Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHIA Shir Reen, CHEW Kit Wayne, SHOW Pau Loke, SIVAKUMAR Manickam, LING Tau Chuan, TAO Yang
- Isolation of protein from Chlorella sorokiniana CY1 using liquid biphasic flotation assisted with sonication through sugaring-out effect
- Journal of Oceanology and Limnology, 37(3): 898-908
- http://dx.doi.org/10.1007/s00343-019-8246-2
Article History
- Received Sep. 13, 2018
- accepted in principle Oct. 19, 2018
- accepted for publication Nov. 15, 2018
2 Department of Chemical and Environmental Engineering, Faculty of Engineering, University of Nottingham Malaysia Campus, Semenyih 43500, Malaysia;
3 Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur 50603, Malaysia;
4 College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
Microalgae are being studied intensively as a potential replacement of feedstock for the next generation in the bioenergy production (Chia et al., 2018a). The carbohydrates and lipid content within most of the microalgae species are the major constituents to produce biofuels through transesterification, biochemical conversion and thermochemical conversion (Xia et al., 2013; Ho et al., 2017; Chia et al., 2018b), while other constituents such as pigments, vitamins, polyphenols and polyunsaturated fatty acids are the high valuable compounds that are beneficial to mankind and animal health (Yang et al., 2010; Ma et al., 2011; Chew et al., 2017) with biological activities such as antitumor, antimicrobial, anti-diabetes etc. (Liu et al., 2010; Guo et al., 2011). Besides these constituents, the protein content in microalgae was identified to be a potential substituent to conventional protein source. Microalgal protein is often discussed and investigated as the consumers are paying more attention to the health benefits and notably it offers an alternative source of protein to vegan or allergenic consumers (Hariskos and Posten, 2014). The protein content of microalgae could be as high as 40% to 70% (Becker, 2007), and has a comparable composition with the lipid content within microalgae. This high composition of protein could possibly substitute the main protein supply for human nutrition due to the rapid growth rate of microalgae and their high photosynthetic efficiencies in specific environments (Markou and Nerantzis, 2013).
Conventional techniques for the extraction of bioactive compounds require longer processing time, cost-consuming with complex scale-up (Asenjo and Andrews, 2012). In order to overcome these significant problems in the extraction of bioactive compounds, an efficient liquid biphasic flotation (LBF) method was proposed. LBF is the formation of two types of immiscible solutions, with the induction of air bubbles to the system. LBF is a combination of the aqueous two-phase system (ATPS) with solvent sublation (SS) (Phong et al., 2017). LBF is proposed mainly due to the wide application of ATPS in the separation of biomolecules and could be performed better with an enhancement of SS (Platis and Labrou, 2009). Among all the downstream processing methods, ATPS is scalable, cost-effective, and a good recovery of biomolecules could be obtained from biomass in various studies (Frampton et al., 2015; Zimmermann et al., 2018). ATPS acts as an effective and simple platform for the purification of biomolecules with good performance, which paves a way towards industrial applications (Soares et al., 2015). On the other hand, SS is able to concentrate the targeted biomolecules from the aqueous solution with a suitable gas flow rate and type of solvents applied in the system (Sobianowska et al., 2009). ATPS is a liquid-liquid fractionation technique with water as the main medium, which prevents the denaturation of biomolecules. The principle of SS is based on nonfoaming adsorptive bubbles separation, by allowing the biomolecules absorbed to the surface of bubbles and the bubbles subsequently float up from the bottom liquid phase to the upper liquid phase (Sobianowska et al., 2009). Therefore, LBF is claimed to have the advantages of both ATPS and SS, as mentioned above.
Apart from the extraction methods of biomolecules, the cell disruption method is equally essential in order to break down the cell wall of microalgae to release the targeted biomolecules. There are several types of cell disruption methods to release protein from the biomass, for example, manual grinding, ultrasonication, bead-beating, high pressure cell disruption and alkaline treatment (Safi et al., 2014). In this study, sonication was exploited to assist in the extraction of protein. The mechanism induced by sonication such as erosion, sonoporation, fragmentation and more (Khadhraoui, 2018), often enhance the extraction of the targeted component. These mechanisms allowed the inner medium of natural sources to be released by damaging the membrane or cell wall of the plant. Sonication is used as "green and innovative" approach in green processing, pasteurization, and extraction as it can be used to overcome the current issues faced in industries, such as low production efficiency, time-consuming procedures and a large quantity of wastewater (Chemat et al., 2017a). The green impacts of sonication for downstream processing are a reduction of wastewater, time and energy-saving (use of the low volume of water, shorten or simplified procedures for heating and stirring, etc.) and elimination of hazardous substances which preserve the environment. Sonication can be performed using a bath sonicator or probe horn. The bath sonicator is often known as indirect sonication while the probe type is considered to be direct sonication. The sonicator probe generally provides more ultrasound energy compared to sonicator bath (Wu et al., 2001). The cell wall structure of microalgae was taken into consideration as its cell wall consists of polysaccharides and glycoprotein matrix, which made the cell wall intrinsic and provides formidable defence towards the environment (Gerken et al., 2013). Hence, sonication was performed using the sonicator probe.
In this study, the extraction of protein from microalgae was carried out by using LBF assisted with probe type sonication. The protein extracted through sugaring-out effect has been investigated with different types and concentrations of sugar as well as the working volume of both phases in the system. Besides, this study was performed with Chlorella sorokiniana, a microalgal that consists of higher protein content. The studies related to sonication such as pulse mode and continuous mode for assisting the extraction of protein were determined to evaluate the optimum parameters during the extraction process.
