Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HOU Dandan, YU Weiting, ZHANG Demeng, ZHAO Lili, LIU Xiudong, MA Xiaojun
- Culture of yeast cells immobilized by alginate-chitosan microcapsules in aqueous-organic solvent biphasic system
- Journal of Oceanology and Limnology, 37(3): 863-870
- http://dx.doi.org/10.1007/s00343-019-8126-9
Article History
- Received May. 9, 2018
- accepted in principle Sep. 20, 2018
- accepted for publication Oct. 15, 2018
2 Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, China;
3 State Key Laboratory of Bioactive Seaweed Substances, Qingdao Brightmoon Seaweed Group Co. Ltd., Qingdao 266400, China;
4 Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
Due to the high selectivity, mild and environmentbenign condition, biocatalysis techniques have been widely applied in industrial biotechnology (Wenda et al., 2011; Schrewe et al., 2013; Bezerra et al., 2015) and substituted some traditional chemical synthetic routes (Wohlgemuth, 2010; De Carvalho, 2011; Reetz, 2013). For instance, microbes using as biocatalysts can convert substrates to bulk chemicals, value-added pharmaceutical intermediates and drugs (Rivero et al., 2015; Ratnayake et al., 2016). However, some biocatalysis processes involve low soluble or hydrophobic substrates/products, which inevitably results in low conversion rate in an aqueous environment or suppression on the activity of biocatalysts. To solve the above problems, nonaqueous media such as organic solvents, aqueous-organic solvents biphasic systems, supercritical carbon dioxide, and ionic liquids have been introduced for efficient biocatalysis and bioconversion (Marques et al., 2010; Yu et al., 2010a; Dennewald et al., 2012). Although nonaqueous media demonstrated superiority compared to traditional aqueous biocatalysis, they can also induce the decrease or even loss of biocatalyst activity in a hostile environment (Yang et al., 2009). Besides the effort on screening of extremophiles or construction of biocatalysts tolerant to organic solvents via directed evolution (Cirino and Sun, 2008; Bornscheuer et al., 2012), microencapsulation technology can improve the activity and stability of entrapped biocatalysts in nonaqueous media by preventing direct contact of biocatalysts from toxic solvents (Bommarius and Paye, 2013). Therefore, it has become a potential technology in industrial biocatalysis processes for the green or sustained production of bulk chemicals, pharmaceutical intermediates and drugs (Eş et al., 2015; Kisukuri and Andrade, 2015).
Among the common microencapsulation carriers, alginate-based gel beads or microcapsules have been proved the competitive ones in traditional aqueous biocatalysis processes especially for cell encapsulation such as cellulose degrading bacteria— Clostridium sulfatireducens CCUG 50825 (Börner et al., 2013), Lactobacillus casei subsp. rhamnosus ATCC11979 (NBIMCC1013) (Goranov et al., 2013), and Saccharomyces pastorianus (carlsbergensis) Saflager S-23 (Naydenova et al., 2014). Alginate is a polysaccharide with the ability to form a threedimensional hydrogel under mild ionotropy effect when it encounters some divalent cations such as Ca2+ (Wang et al., 2006). A few of reports have demonstrated calcium alginate gel beads can be used to immobilize microbial cells for biotransformation in nonaqueous media such as aqueous-organic solvent biphasic systems (Kansal and Banerjee, 2009; Arabi et al., 2010; Zhang et al., 2010). Alginate beads helped to improve the bioconversion efficacy to some extent compared to that in aqueous biocatalysis, while there were obvious problems such as activity reduction or loss of cells due to the weak structure stability of alginate beads and the toxicity from solvents (Garikipati et al., 2009). Fortunately, the mechanical and physicochemical properties of alginate hydrogel can be improved using polymers such as chitosan (Yu et al., 2010b) and poly-l-lysine (Cui et al., 2006) through forming microcapsule with polyelectrolyte complex (PEC) membrane under electrostatic interaction. In one word, the mild preparation conditions and the biocompatible living environment of microcapsules are beneficial for the maintenance of structure stability and the activity of biocatalysts (Shih et al., 2010; Rathore et al., 2013).
