gelidium microdon (Rhodophyta) (Silva et al., 2013) but these seaweed extracts were apparently less active than B. bifurcata, though direct comparisons are difficult as the maximum concentration used was 256 μg/mL and the fungal strains used were not the same. Nevertheless, B. bifurcata (BbPe) had higher antifungal activity than U. rigida and G. microdon extracts against T. mentagrophytes FF7 and E. floccosum FF9 (Silva et al., 2013). Lopes et al. (2012) used eleven brown algae (Ochrophyta-Phaeophyceae) to obtain phlorotanninenriched extracts, but their lowest MIC and MLC values were 3.9 and 7.8 mg/mL respectively from Cystoseira nodicaulis extracts, substantially higher to our best results.
Although the antifungal activity from the B. bifurcata extracts was not greater than Fluconazole, the samples used were crude MeOH extracts and purification of the active components may be relevant, and some studies suggest the possible origin of the group of metabolites that cause the antifungal activity. Twelve purified extracts of acyclic diterpenes and complex mixtures of non-acetylated diterpenes from B. bifurcata from four different geographical locations (Quiberon, France; Port Sail, France; Oualidia, Morocco; and Black Head, Ireland), were tested for antifouling capacity (Hellio et al., 2001) and they revealed antifungal effects against marine fungi cultures (Corollospora maritima, Lulworthia sp. and Dendryphiella salina) with MIC values of 8 μg/mL (Hellio et al., 2001). Although this antifungal activity was not against dermatophytes, this suggests that the antifungal activity in our extracts may be associated with diterpenes.
In an interesting work, Gallé et al. (2013) selected twenty seaweed species to evaluate bioactivity against Trypanosoma brucei rhodesiense trypomastigotes and reported impressive results from hydro-alcoholic and ethyl acetate extracts from B. bifurcata. This might suggest that the antifungal effect of BbPe might arise from a general cytotoxic effect, not specific for fungi but for other microorganisms as well. It is notable that after fractionation, the most active extract contained the diterpene eleganolone. However, when this fraction was tested alone, the activity was decreased compared to the whole extracts, which suggests a synergistic effect between different B. bifurcata metabolites (Maréchal et al., 2004; Gallé et al., 2013).
Fractionation of the BbPe MeOH extract could determine which fraction or fractions, alone or mixed, were responsible for the antifungal activity. Use of metabolomics strategies with liquid chromatography coupled to mass spectrometry (LC-MS) could elucidate the metabolites present in these tested fractions that might be responsible for the antifungal effect. This approach could also explain the differences between the three different B. bifurcata samples in this work (BbPe, BbAg, and BbLC).
Another subject where a metabolomics approach would help is in determining if the seasonal and geographical variation, as reported in by Hellio et al., (2001) and Maréchal et al., (2004), might influence the different antifungal activities registered from the B. bifurcata extracts of this work (BbPe, BbAg, and BbLC). Seaweed metabolism changes as the local climate changes during the year. The sunny winters common in Portugal encourage biomass production in perennial seaweed species, depending on wave hydrodynamics. Indeed, Bifurcaria bifurcata is perennial and adult specimens can be easily found during winter if the location is somehow protected against the strong waves.
However, looking for effects due to seasonal variation among our bioactivity results is hampered as we can only compare BbPe (harvested in January) with BbAg (harvested October) and these samples were obtained from different locations. Therefore, we cannot confirm if the higher antifungal activity of winter-harvested BbPe (January) over autumnharvested BbAg (October) was due to location or season. Indeed, a future perspective would be a geographical and seasonality study with the collection of samples at different times of a year and from different places to compare activity results and extrapolate seasonal influence among the optimal period of a year and optimal climatic factors for harvesting. This information could direct future harvesting or culturing protocols for maximizing antifungal components from B. bifurcata. The fact that the bioactivity of the cultured sample (BbLC) was inferior to the wild-type sample BbPe might suggest that encouragement of vegetative growth in culture does not encourage the production of the metabolites responsible for the antifungal activity.