2 MATERIAL AND METHOD 2.1 MaterialGlucose, sucrose, maltose, fructose, acetonitrile and Bradford reagent were obtained from R & M Chemicals (Malaysia). The solutions used for protein recovery were prepared from distilled water and the medium used for microalgae cultivation was prepared from deionized water. The working standard solutions of BSA were prepared by appropriately diluting the stock solution of 2 mg/mL using distilled water. All the purchased chemicals and solvents were analytical grade.
2.2 ApparatusA liquid biphasic flotation (LBF) equipment with a total volume of 500 mL was connected to the oilless air compressor (Model: PAC750-240F) with a capacity of 24-L tank, motor speed of 1 450 r/min, maximum pressure of 8 bar and an air displacement of 107 L/min for the generation of air bubbles. The LBF equipment is a 15-cm height of glass column with an inner diameter of 7 cm, equipped with the sintered disk of G4 porosity at the bottom of the glass column. The thickness of the glass column is 0.4 cm. The air flowrate of the system was regulated using the rotameter (Dwyer, USA) in a range from 25 to 250 mL/min. The ultrasound treatment was introduced using Bandelin Sonopuls (UW 2200, Germany) with a titanium horn sonotrode (TT 13/FZ). The schematic of LBF equipment is as shown below (Fig. 1).
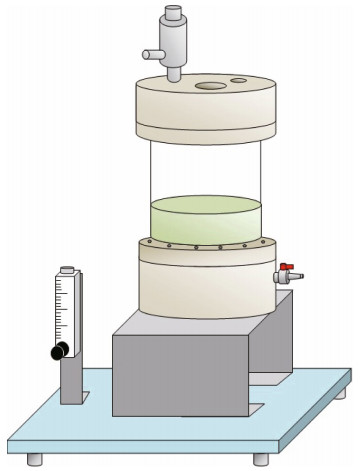
|
| Fig.1 Schematic of the equipment used in liquid biphasic flotation (LBF) |
A green microalgae strain, Chlorella sorokiniana CY-1, was selected in this study. The medium used in the pre-cultivation and batch cultivation for the microalgae is BG-11 medium with a continuous supply of 2.5% CO2. The composition of BG-11 medium is as follows: 1.5 g/L of NaNO3, 0.03 g/L of K2HPO4, 0.075 g/L of MgSO4∙7H2O, 0.006 g/L of citric acid, 2 g/L of Na2CO3, 3.6 g/L of aCl2∙2H2O, 0.6 g/L of ferric ammonium nitrate, 0.1 g/L of EDTA, 2.86 g/L of H3BO3, 1.81 g/L of MnCl2∙4H2O, 0.222 g/L of ZnSO4∙7H2O, 0.39 g/L Na2MoO4∙2H2O, 0.079 g/L of CuSo4∙5H2O and 0.049 g/L of Co(NO3)2∙6H2O.
The initial culturing of microalgae and the transferring of inoculums into photobioreactor were carried out within the laminar flow chamber to reduce the possibility of biological contamination. The precultivation of microalgae was performed for one week, whereas the batch cultivation was performed within 10 to 14 days. The nitrogen content was determined on daily basis and the wet microalgae were harvested when the nitrogen content of microalgae was about 10% of the original nitrogen content at Day 1 of batch cultivation (nitrogen starvation). The nitrogen content of microalgae biomass was determined by centrifuging the harvested biomass at 6 000 r/min for 5 min (Eppendorf, 5430). The supernatant was obtained and 10 times dilution was performed prior to analysis. The sample was tested at a wavelength of 220 nm by using UV-Vis spectrophotometer (Chew et al., 2018).
2.4 Determination of protein contentThe protein content of microalgae biomass was examined using a modified Bradford method (Bradford, 1976). 0.25 mL of sample was mixed with 2.5 mL of Bradford reagent to measure the extracted protein content from microalgae. A wavelength of 595 nm was used to determine the extracted protein by using UV-Vis spectrophotometer. The concentration of protein extracted was determined by converting the obtained absorbance values via calibration between OD595 and BSA protein concentrations. The protein calibration curve was obtained using BSA as a standard and the working standard solutions are prepared in a range of 0.025 to 2 mg/mL.
2.5 Determination of separation efficiency (E), total protein content and yield (Y)The separation efficiency (E) for the extraction of protein was calculated using Eq.1:
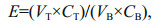 (1)
(1)where VT is the volume obtained for the top phase, VB is the volume obtained for bottom phase, CT is the concentration of the top phase and CB is the concentration of bottom phase.
The total protein content in the microalgae (PT) was calculated using Eq.2:
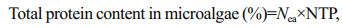 (2)
(2)where Nea is the total nitrogen (%) of Chlorella sorokiniana and a value of 7.05% was obtained by elemental analysis; NTP is a constant value of the nitrogen-to-protein conversion factor, which is 4.78.
The yield of protein (Y) from microalgae was calculated using Eq.3: Total protein recovered in top phase 100%.
 (3)
(3)Sonication-assisted LBF composed of sugar solution and alcohol was implemented for the extraction of protein from microalgae. Firstly, the sugar solution was prepared and mixed with the weighed microalgae biomass. The mixture was poured into LBF equipment, followed by a measured volume of alcohol was added. The flotation time initially was set to 5 min with an air flowrate of 10 mL/min. The compressor was supplying at 0.5 bar and the air was filtered through a sterile air filter. The filtered air will be connected to the bottom joint of equipment, where the generated air bubbles passed through the solution (consist of sugar solution, acetonitrile, and wet microalgae biomass) from the bottom of the system. The sonicator probe was inserted into the system and was placed between the interface of the top and bottom phase. The targeted product will be brought to the upper phase of the system and took for further analysis.