In our previous reports, yeast cells have been entrapped successfully in calcium alginate gel beads and alginate-chitosan (AC) microcapsules. During the culture process in aqueous media, the entrapped low-density cells demonstrated superiority in growth and improved functions (Yu et al., 2011). Especially, when the entrapped low density cells (as potential probiotics products) were evaluated in some hostile environments, they displayed much better stress resistance in free-drying and simulated gastric fluid (Song et al., 2013, 2014; Gao et al., 2016). These findings inspired us the idea to investigate the performance of AC microencapsulated microbial cells in nonaqueous media for potential biocatalysis, which is seldom concerned and testified so far. Therefore, AC microcapsules were prepared as immobilization carrier by emulsification-internal gelation and complexation reaction. After screening solvents to form culture medium-solvent biphasic systems, AC microencapsulated yeast cells were put into the biphasic system and cultured. The properties of cell activity, growth, and basic metabolism were evaluated. Moreover, the influence of process parameters regarding microcapsule preparation and cell culture in biphasic systems on the cell activity and growth was investigated. The purpose is to get basic information for the further production of aromatic alcohol via biocatalysis in the aqueoussolvent biphasic system.
2 MATERIAL AND METHOD 2.1 Materials and cellsSodium alginate was purchased from Qingdao Crystal Rock Biotechnological Development Co. Ltd. (Qingdao, China), whose viscosity is over 0.02 Pa·s when dissolved to form a 1.5% (w/v) aqueous solution at 20℃. The compositions ratio of α-L-guluronic acid (G) residues and β-D-mannuronic acid (M) residues of alginate were characterized by 1H NMR as G/M ratio of 34/66, and the molecular weight (Mw) was 430 kDa. The deacetylation degree (DD) of chitosan samples was 96%, and Mw ranged from 20 to 158 kDa, which was degraded from raw chitosan (Yuhuan Ocean Biomaterials Corporation, China) with gamma (γ) rays irradiation by Key Laboratory of Nuclear Analysis Techniques, Chinese Academy of Sciences. Dibutyl sebacate (DBS) were purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). All other reagents and solvents of reagent grade were used without further purification.
Yeast cells (Saccharoinyces cerevisiae) BY4741 were obtained from the Institute of Applied Ecology, Chinese Academy of Science (Shenyang, China). The strain was maintained in YPD medium (20 g of glucose, 10 g of polypepton and 10 g of yeast extract in 1 L distilled water at 30℃).
2.2 Preparation of immobilized yeast cells in alginate-chitosan microcapsules by emulsificationinternal gelation coupled electrostatic complexationThe alginate beads were prepared by emulsificationinternal gelation technique according to our recent paper (Song et al., 2013) to immobilize yeast cells. Sodium alginate solution at a concentration of 1.5% (w/v) was filtered with a 0.22-μm membrane filter and stored overnight before use to facilitate deaeration. Then yeast cells in late exponential phase were added in alginate solution, which was further mixed with CaCO3 powder to form a finely dispersed suspension. Next, alginate—CaCO3-cell mixture was dispersed in liquid paraffin containing 0.5% (v/v) Span 85 at 1:5 (v/v) to form emulsion by stirring at 200 r/min. After emulsification for 30 min, glacial acetic acid was slowly added into the emulsion to liberate Ca2+ for gelation. The calcium alginate beads entrapping yeast cells were collected and successively rinsed with 1% (v/v) Tween 80 solution.
The calcium alginate beads entrapping yeast cells were further immersed in 0.5% (w/v) chitosan solution dissolved in 0.1 mol/L sodium acetate-acetic buffer at the ratio of 1:10 (beads/solution) to form alginate-chitosan (AC) microcapsules, followed by rinsing with 0.9% (w/v) NaCl solution. After being liquefied by 0.055 mol/L sodium citrate and rinsed three times with 0.9% (w/v) NaCl solution, AC microcapsules entrapping yeast cells were formed. Moreover, different time of membrane formation of AC microcapsules and chitosan samples of different Mw were used in the experiments.
2.3 Characterization of the morphology of alginate beads and AC microcapsules with immobilized yeast cellsAfter the formation of alginate beads and AC microcapsules with immobilized yeast cells, they were transferred into culture medium-DBS biphasic system and cultured for 24 h. Then they were rinsed and observed with a Nikon Eclipse TE2000 Inverted Research Microscope (Nikon Corp., Japan).