In summary, B. bifurcata MeOH extracts demonstrated antifungal capacity against human dermatophyte fungi and appear to be the most effective seaweed in published literature. It would be interesting to extend the screen for antifungal activity to other important fungal species. The effectiveness of the crude extracts suggests the potential for pharmaceutical studies to purify and identify candidate antifungal components. Previous work strongly suggests that the compounds responsible for this activity might be diterpenes. This work indicates that bioactivity might be seasonally or geographically influenced, and the next steps could be to apply a metabolomics approach using a seasonal and geographical sensitive sampling method. This way, characterization and identification of the responsible metabolites for antifungal effect will be achieved together with the knowledge on the optimal growth conditions for culturing this seaweed should the antifungal properties justify exploitation.
4 CONCLUSIONFor the first time, we report the antifungal capacity of B. bifurcata against human pathogen dermatophyte fungi strains, by evaluating the Minimum Inhibitory Concentrations (MICs) and Minimum Lethal Concentrations (MLCs). Such bioactivity needs to be more explored to discover the active compounds and to understand the effect of external factors such as climate and local geography on this bioactivity. A metabolomics approach together with a strategic seasonal and geographical harvest of samples would be a promising strategy to discover which metabolites are responsible for the activity. Also, testing these extracts against a wider range of fungi, perhaps those which cause problems on crops and/or food would be of major interest.
5 DATA AVAIBILITY STATEMENTThe data that support the findings of this study are available in the repository "Estudo Geral" from the University of Coimbra, with the identifier http://hdl.handle.net/10316/24928 (in Portuguese).
6 ACKNOWLEDGEMENTThis work had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic project UID/MAR/04292/2013 granted to MARE. This work had also the support from the European Union through EASME Blue Labs project AMALIA—Algae-to-MArket Lab IdeAs (EASME/EMFF/2016/1.2.1.4/03/SI2.750419). This work had also received funding from European Structural & Investment Funds through the COMPETE Programme and from National Funds through FCT—Fundação para a Ciência e a Tecnologia under the Programme grant SAICTPAC/0019/2015. We would like to acknowledge the support of ALGAplus for giving the opportunity of culturing the B. bifurcata sample that was used for this work. We also want to especially thank Dr. Gordon McDougall from The James Hutton Institute, Scotland, for reviewing this manuscript.
Abboud Y, Abourriche A, Ainane T, Charrouf M, Bennamara A, Tanane O, Hammouti B. 2009. Corrosion inhibition of carbon steel in acidic media by Bifurcaria bifurcata extract. Chemical Engineering Communications, 196(7): 788-800.
DOI:10.1080/00986440802589875 |
Cardoso S M, Carvalho L G, Silva P J, Rodrigues M S, Pereira O R, Pereira L. 2014. Bioproducts from seaweeds: a review with special focus on the Iberian Peninsula. Current Organic Chemistry, 18(7): 896-917.
DOI:10.2174/138527281807140515154116 |
Carvalho L G, Pereira L. 2015. Review of marine algae as source of bioactive metabolites. In: Pereira L, Neto J M eds. Marine Algae Biodiversity, Taxonomy, Environmental Assessment and Biotechnology. CRC Press, Boca Raton, FL. p.192-224.
|
CLSI. 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous fungi; Approved Standard M38-A2. 2nd edn. National Committee for Clinical Laboratory Standards, Wayne, Pennsylvania.
|
Gallé J-B, Attioua B, Kaiser M, Rusig A M, Lobstein A, Vonthron-Sénécheau C. 2013. Eleganolone, a diterpene from the French marine alga Bifurcaria bifurcata inhibits growth of the human pathogens Trypanosoma brucei and Plasmodium falciparum. Mar ine Drugs, 11(12): 599-610.
DOI:10.3390/md11030599 |
Hellio C, Thomas-Guyon H, Culioli G, Piovettt L, Bourgougnon N, Le Gal Y. 2001. Marine antifoulants from Bifurcaria bifurcata(Phaeophyceae, Cystoseiraceae) and other brown macroalgae. Biofouling, 17(3): 189-201.