The initial condition for sonication-assisted LBF was stated as followed: 150 mL of 200 g/L sugar solution (glucose), 150 mL of pure acetonitrile, 0.5 g of wet microalgae biomass, 20% amplitude of a 200 W maximal power, pulse mode (5 s ON / 10 s OFF), sonication time of 5 min and flotation time of 5 min. The initial volume ratio used is 1:1 as reported in the previous work which used a small working volume (Dhamole et al., 2010a). The air flowrate was remained constant throughout the experiment to avoid any disturbance caused to the equilibrium of the twophase system.
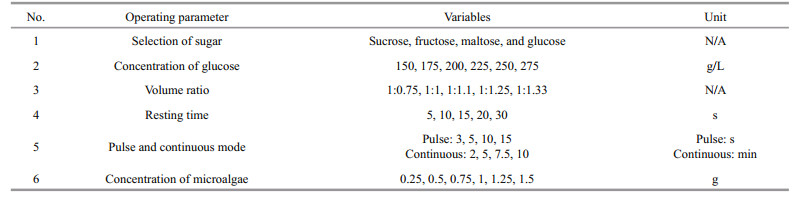
The operating parameters of sonication-assisted LBF were performed as followed: selection and concentration of sugar, the volume ratio of the system, resting time for sonication, pulse and continuous mode and concentration of microalgae, were shown in Table 1. These parameters were studied and optimised using one-factor-at-a-time (OFAT) approach.
|
In liquid biphasic flotation (LBF), the common chemicals used as phase construction materials are polymers, salts, and alcohols. Other than salt solutions, sugar solutions could be used to form the two phases with alcohols as well. In this case, the targeted product that had to be isolated from the source would be separated from the biomass through sugaring-out effect. The sugaring-out effect is similar to the saltingout effect, where the types of sugars are the crucial phase constructing components in the system. The effect of sugaring-out agents could result in variations in the ability of phase separation as reported earlier (Zhang et al., 2012; Tu et al., 2018).
The protein partitioning was determined using different types of sugars, for example, sucrose, fructose, glucose, and maltose. These sugars were chosen as they are common sugars that are either fall under the category of monosaccharide or disaccharide. Glucose and fructose are monosaccharides while sucrose and maltose are disaccharides. Acetonitrile has been chosen to be the top phase of the system due to the suitability and stability of forming phases with the selected sugar solution (Dhamole et al., 2010b). In addition, the phase separation of acetonitrile and water can be induced at very low temperatures such as 1℃ using sugars (Wang et al., 2008a), which can also be achieved at low and at room temperatures in the range of 6 to 24℃ (Dhamole et al., 2010a). For this experiment, pure acetonitrile with a similar working volume of sugar solution was used and the experiment was performed at room temperature.
Figure 2 shows the protein yield (Y) and separation efficiency (E) of protein from the biomass. The highest Y recovered from the biomass was around 52% and the lowest Y was around 33%, for glucose and fructose respectively. Glucose as the bottom phase gave the highest Y but comparable E of protein with the system consisting of fructose. The performance of both monosaccharides i.e. glucose and fructose in protein partitioning, were completely different, as glucose had the best performance on Y while fructose was not. The study of Cardoso et al. (2013) suggested that the hydration ability of sugars is proportional to the tendency of phase separation (Cardoso et al., 2013). According to their study, glucose (an aldose with a 6-sided ring of carbon atoms) is more effective in inducing the formation of two aqueous phases compared to fructose, due to the presence of ketose. On the other hand, the system using maltose as the bottom phase had the highest E compared to other systems. The disaccharides have comparable Y and E, and they had slightly better performance than fructose either in Y or E. The disaccharides showed similar capabilities in isolating protein from biomass due to their structure. Sucrose consists of glucose and fructose while maltose consists of two units of glucose. As disaccharides and glucose have lower compatibility with acetonitrile molecules, more of these acetonitrile molecules were separated out from aqueous solutions, resulting in the higher amount of protein being transferred to the acetonitrile-rich phase (Zhang et al., 2012). Glucose has been chosen as the bottom phase of LBF as highest Y and comparable E were obtained among another type of sugars.
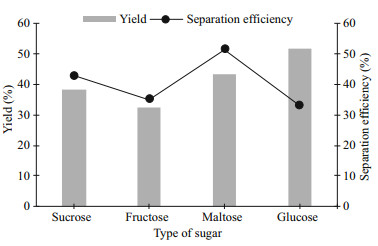
|
| Fig.2 Effect of type of sugar on the protein yield and separation efficiency |
From the study related to types of sugars, glucose has been chosen among the carbohydrates due to the high recovery of protein and comparable separation efficiency (E) obtained. The induction of two phase depends on the concentration of glucose as well. In this study, the concentration of glucose as the bottom phase was altered as, 150, 175, 200, 225, 250, and 275 g/L. The initial glucose concentration was 200 g/L as followed in the study of Shishov et al. (2017). In the mixture of acetonitrile and water, Shishov et al. (2017) reported that the addition of glucose above a critical concentration was able to induce two phases. The sugar concentration of 200 g/L and above extracted amylmetacresol effectively in both phases (Shishov et al., 2017). Hence, lower sugar concentrations (150 and 175 g/L) were examined along with the high concentration of sugar for larger working volume to investigate the potential of utilizing lower glucose concentration to extract the targeted product. However, a faint line was observed in both the systems containing 150 and 175 g/L of glucose in the bottom phases. As the glucose concentration increased, phase separation in the system was clearly observed. This finding corresponds to the observations of Timofeeva et al. (2017), where a complete phase separation was only observed at the concentration of glucose in an aqueous solution which was higher than 200 g/L (Timofeeva et al., 2017).