2.4 Culture of yeast cells immobilized by alginate beads and AC microcapsules in aqueous-organic solvent biphasic system 2.4.1 Yeast cells immobilized by alginate beadsOne mL alginate beads with immobilized yeast cells (Saccharoinyces cerevisiae, BY4741) were added into 10 mL culture medium-DBS biphasic system (at a ratio of 1:1 v/v). Then the cells immobilized by alginate beads were cultured in a shaking incubator at 28℃ and 170 r/min for 30 h. At a time interval of 3 h, 0.5 mL medium containing uniformly dispersed alginate beads were collected and broken in 0.055 mol/L sodium citrate medium to release the entrapped cells, and then the cell density was determined at 600 nm (OD600). Meantime, the same inoculation amount of free yeast cells as that in 1-mL alginate beads was cultured in the biphasic system as control and the same volume of cells was evaluated.
2.4.2 Yeast cells immobilized by AC microcapsulesOne mL AC microcapsules with immobilized yeast cells (Saccharoinyces cerevisiae, BY4741) were added into the 10-mL culture medium-DBS biphasic system (at a ratio of 1:1 v/v). Then the immobilized cells were cultured in a shaking incubator at 28℃ and 170 r/min for 24 h. 0.5 mL AC microcapsules were collected and broken to release the entrapped cells, and then the cell density was determined at 600 nm (OD600). The consumption of glucose in the medium was also determined to evaluate the metabolic ability of cells. Meantime, the same amount of AC microencapsulated yeast cells was cultured in 10 mL of aqueous media as control and evaluated. All cell experiments were carried out in triplicate samples.
3 RESULT AND DISCUSSION 3.1 Growth characteristic of yeast cells immobilized by alginate beads and AC microcapsules in aqueous-organic solvent biphasic systemIn our previous report (Hou et al., 2012), both calcium alginate beads and alginate-chitosan (AC) microcapsules were prepared by emulsificationinternal gelation and complexation reaction. Five organic solvents including DBS, ethyl oleate and isopropyl myristate were selected according to log P value to form culture medium-solvent biphasic systems with the potential production of aromatic alcohol. It was found that AC microcapsules could keep stable size and high membrane strength in culture medium-DBS biphasic system, which was thought to be beneficial for protecting the activity of biocatalysts.
Yeast cells (BY4741) were entrapped in alginate beads and AC microcapsules respectively according to above-mentioned methods, and then two kinds of immobilized yeast cells were cultured in the mediumDBS biphasic system and evaluated for the growth characteristic with free cells as a control.
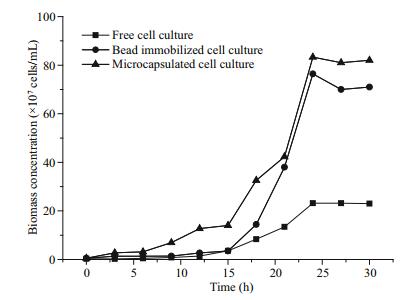
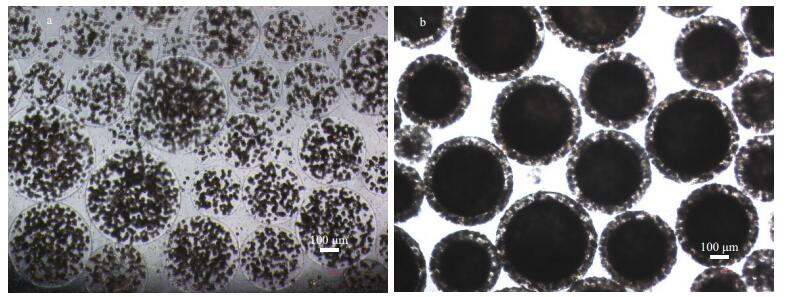
Figure 1 shows the growth curves of yeast cells in a free state, in alginate beads and AC microcapsules. They all experience a slow growth period in the early stage of 12 h. In the following 12 h, the free yeast cells grow gradually and then maintain stable biomass at around 20×107 cells/mL. While yeast cells entrapped in alginate beads and AC microcapsules demonstrate a sharp increase in biomass and reach around 75×107 cells/mL and 85×107 cells/mL respectively. These results indicate that the growth kinetics of entrapped yeast cells are obviously superior to that of free cells, which means the immobilization carriers can confer yeast cells the ability to resist the adverse effect of solvent. The optical images also display the good morphology and integrate structure of alginate beads and AC microcapsules (Fig. 2), which is consistent with our previous stability evaluation of microcapsules (Hou et al., 2012). The images also show that cell density in AC microcapsules is clearly higher than that in alginate beads, suggesting a higher growth rate of cells in microcapsules.