DOI:10.1080/08927010109378478 |
Lavanya R, Veerappan N. 2012. Pharmaceutical properties of marine macroalgal communities from Gulf of Mannar against human fungal pathogens. Asian Pacific Journal of Tropical Disease, 2(S1): S320-S323.
DOI:10.1016/S2222-1808(12)60174-1 |
Lopes G, Sousa C, Silva L R, Pinto E, Andrade P B, Bernardo J, Mouga T, Valentão P. 2012. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions?. PLoS One, 7(2): e31145.
DOI:10.1371/journal.pone.0031145 |
Manivannan K, Karthikai devi G, Anantharaman P, Balasubramanian T. 2011. Antimicrobial potential of selected brown seaweeds from Vedalai coastal waters, Gulf of Mannar. Asian Pacific Journal of Tropical Biomedicine, 1(2): 114-120.
DOI:10.1016/S2221-1691(11)60007-5 |
Maréchal J P, Culioli G, Hellio C, Thomas-Guyon H, Callow M E, Clare A S, Ortalo-Magné A. 2004. Seasonal variation in antifouling activity of crude extracts of the brown alga Bifurcaria bifurcata(Cystoseiraceae) against cyprids of Balanus amphitrite and the marine bacteria Cobetia marina and Pseudoalteromonas haloplanktis. Journal of Experimental Marine Biology and Ecology, 313(1): 47-62.
DOI:10.1016/j.jembe.2004.07.016 |
Mhadhebi L, Chaieb K, Bouraoui A. 2012. Evaluation of antimicrobial activity of organic fractions of six algae from Tunisian Mediterranean coasts. International Journal of Pharmacy and Pharmaceutical Sciences, 4(1): 534-537.
|
Oumaskour K, Boujaber N, Etahiri S, Assobhei O. 2012. Screening of antibacterial and antifungal activities in green and brown algae from the coast of Sidi Bouzid(El Jadida, Morocco). African Journal of Biotechnology, 11(4): 16 831-16 837.
DOI:10.5897/AJB11.3761 |
Pereira L. 2015. Seaweed flora of the European north Atlantic and Mediterranean. In: Kim S-K ed. Springer Handbook of Marine Biotechnology. Springer, Berlin, Heidelberg. p.65-178, https: //doi.org/10.1007/978-3-642-53971-8_6.
|
Pereira L. 2016. Edible Seaweeds of the World. Science Publishers, An Imprint of CRC Press/Taylor & Francis Group, Boca Raton, FL. 448p.
|
Pereira L. 2018a. Bifurcaria bifurcata. MACOI-Portuguese Seaweeds Website, MARE, University of Coimbra, Portugal. http://macoi.ci.uc.pt/spec_list_detail.php?spec_id=8. Accessed on 2018-01-11.
|
Pereira L. 2018b. Baleal, Peniche(Portugal). MACOI-Portuguese Seaweeds Website, MARE, University of Coimbra, Portugal, http://macoi.ci.uc.pt/local_detail.php?loc_id=2&searchSite=baleal%2C+portugal. Accessed on 2018-01-11.
|
Pereira L. 2018c. Aguda, Gaia(Portugal). MACOI-Portuguese Seaweeds Website, MARE, University of Coimbra, Portugal, http://macoi.ci.uc.pt/local_detail.php?loc_id=12&searchSite=aguda%2C+portugal. Accessed on 2018-01-11.
|
Silva M, Vieira L M M, Almeida A P, Silva A M S, Seca A M L, Barreto M C, Pedro A I N M, Pinto E, Kijjoa A. 2013. Chemical study and biological activity evaluation of two Azorean macroalgae: Ulva rigida and Gelidium microdon. Oceanography, 1(1): 1-7.
DOI:10.4172/2332-2632.1000102 |
 2019, Vol. 37
2019, Vol. 37