From Fig. 3, the protein recovered using 150 and 175 g/L of glucose were comparatively low, which were only able to recover 8.86% to 12.45% of protein yield (Y) compared to using a higher concentration of glucose. The results obtained had shown that Y was increasing from 150 g/L to 175 g/L with an increase in the volume of organic extract due to an increase in the concentration of glucose. The increasing trend of Y and E was observed from 150 g/L to 200 g/L and from 250 g/L to 275 g/L of glucose. It is believed that the sonication along the process breaks down the cell wall and the bubbling effect (flotation) could bring the soluble proteins from the glucose solution (bottom phase) to the acetonitrile-rich phase. In addition, the higher glucose concentration contributed in breaking the cell wall of microalgae partially before performing the extraction (sonication and flotation), which showed a significant difference of E and Y between low and high glucose concentration. However, the highest glucose concentration did not recover most of the protein from the biomass, instead 200 g/L of glucose was able to recover the highest percentage of protein among all the parameters. The systems consisted of glucose concentration higher than 200 g/L have a lesser water content in the glucose mixture as the percentage of glucose is higher. Thus, it is reasonable to speculate that the maximum capacity of protein soluble in the water was reduced due to the limited water content (Tu et al., 2018). This would reduce the amount of soluble protein in the acetonitrile-rich phase under sonication and flotation. Indeed, the separation efficiency of protein was at a peak in 275 g/L of glucose and was the lowest in 150 g/L of glucose. The volume ratio of acetonitrilerich phase and glucose were varied from the initial volume ratio, where most of the higher glucose concentration formed a higher volume of acetonitrilerich phase. This is similar to the salting-out effect, where a higher concentration of glucose resulted in an increased partition behaviour of protein to acetonitrilerich phase (Goja et al., 2013). In this study, the glucose concentration of 200 g/L was chosen as the optimum concentration for protein extraction.

|
| Fig.3 Effect of sugar concentration on the protein yield and separation efficiency |
From previous studies, glucose with 200 g/L was chosen to study the volume ratio in the system. This is an important study for determining the optimum volume ratio required to isolate the protein without denaturing and to obtain an optimum yield (Y) and separation efficiency (E) from the biomass. By using acetonitrile as top phase, the volume ratio was altered in the ratio of 1:0.75/ 1/ 1.1/ 1.25 and 1.33 (volume of bottom phase: volume of top phase). The volume of top phase was altered due to more protein which was separated to the top phase compared to the bottom phase (data not shown) and the increasing working volume of top phase would possibly improve Y and E of protein. The total working volume of the system remained the same (300 mL) similar to studies shown in section 3.1 and 3.2. The volume ratio of acetonitrile to water mixture with the addition of sugars is 1:1 for conducting the sugaring-out effect (Wang et al., 2008b; Nugbienyo et al., 2017). Therefore, the initial volume ratio of 1:1 was used as the standard and the range was set as shown in Fig. 4.
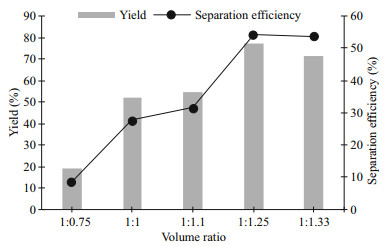
|
| Fig.4 Effect of volume ratio on the protein yield and separation efficiency |
The results obtained in this study are not similar to both the previous studies. The Y and E have been shown to be in an increasing manner with an increase in the working volume of acetonitrile. The higher Y in acetonitrile-rich phase was obtained due to more soluble protein released from the biomass through sonication and flotation was able to solubilise in the acetonitrile-rich phase. However, a slight decrement of both Y and E was observed in the highest volume ratio (1:1.33) of the system (Fig. 4). As the initial volume of acetonitrile-rich phase greater, the lower the working volume of glucose-rich phase. The lower working volume of glucose-rich phase was not consisting of a similar amount of water content as with another volume ratio. Thus, the lower water content in glucose-rich phase was not capable to solubilise a higher amount of protein extracted from microalgae biomass through flotation even sonication was performed to disrupt the cell wall (Naveena et al., 2015). Furthermore, the working volume of acetonitrile should be at least the same working volume with the bottom phase to obtain more than 50% of recovered protein. The low working volume of acetonitrile was not able to extract more protein from the biomass even in the presence of sonication. The protein released through sonication may not be able to be extracted into the acetonitrile-rich phase as only a certain amount of protein can be solubilised into the limited capacity of acetonitrile-rich phase. With the discussion mentioned above, the volume ratio of 1:1.25 has been chosen as the best condition to perform the next study, the resting time of ultrasonication.
3.4 Resting timeThe studies related to liquid biphasic flotation (LBF) were discussed in detail in Section 3.1 to 3.3. Hence, the studies focusing on sonication were investigated for the integrated extraction. Resting time is the resting period in between the sonication process during the pulse mode. The resting time of sonication would influence the extraction as the sonicator probe would have a higher temperature if longer sonication time is applied for the extraction. Besides, the physical and chemical effects of ultrasound may vary depending on the resting time in between the sonication period. Generally, the occurrence of cavitation bubbles for longer resting time might not be as much compared to the shorter resting time between the sonication period. Hence, the effect of resting time on the protein extraction was investigated as the cavitation effect generated reduces during the ultrasonication. In this study, only the resting time was altered while the sonication time was fixed at 10 s in a total extraction time of 5 min. The resting time in between the sonication time was ranged from 5 s to 30 s in order to sustain sufficient cavitation effect for breaking the cell wall of microalgae.