|
| Fig.1 Growth curve of yeast cells (BY4741) immobilized in alginate beads or AC microcapsules in a mediumDBS biphasic system with free cells as the control |
|
| Fig.2 Optical images of alginate beads immobilized yeast cells (a) and AC microencapsulated yeast cells (b) in the mediumDBS biphasic system after culturing for 24 h (40×) |
It has been well known that organic solvents are usually harmful to biocatalysts by reducing their activity or even making them inactivity (Kansal and Banerjee, 2009; Arabi et al., 2010). This explains the low growth behavior of free yeast cells in the mediumDBS biphasic system. Both alginate hydrogel beads and AC microcapsules provide three-dimensional (3D) gel network, which can protect the entrapped yeast cells from direct contact with a solvent (DBS) and avoid the phase toxicity of solvent. Therefore, they help maintain the activity and stability of cells in biphasic systems. However, when there exist some phosphate, lactate, citrate or anti-gelation sodium ions in culture media, alginate hydrogel beads always become instable in structure resulting in solvent toxicity to cells (Gaumann et al., 2000; Liouni et al., 2008). While the formation of the microcapsule membrane by complexing alginate beads with polycations such as chitosan can effectively improve the structure stability and diffusion resistance, which protects the entrapped cells from solvent toxicity. The findings give further support that AC microcapsules can provide microbial cells better stress resistance not only in free-drying and simulated gastric fluid (Song et al., 2013, 2014; Gao et al., 2016) but also in nonaqueous media such as solvent containing biphasic systems.
3.2 The influence of chitosan Mw on growth behavior of AC microencapsulated cells in the medium-DBS biphasic systemThe advantage of AC microcapsules for the protection of entrapped cells lies in the outer PEC membrane, which is formed under electrostatic interaction between positively charged amino of chitosan and negatively charged carboxyl of alginate. The materials and structure parameters usually have a direct influence on the properties of PEC membrane such as permeation and mechanical stability, and subsequently, have an influence on the entrapped cells. The Mw of chitosan has been found an important factor for controlling the structure and permeability of membrane in our previous report (Yu et al., 2010b). Herein, AC microcapsules with chitosan of different Mw are prepared to entrap yeast cells and cultured in medium-DBS biphasic systems. When the Mw of chitosan is below 100 000 Da, the biomass in AC microcapsules after culturing for 24 h almost reaches the same level of 80×107 cells/mL, except the highest level of near 100×107 cells/mL with Mw of 40 000 Da and decreased level with Mw of 158 000 Da (Fig. 3a). Correspondingly, the residual rate of glucose (main substrate in medium) shows the lowest value with Mw of 40 000 Da and highest value with Mw of 158 000 Da (Fig. 3b), that is, the highest amount of glucose is consumed by yeast cells in AC microcapsules with chitosan Mw of 40 000 Da. Generally, when the membrane formation time is maintained the same, chitosan molecules with low Mw diffuse easily into 3D gel network of alginate beads to form a denser membrane, which helps to resist the diffusion of solvent in biphasic system and benefits for the growth and metabolism of entrapped yeast cells. These results suggest that PEC membrane formed with chitosan Mw of 40 000 Da provides a balance condition protecting entrapped yeast cells from the harmful damage of solvent in a biphasic system, as well facilitating the inward diffusion of glucose for cell growth and metabolism.