Figure 5 shows that 10 to 30 s had similar protein yield (Y) recovered from the biomass except for a resting time of 15 s. The Y was found to be decreasing drastically at 15 s of resting time, which was around 30%, even 5 s of resting time had higher protein yield than 15 s. This is mainly due to the times of sonication generated in 5 min as more times of sonication generated in 5 s of resting time than in 15 s of resting time. The continuous oscillatory motion of liquid medium generated by the radial movement of cavitation bubbles, namely micro-convection, which subsequently result in the growth of nuclei. When the cavitation bubbles grow to the maximum size (4– 300 mm diameter) and acoustic energy reached sufficient intensity, these microbubbles become unstable and collapse. The times of sonication generated was critical to the breakage of cell wall as the collapse of these microbubbles create microjets with a velocity of more than 100 m/s (Naveena et al., 2015). As the resting time in between sonication increases, the times of sonication decreases. However, 20 to 30 s of resting time has better Y and separation efficiency (E) of protein than 15 s of resting time. The mixing process induced by flotation and sonication for 15 s of resting time was more vigorous than 20 and 30 s of resting time, resulting into turbulence in the medium which pushes the extracted protein away from the surface of microbial cells. Furthermore, the vigorous mixing process causes the extracted protein dispersed in between the two phases, without remaining in the acetonitrile-rich phase. Besides, the E of each resting time was different. The higher resting time had similar E, but the lowest resting time had a similar result with the moderate resting time (15 s). This is due to the volume ratio of both phases which affected through the turbulence generated by flotation and sonication, as indicated in the previous paragraph. The 10 s of resting time was the most suitable for pulse mode and was chosen to be studied for the next parameter as the highest E and Y was obtained.
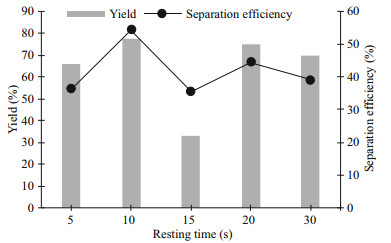
|
| Fig.5 Effect of resting time of ultrasonication on the protein yield and separation efficiency |
The pulse and continuous mode of sonication were studied to examine the suitable mode for the experiments after investigating the resting time in between sonication. The pulse mode of sonication was examined from 3 to 15 s with a fixed resting time of 10 s and the continuous mode was studied from 2 to 10 min of non-stop sonication without any resting time in between. As reported by Chemat et al. (2017b) and Sivakumar et al. (2017), the extraction of components through different modes of sonication could affect the yield of components extracted (Chemat et al., 2017b; Sivakumar et al., 2017). The range selected for continuous mode was lower than the ranges studied in the previous reports (Pradal et al., 2016; Shirsath et al., 2017), due to the mechanisms as both sonication and flotation lead to mixing in the system.
The obtained results for pulsed and continuous mode of sonication assisted with flotation have been shown in Fig. 6. Among all the sonication time set for pulse mode, 5 s of sonication time could achieve the highest protein yield (Y) and separation efficiency (E). The sonication time of 3 s was not sufficient for the complete breakage of the cell wall, causing low Y and E of protein obtained. However, similar results were obtained by 10 and 15 s of sonication time with 3 s of sonication time, only 5 s of sonication time was the best. Five seconds of sonication time has higher Y than 10 s and 15 s of sonication time as the occurrence of sonication regularity at a given time had been increased in shorter pulse mode, leads to a better Y and E (Sankaran et al., 2018). Overall, the average yield obtained by using continuous mode was better than pulse mode, yet the highest yield and separation efficiency was obtained using pulse mode. The pulse mode of the sonicator probe could perform better than the continuous mode in extracting the protein due to the cavitational events. The gas content of the liquid increased as pulse mode was applied to the system, which creates a higher number of cavities resulting in higher cavitational events, thereby enhancing the Y (Sivakumar et al., 2002). As for the continuous mode, the decrement of Y was caused by the application of continuous ultrasound, where the nuclei were destroyed rapidly and resulted in degassing of the cavitation bubbles (Kumar et al., 2000). Besides, the continuous mode with 10 min of sonication obtained the lowest Y as well as E, which is mainly due to the vigorous mixing process, as mentioned in Section 3.4.
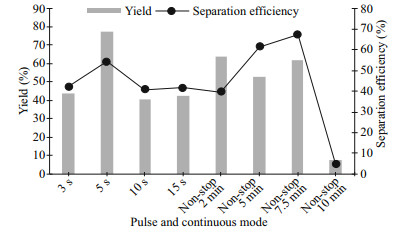
|
| Fig.6 Effect of pulse and continuous modes of ultrasonication on the protein yield and separation efficiency |
The study related to the sample weight was carried out and the examined weight of biomass was ranged from 0.25 to 1.5 g of wet microalgae. This study was performed due to the previous studies which stated that the concentration or weight of crude biomass does affect the targeted product recovered from the crude biomass (Show et al., 2011; Phong et al., 2017). The partition behaviour of protein altered accordingly when biomass was loaded into the system. In the study of Show et al. (2011), the separation efficiency was decreased as the higher amount of feedstock was loaded to the system, which influenced the composition of the system (Show et al., 2011). Furthermore, the protein recovered from the biomass should be increasing as the crude load of biomass was increased. In fact, the obtained result was not corresponding to the hypothesis. Figure 7 illustrates that the yield (Y) and separation efficiency (E) were correlated to each other as the lower yield has lower separation efficiency for 0.75 to 1.5 g of biomass used. However, the highest separation efficiency did not correspond to the highest yield obtained in this study. The precipitation of cell debris or unwanted components may occur at the interface of the system, resulting in the protein not to be sugaring-out from the bottom phase (glucose-rich phase) (Selvakumar et al., 2010).