|
| Fig.3 The effect of molecular weight (Mw) of chitosan on the biomass concentration (a) and glucose residual rate (b) of AC microencapsulated yeast cells in the medium-DBS biphasic system after culturing for 24 h |
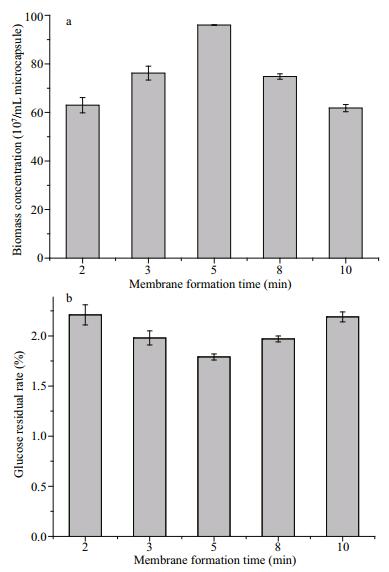
The PEC membrane of AC microcapsules actually serves as a physical barrier to separate the inner cells from outer media. The membrane formation time can also affect the membrane structure (such as thickness, pore size) and performance (such as mechanical stability, permeation), which certainly will have an effect on the entrapped cells. By using chitosan with Mw of 40 000 Da to form AC microcapsules, the membrane formation time is extended from 2 min to 10 min. It can be seen that the biomass in AC microcapsules after culturing in the medium-DBS biphasic system for 24 h increases firstly from 2 min to 5 min, reaches the peak and then decreases from 5 min to 10 min (Fig. 4a). Accordingly, the residual rate of glucose displays a reverse trend, and give the lowest value at 5 min (Fig. 4b). With the extension of membrane formation time, more chitosan molecules can diffuse inward and complex with alginate to form a denser membrane for the resistance of solvent. However, the denser membrane also increases the diffusion resistance of the substrate so that the biomass decreases at longer time. Therefore, it should reduce the membrane formation time on the condition that the cell viability and metabolic ability can be maintained.
|
| Fig.4 The effect of membrane formation time on the biomass concentration (a) and glucose residual rate (b) of AC microencapsulated yeast cells in the medium-DBS biphasic system after culturing for 24 h |
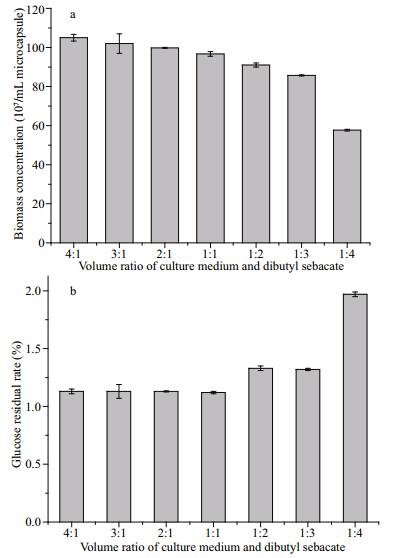
It has been found that the change of volume ratio between water and solvent can affect the interface area and then affect the activity of biocatalysts or the extraction of hydrophobic products by solvent (Stark et al., 2002; Kansal and Banerjee, 2009). Here, AC microencapsulated yeast cells are cultured in a mediumDBS biphasic system with a fixed medium volume of 5 mL and varied volume ratios of medium-DBS from 4:1 to 1:4. With the decrease of the volume ratio of medium-DBS, the biomass in AC microcapsules shows a decreasing trend after culturing in a biphasic system for 24 h (Fig. 5a). When the volume ratio is above 1:1, the aqueous phase (culture medium) is in dominant condition so that the decrease of biomass is not obvious with the decrease of volume ratio from 4:1 to 1:1. While the volume ratio decreases from 1:1 to 1:4, the biomass significantly reduces and only keeps as half as that at 4:1. Meantime, the residual rate of glucose demonstrates the lowest level at a volume ratio of 4:1 but the highest level at 1:4 (Fig. 5b). The optical images also confirm the clear difference. Yeast cells grow well and occupy all the inside space of AC microcapsules after culturing for 24 h in the medium-DBS biphasic system at a volume ratio of 4:1, while cells only occupy half space even less of AC microcapsules at a volume ratio of 1:4 (Fig. 6).
|
| Fig.5 The effect of the volume ratio of medium-DBS on the biomass concentration (a) and glucose residual rate (b) of AC microencapsulated yeast cells in the medium-DBS biphasic system after culturing for 24 h |

|
| Fig.6 Optical images of AC microencapsulated yeast cells in different volume ratios of medium-DBS after culturing for 24 h (40×) |
In our system, the PEC membrane of AC microcapsules provides the physical barrier for the maintenance of cell activity. The excess solvent, however, can increase the contact chance between solvent and microcapsules, which will go against the structure integrity of microcapsules and the growth behavior of entrapped cells. Therefore, the higher volume ratio between medium and solvent in a biphasic system is beneficial for both cell growth and metabolism with the protection of AC microcapsules. The much higher volume ratio is not recommended considering the cost and separation procedures in industrial application.
Moreover, solvent (DBS) is added separately into the culture medium at volume ratio of 1:1 to form biphasic system when AC microencapsulated yeast cells grow in lag phase (0 h), early stage of log phase (12 h), mid stage of log phase (18 h) and late stage of log phase (21 h), it clearly demonstrates that the addition time point of DBS at different cell growth phases has no significant adverse effect on the growth and metabolism of AC microencapsulated yeast cells. While the free cells in the mid or late stage of log growth phase usually have better tolerance to solvent than those in other phases (Wu et al., 1998).
4 CONCLUSIONWith the purpose of applying microencapsulated microbes for biocatalysis and biotransformation in the aqueous-solvent biphasic system, yeast cells (BY4741) are firstly entrapped in calcium alginate hydrogel beads prepared by emulsification-internal gelation technology, followed by coating with chitosan to form AC microcapsules. After culture in the medium-solvent biphasic system for 24 h, the biomass concentrations in AC microcapsules and alginate beads reach the 4.25- fold and 3.75-fold level to free cells. These results indicate that AC microcapsules as the immobilization carriers can confer yeast cells the ability to resist the adverse effect of solvent and show obviously superior growth behavior to free cells. Moreover, the cell activity, growth, and basic metabolism in AC microcapsules could be improved by adjusting the process parameters of PEC membrane formation and culture condition in biphasic systems. The formation of outer PEC membrane under the balance of resisting solvent and keeping inward substrate diffusion is important, and AC microcapsules help cells at different growth stages to tolerate solvent toxicity, which is superior to free cells in the biphasic system.
5 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are included in this published article. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
6 ACKNOWLEDGEMENTWe thank Dr. Xin Xiong (Natural and Medical Sciences Institute at University of Tübingen, Markwiesenstr. 55, 72770 Reutlingen, Germany), and Prof. Dr. Rumen Krastev (University Reutlingen, Alteburgstr. 150, 72762 Reutlingen, Germany) for the constructive discussion and communication about biocatalysis-related issues.
Arabi H, Yazdi M T, Faramarzi M A. 2010. Influence of whole microalgal cell immobilization and organic solvent on the bioconversion of androst-4-en-3, 17-dione to testosterone by Nostoc muscorum. Journal of Molecular Catalysis B: Enzymatic, 62(3-4): 213-217.
DOI:10.1016/j.molcatb.2009.10.006 |
Bezerra C S, De Farias Lemos C M G, De Sousa M, Gonçalves L R B. 2015. Enzyme immobilization onto renewable polymeric matrixes: past, present, and future trends. Journal of Applied Polymer Science, 132(26): 42125.
|
Bommarius A S, Paye M F. 2013. Stabilizing biocatalysts. Chemical Society Reviews, 42(15): 6534-6565.
DOI:10.1039/c3cs60137d |
Börner R A, Aliaga M T A, Mattiasson B. 2013. Microcultivation of anaerobic bacteria single cells entrapped in alginate microbeads. Biotechnology Letters, 35(3): 397-405.
DOI:10.1007/s10529-012-1094-1 |
Bornscheuer U T, Huisman G W, Kazlauskas R J, Lutz S, Moore J C, Robins K. 2012. Engineering the third wave of biocatalysis. Nature, 485(7397): 185-194.
DOI:10.1038/nature11117 |
Cirino P C, Sun L H. 2008. Advancing biocatalysis through enzyme, cellular, and platform engineering. Biotechnology Progress, 24(3): 515-519.
DOI:10.1021/bp070387a |
Cui J H, Cao Q R, Choi Y J, Lee K H, Lee B J. 2006. Effect of additives on the viability of bifidobacteria loaded in alginate poly-l-lysine microparticles during the freeze-drying process. Archives of Pharmacal Research, 29(8): 707-711.