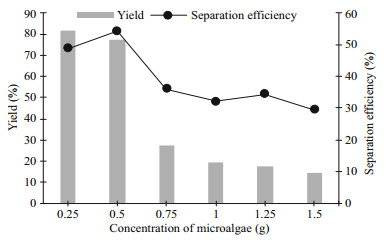
|
| Fig.7 Effect of concentration of microalgae on the protein yield and separation efficiency |
The best performance of the system was observed using the lightest biomass weight (0.25 g) to obtain the highest Y of protein, around 80% with a comparable E of protein with 0.5 g of biomass. This may be due to the complete sonicated biomass by using the sonicator, which causes a higher amount of protein extracted from the biomass. Nonetheless, the biomass weight with 0.75 g to 1.5 g was only capable to extract 10% to 30% of protein from the biomass which was much lesser than using 0.25 and 0.5 g of biomass in the system. The Y for biomass weight from 0.75 to 1.5 g decreased drastically while having similar E of protein in these systems. These phenomena would probably occur as the volume ratio of systems was similar after the extraction process but obtaining lesser protein in the top phase (acetonitrile-rich phase).
3.7 Potential industrial application and up-scalingSonication process often shows green impact such as lower energy consumption, reduction of ecofootprint of process and act as an economical model. Therefore, the parameters of sonication along with the parameters of LBF were included in this work. In order to utilize the energy input effectively, the resting time of sonication and mode of sonication have been studied and optimized. The optimum condition to extract protein is resting time of 10 s and pulse mode in sonication-assisted LBF. The resting time of sonication is significant as it implies the energy required for the sonication process, whereby more energy input is needed for the shorter resting time of sonication and vice versa. Besides, sonication in pulse mode requires lower energy input from the generator compared to the sonication in a continuous mode as it requires a continuous supply of energy to perform the sonication.
A preliminary assessment of the energy consumption for both conventional and sonication-treated extraction of seeds have been performed in the study of Sicaire et al. (2016). The study takes into account preparation of seeds, desolventization step, distillation step and heat recovered from the gas from total distillation. Reduction of heat and steam consumption was observed which subsequently leads to lower global environmental impact by using sonication-treated extraction.
This work has indicated potential industrial application as the sonication-assisted process have speed-up the processing time, where the extraction only required 5 min of sonication with 5 s of sonication and 10 s of resting time. A significant time reduction in extraction process could lead to a reduction of processing cost implied in energy production. Hence, the sonication-assisted LBF is highly recommended as the extraction method, LBF is ease to scale up to industrial scale. However, further investigation is required as the lab scale extraction may vary with the industrial scale extraction. The impact in industrial has to be investigated as it might clearly to be beneficial to the industry.
4 CONCLUSIONIn conclusion, this study demonstrates an effective technique to extract the protein content from green microalga, Chlorella sorokiniana CY1, assisted with sonication that aids in breaking down the cell wall during extraction. The results presented in this work show that the protein could be isolated from microalgae through sugaring-out effect. In liquid biphasic flotation (LBF), the type of sugar solution, the concentration of sugar, the weight of wet microalgae were investigated as the factors influencing the performance of extraction. Besides, the protein extraction was influenced by the working volume of both phases significantly, obtaining around 77% of protein yield and 54% of separation efficiency. It has also been found that the protein extraction is higher for pulse mode compared to the protein extracted by the continuous mode. The highest protein yield of 80% and the corresponding separation efficiency of 49%, were achieved after optimisation through various parameters, suggesting that the high content of protein within microalgae could be extracted on an industrialscale, as LBF is easy to be scaled up with lower cost.
5 DATA TRANSFER STATEMENTThe datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Asenjo J A, Andrews B A. 2012. Aqueous two-phase systems for protein separation:phase separation and applications. Journal of Chromatography A, 1238: 1-10.
DOI:10.1016/j.chroma.2012.03.049 |
Becker E W. 2007. Micro-algae as a source of protein. Biotechnology Advances, 25(2): 207-210.
DOI:10.1016/j.biotechadv.2006.11.002 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Cardoso G D B, Mourão T, Pereira F M, Freire M G, Fricks A T, Soares C M F, Lima Á S. 2013. Aqueous two-phase systems based on acetonitrile and carbohydrates and their application to the extraction of vanillin. Separation and Purification Technology, 104: 106-113.
DOI:10.1016/j.seppur.2012.11.001 |
Chemat F, Rombaut N, Meullemiestre A, Turk M, Perino S, Fabiano-Tixier A S, Abert-Vian M. 2017a. Review of green food processing techniques. Preservation, transformation, and extraction. Innovative Food Science& Emerging Technologies, 41: 357-377.
DOI:10.1016/j.ifset.2017.04.016 |
Chemat F, Rombaut N, Sicaire A G, Meullemiestre A, FabianoTixier A S, Abert-Vian M. 2017b. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34: 540-560.
DOI:10.1016/j.ultsonch.2016.06.035 |
Chew K W, Chia S R, Show P L, Ling T C, Arya S S, Chang J S. 2018. Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris. Bioresource Technology, 267: 356-362.
DOI:10.1016/j.biortech.2018.07.069 |
Chew K W, Yap J Y, Show P L, Suan N H, Juan J C, Ling T C, Lee D J, Chang J S. 2017. Microalgae biorefinery:high value products perspectives. Bioresource Technology, 229: 53-62.
DOI:10.1016/j.biortech.2017.01.06 |
Chia S R, Chew K W, Show P L, Yap Y J, Ong H C, Ling T C, Chang J S. 2018b. Analysis of economic and environmental aspects of microalgae biorefinery for biofuels production:a review. Biotechnology Journal, 13(6): 1 700 618.