DOI:10.1007/BF02968256 |
De Carvalho C C C R. 2011. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnology Advances, 29(1): 75-83.
DOI:10.1016/j.biotechadv.2010.09.001 |
Dennewald D, Hortsch R, Weuster-Botz D. 2012. Evaluation of parallel milliliter-scale stirred-tank bioreactors for the study of biphasic whole-cell biocatalysis with ionic liquids. Journal of Biotechnology, 157(1): 253-257.
DOI:10.1016/j.jbiotec.2011.10.008 |
Eş I, Vieira J D G, Amaral A C. 2015. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Applied Microbiology and Biotechnology, 99(5): 2065-2082.
DOI:10.1007/s00253-015-6390-y |
Gao M, Song H Y, Zheng H Z, Ren Y, Li S, Liu X D, Yu W T, Ma X J. 2016. Culture of low density E. coli cells in alginate-chitosan microcapsules facilitates stress resistance by up-regulating luxS/AI-2 system. Carbohydrate Polymers, 141: 160-165.
|
Garikipati S V B J, McIver A M, Peeples T L. 2009. Whole-cell biocatalysis for 1-naphthol production in liquid-liquid biphasic systems. Applied and Environmental Microbiology, 75(20): 6545-6452.
DOI:10.1128/AEM.00434-09 |
Gaumann A, Laudes M, Jacob B, Pommersheim R, Laue C, Vogt W, Schrezenmeir J. 2000. Effect of media composition on long-term in vitro stability of barium alginate and polyacrylic acid multilayer microcapsules. Biomaterials, 21(18): 1911-1917.
DOI:10.1016/S0142-9612(00)00071-5 |
Goranov B, Blaziiva D, Kostov G, Denkova Z, Germanova Y. 2013. Lactic acid fermentation with encapsulated Lactobacillus Casei ssp. Rhamnosus ATCC11979 (NBIMCC1013) in alginate-chitosan matrices. Bulg. J. Agric. Sci., 19(2): 101-104, https://www.researchgate.net/publication/258047099_Lactic_acid_fermentation_with_encapsulated_Lactobacillus_casei_SSP_rhamnosus_ATCC_11979_NBIMCC_1013_in_alginate_chitosan_matrices.
|
Hou D D, Li H J, Song H Y, Dai X M, Yu W T, Liu X D, Ma X J. 2012. Size and strength of alginate-chitosan microcapsules in nonaqueous system. CIESC Journal, 63(5): 1522-1528.
(in Chinese with English abstract) |
Kansal H, Banerjee U C. 2009. Enhancing the biocatalytic potential of carbonyl reductase of Candida viswanathii using aqueous-organic solvent system. Bioresource Technology, 100(3): 1041-1047.
DOI:10.1016/j.biortech.2008.08.042 |
Kisukuri C M, Andrade L H. 2015. Production of chiral compounds using immobilized cells as a source of biocatalysts. Organic & Biomolecular Chemistry, 13(40): 10086-10107.
|
Liouni M, Drichoutis P, Nerantzis E T. 2008. Studies of the mechanical properties and the fermentation behavior of double layer alginate-chitosan beads, usingSaccharomyces cerevisiae entrapped cell. World Journal of Microbiology and Biotechnology, 24(2): 281-288.
DOI:10.1007/s11274-007-9467-7 |
Marques M P C, De Carvalho C C C R, Cabral J M S, Fernandes P. 2010. Scaling-up of complex whole-cell bioconversions in conventional and non-conventional media. Biotechnology and Bioengineering, 106(4): 619-626.
DOI:10.1002/bit.v106:4 |
Naydenova V, Badova M, Vassilev S, Iliev V, Kaneva M, Kostov G. 2014. Encapsulation of brewing yeast in alginate/chitosan matrix lab-scale optimization of lager beer fermentation. Biotechnology & Biotechnological Equipment, 28(2): 277-284.
|
Rathore S, Desai P M, Liew C V, Chan L W, Heng P W S. 2013. Microencapsulation of microbial cells. Journal of Food Engineering, 116(2): 369-381.
DOI:10.1016/j.jfoodeng.2012.12.022 |
Ratnayake N D, Theisen C, Walter T, Walker K D. 2016. Whole-cell biocatalytic production of variously substituted β-aryl-and β-heteroaryl-β-amino acids. Journal of Biotechnology, 217: 12-21.