DOI:10.1002/biot.201700618 |
Chia S R, Ong H C, Chew K W, Show P L, Phang S M, Ling T C, Nagarajan D, Lee D J, Chang J S. 2018a. Sustainable approaches for algae utilisation in bioenergy production. Renewable Energy, 129: 838-852.
DOI:10.1016/j.renene.2017.04.001 |
Dhamole P B, Mahajan P, Feng H. 2010a. Sugaring out:a new method for removal of acetonitrile from preparative RPHPLC eluent for protein purification. Process Biochemistry, 45(10): 1 672-1 676.
DOI:10.1016/j.procbio.2010.06.020 |
Dhamole P B, Mahajan P, Feng H. 2010b. Phase separation conditions for sugaring-out in acetonitrile-water systems.J. Chem. Eng. Data, 55(9): 3 803-3 806.
DOI:10.1021/je1003115 |
Frampton J P, Tsuei M, White J B, Abraham A T, Takayama S. 2015. Aqueous two-phase system-mediated antibody micropatterning enables multiplexed immunostaining of cell monolayers and tissues. Biotechnology Journal, 10(1): 121-125.
DOI:10.1002/biot.201400271 |
Gerken H G, Donohoe B, Knoshaug E P. 2013. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta, 237(1): 239-253.
DOI:10.1007/s00425-012-1765-0 |
Goja A M, Yang H, Cui M, Li C. 2013. Aqueous two-phase extraction advances for bioseparation. Journal of Bioprocessing & Biotechniques, 4: 140.
DOI:10.4172/2155-9821.1000140 |
Guo S J, Li J, Li T, Shi D Y, Han L J. 2011. Synthesis of three bromophenols from red algae as PTP1B inhibitors.
|
Chinese Journal of Oceanology and Limnology, 29(1): 68-74, https://doi.org/10.1007/s00343-011-9996-7.
|
Hariskos I, Posten C. 2014. Biorefinery of microalgaeopportunities and constraints for different production scenarios. Biotechnology Journal, 9(6): 739-752.
DOI:10.1002/biot.201300142 |
Ho S H, Chen Y D, Chang C Y, Lai Y Y, Chen C Y, Kondo A, Ren N Q, Chang J S. 2017. Feasibility of CO2 mitigation and carbohydrate production by microalga Scenedesmus obliquus CNW-N used for bioethanol fermentation under outdoor conditions:effects of seasonal changes. Biotechnology for Biofuels, 10: 27.
DOI:10.1186/s13068-017-0712-5 |
Khadhraoui B, Turk M, Fabiano-Tixier A S, Petitcolas E, Robinet P, Imbert R, El Maâtaoui M, Chemat F. 2018. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrasonics Sonochemistry, 42: 482-492.
DOI:10.1016/j.ultsonch.2017.11.029 |
Kumar P S, Kumar M S, Pandit A. 2000. Experimental quantification of chemical effects of hydrodynamic cavitation. Chemical Engineering Science, 55(9): 1 633-1 639.
DOI:10.1016/S0009-2509(99)00435-2 |
Liu J G, Zhang X L, Sun Y H, Lin W. 2010. Antioxidative capacity and enzyme activity in Haematococcus pluvialis cells exposed to superoxide free radicals. Chinese Journal of Oceanology and Limnology, 28(1): 1-9.
DOI:10.1007/s00343-010-9244-6 |
Ma X L, Yu J Z, Zhu B H, Pan K H, Pan J, Yang G P. 2011. Cloning and characterization of a delta-6 desaturase encoding gene from Nannochloropsis oculata. Chinese Journal of Oceanology and Limnology, 29(2): 290-296.
DOI:10.1007/s00343-011-0048-0 |
Markou G, Nerantzis E. 2013. Microalgae for high-value compounds and biofuels production:a review with focus on cultivation under stress conditions. Biotechnology Advances, 31(8): 1 532-1 542.
DOI:10.1016/j.biotechadv.2013.07.011 |
Naveena B, Armshaw P, Pembroke J T. 2015. Ultrasonic intensification as a tool for enhanced microbial biofuel yields. Biotechnology for Biofuels, 8: 140.
DOI:10.1186/s13068-015-0321-0 |
Nugbienyo L, Malinina Y, Garmonov S, Kamencev M, Salahov I, Andruch V, Moskvin L, Bulatov A. 2017. Automated sugaring-out liquid-liquid extraction based on flow system coupled with HPLC-UV for the determination of procainamide in urine. Talanta, 167: 709-713.
DOI:10.1016/j.talanta.2017.02.051 |
Phong W N, Show P L, Teh W H, Teh T X, Lim H M Y, Nazri N S B, Tan C H, Chang J S, Ling T C. 2017. Proteins recovery from wet microalgae using liquid biphasic flotation (LBF). Bioresource Technology, 244: 1 329-1 336.
DOI:10.1016/j.biortech.2017.05.165 |
Platis D, Labrou N E. 2009. Application of a PEG/salt aqueous two-phase partition system for the recovery of monoclonal antibodies from unclarified transgenic tobacco extract. Biotechnology Journal, 4(9): 1 320-1 327.
DOI:10.1002/biot.200800359 |
Pradal D, Vauchel P, Decossin S, Dhulster P, Dimitrov K. 2016. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products:extraction and energy consumption optimization. Ultrasonics Sonochemistry, 32: 137-146.
DOI:10.1016/j.ultsonch.2016.03.001 |
Safi C, Ursu A V, Laroche C, Zebib B, Merah O, Pontalier P Y, Vaca-Garcia C. 2014. Aqueous extraction of proteins from microalgae:effect of different cell disruption methods. Algal Research, 3: 61-65.
DOI:10.1016/j.algal.2013.12.004 |
Sankaran R, Manickam S, Yap Y J, Ling T C, Chang J S, Show P L. 2018. Extraction of proteins from microalgae using integrated method of sugaring-out assisted liquid biphasic flotation (LBF) and ultrasound. Ultrasonics Sonochemistry, 48: 231-239.
DOI:10.1016/j.ultsonch.2018.06.002 |
Selvakumar P, Ling T C, Walker S, Lyddiatt A. 2010.A practical implementation and exploitation of ATPS for intensive processing of biological feedstock: a novel approach for heavily biological feedstock loaded ATPS.
|
Separation and Purification Technology, 75(3): 323-331, https://doi.org/10.1016/j.seppur.2010.08.022.
|
Shirsath S R, Sable S S, Gaikwad S G, Sonawane S H, Saini D R, Gogate P R. 2017. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach:effect of different operating parameters. Ultrasonics Sonochemistry, 38: 437-445.
DOI:10.1016/j.ultsonch.2017.03.040 |
Shishov A, Nechaeva D, Moskvin L, Andruch V, Bulatov A. 2017. Automated solid sample dissolution coupled with sugaring-out homogenous liquid-liquid extraction. Application for the analysis of throat lozenge samples. Journal of Molecular Liquids, 233: 149-155.
DOI:10.1016/j.molliq.2017.03.022 |
Show P L, Tan C P, Anuar M S, Ariff A, Yusof Y A, Chen S K, Ling T C. 2011. Direct recovery of lipase derived from Burkholderia cepacia in recycling aqueous two-phase flotation. Separation and Purification Technology, 80(3): 577-584.
DOI:10.1016/j.seppur.2011.06.013 |
Sicaire A G, Vian M A, Fine F, Carré P, Tostain S, Chemat F. 2016. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrasonics Sonochemistry, 31: 319-329.
DOI:10.1016/j.ultsonch.2016.01.011 |
Sivakumar M, Senthilkumar P, Majumdar S, Pandit A B. 2002. Ultrasound mediated alkaline hydrolysis of methyl benzoate - reinvestigation with crucial parameters. Ultrasonics Sonochemistry, 9(1): 25-30.
DOI:10.1016/S1350-4177(01)00099-2 |
Sivakumar V, Rani K, Kumari M. 2017. Efficient extraction of natural dye from red sandal wood (Pterocarpus Sandalinus) using ultrasound. International Wood Products Journal, 8(1): 6-9.
DOI:10.1080/20426445.2016.1214380 |
Soares R R, Azevedo A M, Van Alstine J M, Aires-Barros M R. 2015. Partitioning in aqueous two-phase systems:analysis of strengths, weaknesses, opportunities and threats. Biotechnology Journal, 10(8): 1 158-1 169.
DOI:10.1002/biot.201400532 |
Sobianowska K, Walkowiak W, Kozłowski C. 2009. Principles and applications of solvent sublation-a review. Ars Separatoria Acta, 7: 23-38.
|
Timofeeva I, Shishov A, Kanashina D, Dzema D, Bulatov A. 2017. On-line in-syringe sugaring-out liquid-liquid extraction coupled with HPLC-MS/MS for the determination of pesticides in fruit and berry juices. Talanta, 167: 761-767.
DOI:10.1016/j.talanta.2017.01.008 |
Tu X J, Sun F Y, Wu S Y, Liu W Y, Gao Z S, Huang S K, Chen W B. 2018. Comparison of salting-out and sugaring-out liquid-liquid extraction methods for the partition of 10-hydroxy-2-decenoic acid in royal jelly and their coextracted protein content. Journal of Chromatography B, 1073: 90-95.
DOI:10.1016/j.jchromb.2017.12.020 |
Wang B, Ezejias T, Feng H, Blaschek H. 2008a. Sugaring-out:a novel phase separation and extraction system. Chemical Engineering Science, 63(9): 2 595-2 600.
DOI:10.1016/j.ces.2008.02.004 |
Wang B, Feng H, Ezeji T, Blaschek H. 2008b. Sugaring-out separation of acetonitrile from its aqueous solution. Chemical Engineering & Technology, 31(12): 1 869-1 874.
DOI:10.1002/ceat.200800003 |
Wu J Y, Lin L D, Chau F T. 2001. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrasonics Sonochemistry, 8(4): 347-352.
DOI:10.1016/S1350-4177(01)00066-9 |
Xia S, Wan L L, Li A F, Sang M, Zhang C W. 2013. Effects of nutrients and light intensity on the growth and biochemical composition of a marine microalga Odontella aurita. Chinese Journal of Oceanology and Limnology, 31(6): 1 163-1 173.
DOI:10.1007/s00343-013-2092-4 |
Yang H C, Zeng M Y, Dong S Y, Liu Z Y, Li R X. 2010. Antiproliferative activity of phlorotannin extracts from brown algae Laminaria japonica Aresch. Chinese Journal of Oceanology and Limnology, 28(1): 122-130.
DOI:10.1007/s00343-010-9054-x |
Zhang C, Huang K, Yu P H, Liu H Z. 2012. Sugaring-out threeliquid-phase extraction and one-step separation of Pt(IV), Pd(Ⅱ) and Rh(Ⅲ). Separation and Purification Technology, 87: 127-134.
DOI:10.1016/j.seppur.2011.11.032 |
Zimmermann S, Gretzinger S, Zimmermann P K, Bogsnes A, Hansson M, Hubbuch J. 2018. Cell separation in aqueous two-phase systems- influence of polymer molecular weight and tie-line length on the resolution of five model cell lines. Biotechnology Journal, 13(2): 1 700 250.
DOI:10.1002/biot.201700250 |
 2019, Vol. 37
2019, Vol. 37