DOI:10.1016/j.jbiotec.2015.10.012 |
Reetz M T. 2013. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. Journal of the American Chemical Society, 135(34): 12480-12496.
DOI:10.1021/ja405051f |
Rivero C W, De Benedetti E C, Lozano M E, Trelles J A. 2015. Bioproduction of ribavirin by green microbial biotransformation. Process Biochemistry, 50(6): 935-940.
DOI:10.1016/j.procbio.2015.03.015 |
Schrewe M, Julsing M K, Bühler B, Schmid A. 2013. Wholecell biocatalysis for selective and productive C-O functional group introduction and modification. Chemical Society Reviews, 42(15): 6346-6377.
DOI:10.1039/c3cs60011d |
Shih I L, Chen L D, Wu J Y. 2010. Levan production using Bacillus subtilis natto cells immobilized on alginate. Carbohydrate Polymers, 82(1): 111-117.
DOI:10.1016/j.carbpol.2010.04.030 |
Song H Y, Yu W T, Gao M, Liu X D, Ma X J. 2013. Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydrate Polymers, 96(1): 181-189.
DOI:10.1016/j.carbpol.2013.03.068 |
Song H Y, Yu W T, Liu X D, Ma X J. 2014. Improved probiotic viability in stress environments with post-culture of alginate-chitosan microencapsulated low density cells. Carbohydrate Polymers, 108: 10-16.
DOI:10.1016/j.carbpol.2014.02.084 |
Stark D, Münch T, Sonnleitner B, Marison I W, Von Stockar U. 2002. Extractive bioconversion of 2-phenylethanol from l-phenylalanine by Saccharomyces cerevisiae. Biotechnology Progress, 18(3): 514-523.
DOI:10.1021/bp020006n |
Wang W, Liu X D, Xie Y B, Zhang H A, Yu W T, Xiong Y, Xie W Y, Ma X J. 2006. Microencapsulation using natural polysaccharides for drug delivery and cell implantation. Journal of Materials Chemistry, 16(32): 3252-3267.
DOI:10.1039/b603595g |
Wenda S, Illner S, Mell A, Kragl U. 2011. Industrial biotechnology-the future of green chemistry?. Green Chemistry, 13(11): 3007-3047.
DOI:10.1039/c1gc15579b |
Wohlgemuth R. 2010. Biocatalysis-key to sustainable industrial chemistry. Current Opinion in Biotechnology, 21(6): 713-724.
DOI:10.1016/j.copbio.2010.09.016 |
Wu Z L, Yuan Y J, Hu P, Hu Z D. 1998. Studies on the twoliquid-phase culture of Japanese yew (Taxus cuspidata) for the production of paclitaxel. Chinese Traditional Herbal Drugs, 29(9): 589-592.
(in Chinese with English abstract) |
Yang Z Y, Ni Y, Sun Z H. 2009. Recent trend of nonaqueous enzymology and biocatalysis in nonaqueous media. Chinese Journal of Biotechnology, 25(12): 1779-1783.
(in Chinese with English abstract) |
Yu H L, Ou L, Xu J H. 2010a. New trends in non-aqueous biocatalysis. Current Organic Chemistry, 14(14): 1424-1432.
DOI:10.2174/138527210791616740 |
Yu W T, Lin J Z, Liu X D, Xie H G, Zhao W, Ma X J. 2010b. Quantitative characterization of membrane formation process of alginate-chitosan microcapsules by GPC. Journal of Membrane Science, 346(2): 296-301.
DOI:10.1016/j.memsci.2009.09.049 |
Yu W T, Song H Y, Zheng G S, Liu X D, Zhang Y, Ma X J. 2011. Study on membrane characteristics of alginatechitosan microcapsule with cell growth. Journal of Membrane Science, 377(1-2): 214-220.
DOI:10.1016/j.memsci.2011.04.053 |
Zhang F, Xue Y, Li L, Wang M. 2010. Asymmetric reduction of 3, 5-Bistrifluoromethylphenyl acetophenone by immobilized Saccharomyces rhodotorula in biphasic system. Pharmaceutical Biotechnology, 17(2): 125-129.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37


